Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patient Selection and Data Collection
2.3. Statistical Analysis
2.3.1. Descriptive Comparison and Grouping
2.3.2. Derivation Stage
2.3.3. Validation Stage
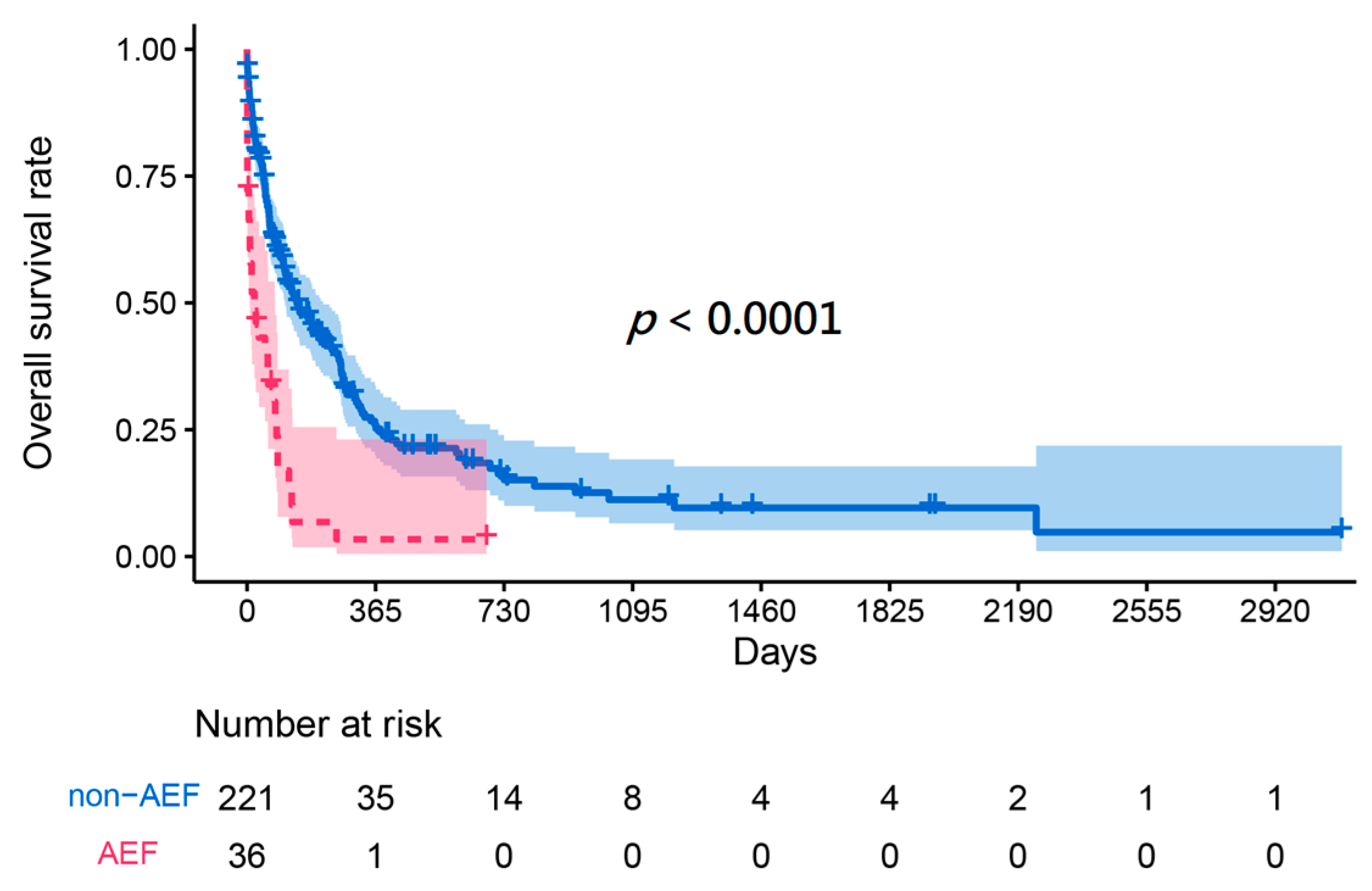
3. Results
3.1. Patient Characteristics of Derivation Cohort and Validation Cohort
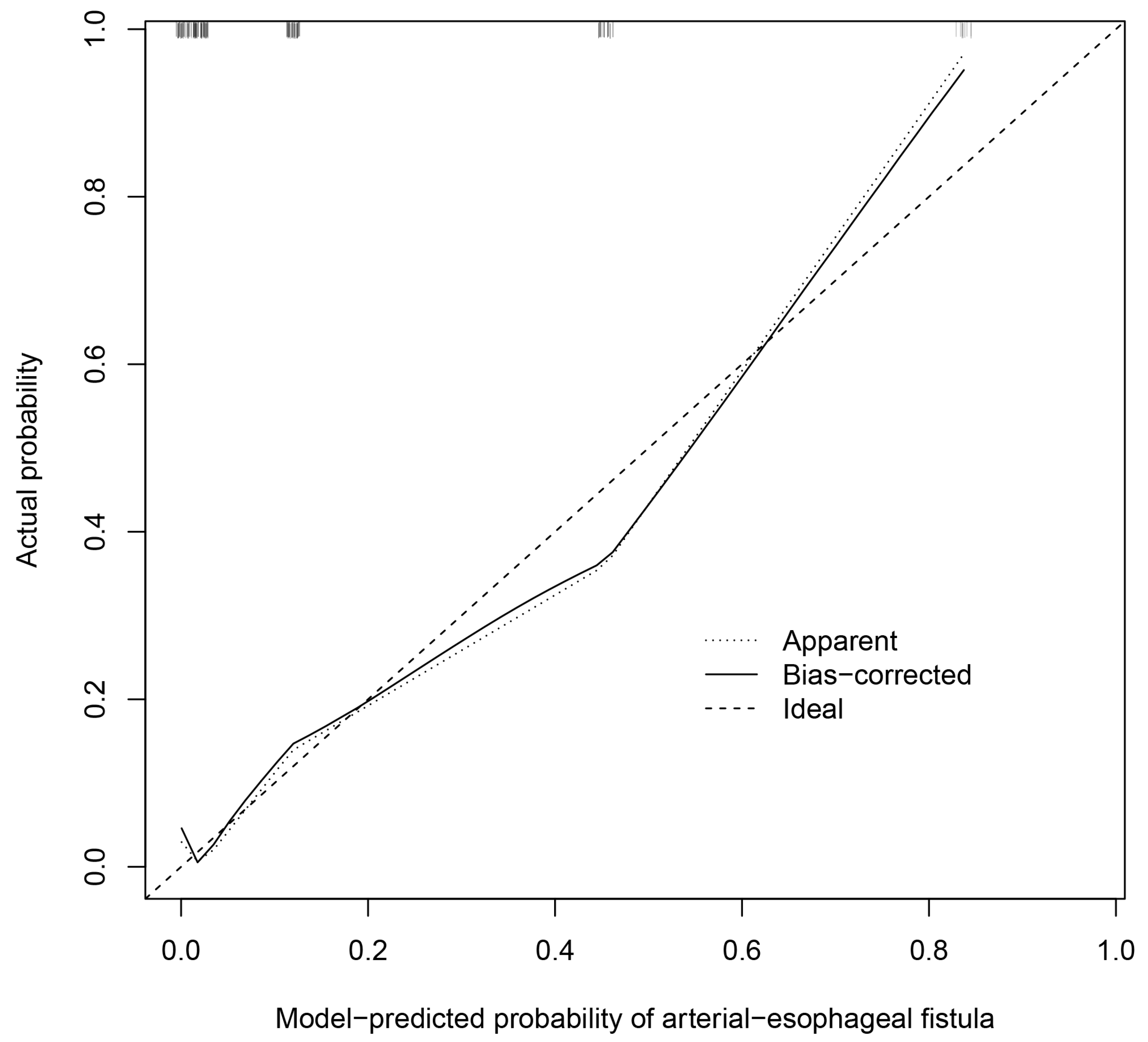
3.2. Derivation Stage
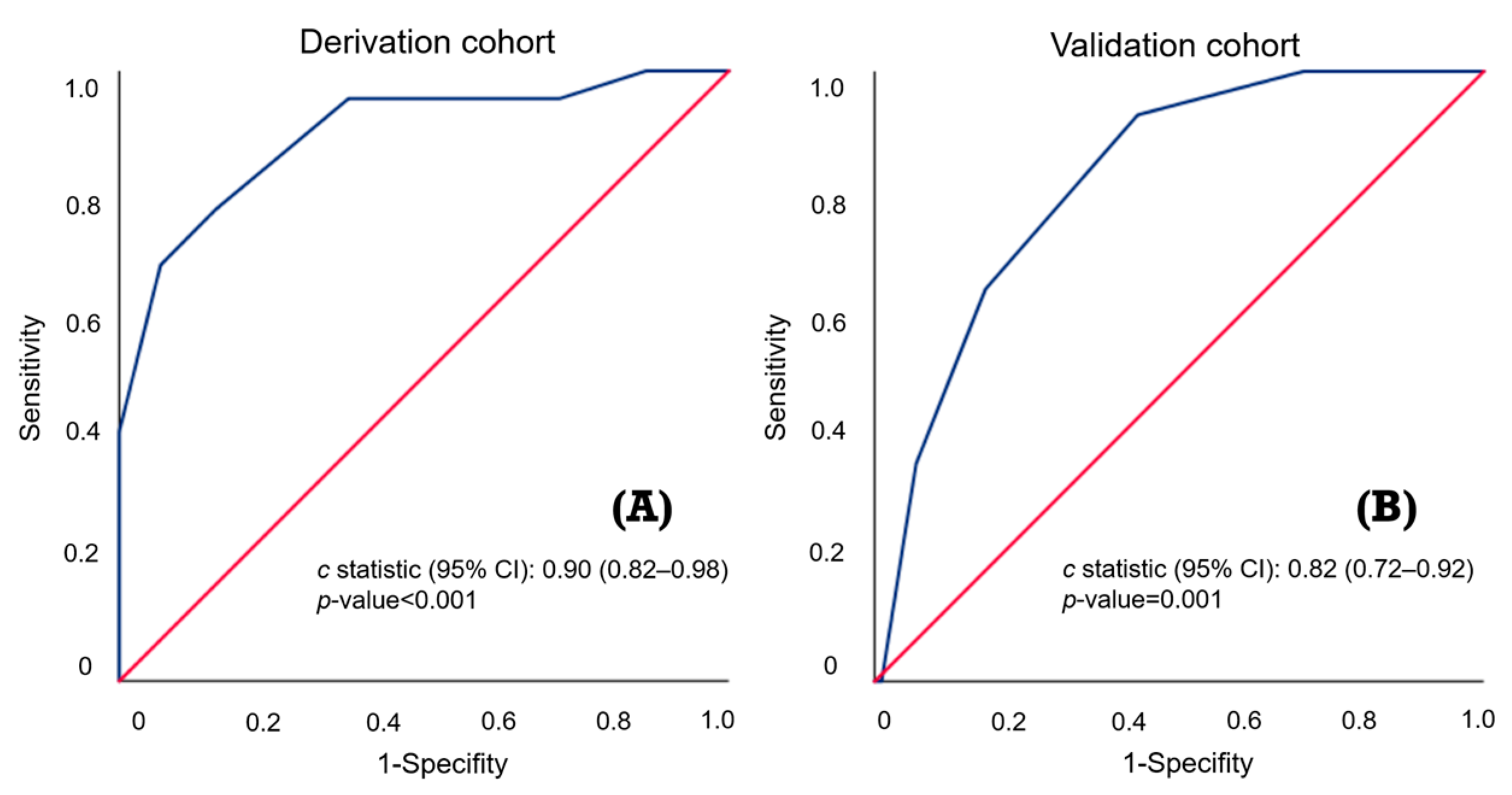
3.3. Validation Stage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.C.; Huang, M.C.; Lee, J.M.; Wu, D.C.; Hsu, H.K.; Wu, M.T. Association between diet and esophageal cancer in Taiwan. J. Gastroenterol. Hepatol. 2004, 19, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S. Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: A meta-analysis of case–control studies. Cancer Causes Control. 2013, 24, 257–265. [Google Scholar] [CrossRef]
- Markar, S.R.; Mackenzie, H.; Jemal, S.; Faiz, O.; Cunningham, D.; Hanna, G.B. Emergency presentation of esophagogastric cancer: Predictors and long-term prognosis. Ann. Surg. 2018, 267, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Pai, C.P.; Yang, T.H.; Lu, J.X.; Hsiao, C.H.; Yen, C.C. Clinical characteristics and risk factors for 30-day mortality in esophageal cancer patients with upper gastrointestinal bleeding: A multicenter study. Front. Oncol. 2023, 13, 1184710. [Google Scholar] [CrossRef]
- Takeno, S.; Ishii, H.; Nanashima, A.; Nakamura, K. Aortoesophageal fistula: Review of trends in the last decade. Surg. Today 2020, 50, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Han, H.C.; Ha, F.J.; Sanders, P.; Spencer, R.; Teh, A.W.; O’Donnell, D.; Farouque, O.; Lim, H.S. Atrioesophageal Fistula: Clinical Presentation, Procedural Characteristics, Diagnostic Investigations, and Treatment Outcomes. Circ. Arrhythm. Electrophysiol. 2017, 10, e005579. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Mulder, G.A.; Snyder, E.N., Jr.; Brewer, L.A., 3rd. Aortoesophageal fistula. Am. J. Surg. 1978, 136, 26–30. [Google Scholar] [CrossRef]
- Flores, J.; Shiiya, N.; Kunihara, T.; Yoshimoto, K.; Yasuda, K. Aortoesophageal fistula: Alternatives of treatment case report and literature review. Ann. Thorac. Cardiovasc. Surg. 2004, 10, 241–246. [Google Scholar] [PubMed]
- Akashi, H.; Kawamoto, S.; Saiki, Y.; Sakamoto, T.; Sawa, Y.; Tsukube, T.; Kubota, S.; Matsui, Y.; Karube, N.; Imoto, K. Therapeutic strategy for treating aortoesophageal fistulas. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Heckstall, R.L.; Hollander, J.E. Aortoesophageal fistula: Recognition and diagnosis in the emergency department. Ann. Emerg. Med. 1998, 32, 502–505. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Tustumi, F.; Kimura, C.M.S.; Takeda, F.R.; Uema, R.H.; Salum, R.A.A.; Ribeiro-Junior, U.; Cecconello, I. Prognostic factors and survival analysis in esophageal carcinoma. ABCD. Arq. Bras. Cir. Dig. 2016, 29, 138–141. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, K.U.; Kim, S.J.; Seo, S.I.; Kim, H.S.; Jang, M.K.; Kim, H.Y.; Shin, W.G. Predictors for the need for endoscopic therapy in patients with presumed acute upper gastrointestinal bleeding. Korean J. Intern. Med. 2019, 34, 288–295. [Google Scholar] [CrossRef]
- Monreal, M.; FalgÁ, C.; ValdÉS, M.; SuÁRez, C.; Gabriel, F.; Tolosa, C.; Montes, J. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: Findings from the RIETE registry. J. Thromb. Haemost. 2006, 4, 1950–1956. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.I.; Healy, B.; Dzik, W.H. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion 2006, 46, 1279–1285. [Google Scholar] [CrossRef]
- Yen, C.C.; Yeh, H.; Ho, C.F.; Hsiao, C.H.; Niu, K.Y.; Yeh, C.C.; Lu, J.X.; Wu, C.C.; Chang, Y.C.; Ng, C.J. Risk factors for 30-day mortality in patients with head and neck cancer bleeding in the emergency department. Am. J. Emerg. Med. 2022, 58, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Ho, C.F.; Wu, C.C.; Tsao, Y.N.; Chaou, C.H.; Chen, S.Y.; Ng, C.J.; Yeh, H. In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding. Medicina 2022, 58, 177. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Shao, S.-C.; Lee, Y.-C.; Hsiao, C.-H.; Yen, C.-C. Risk factors for peri-intubation cardiac arrest: A systematic review and meta-analysis. Biomed. J. 2023, 100656. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Ho, C.F.; Niu, K.Y.; Wu, C.C.; Chang, Y.C.; Hsiao, C.H.; Yen, C.C. Risk factors for rebleeding and long-term outcomes in patients with head and neck cancer bleeding: A multicenter study. BMC Cancer 2022, 22, 841. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Bleeker, S.E.; Moll, H.A.; Grobbee, D.E.; Moons, K.G. Internal and external validation of predictive models: A simulation study of bias and precision in small samples. J. Clin. Epidemiol. 2003, 56, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.E.; Quick, G. Aortoesophageal fistula: A comprehensive review of the literature. Am. J. Med. 1991, 91, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Park, S.I.; Do, Y.S.; Yoon, H.K.; Sung, K.B.; Sohn, K.H.; Min, Y.I. Expandable metallic stent placement in patients with benign esophageal strictures: Results of long-term follow-up. Radiology 1997, 203, 131–136. [Google Scholar] [CrossRef]
- Tian, D.; Wen, H.; Fu, M. Comparative study of self-expanding metal stent and intraluminal radioactive stent for inoperable esophageal squamous cell carcinoma. World J. Surg. Oncol. 2016, 14, 18. [Google Scholar] [CrossRef]
- Hubmann, R.; Bodlaj, G.; Czompo, M.; Benkö, L.; Pichler, P.; Al-Kathib, S.; Kiblböck, P.; Shamyieh, A.; Biesenbach, G. The use of self-expanding metal stents to treat acute esophageal variceal bleeding. Endoscopy 2006, 38, 896–901. [Google Scholar] [CrossRef]
- Bilal, S.; Saeed, S.M.; Siddique, M.Z.; Saqib, M.; Mehmood, S.; Yusuf, M.A. Salvage therapy of bleeding esophageal tumor by fully covered self-expandable metallic stent: A case report. SAGE Open Med. Case Rep. 2021, 9, 2050313x21997198. [Google Scholar] [CrossRef]
- Siersema, P.D.; Tan, T.G.; Sutorius, F.F.; Dees, J.; van Blankenstein, M. Massive hemorrhage caused by a perforating Gianturco-Z stent resulting in an aortoesophageal fistula. Endoscopy 1997, 29, 416–420. [Google Scholar] [CrossRef]
- Unosawa, S.; Hata, M.; Sezai, A.; Niino, T.; Yoda, M.; Shimura, K.; Furukawa, N.; Minami, K. Surgical treatment of an aortoesophageal fistula caused by stent implantation for esophageal stenosis: Report of a case. Surg. Today 2008, 38, 62–64. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, Z. Massive hemorrhage from an aortoesophageal fistula caused by esophageal stent implantation: A case report and literature review. Medicina 2019, 98, e18303. [Google Scholar] [CrossRef]
- McGinnis, G.J.; Holland, J.M.; Thomas, C.R., Jr.; Nabavizadeh, N. Massive hemorrhage following definitive esophageal chemoradiation: Teaching case of a fatal aortoesophageal fistula and lessons learned. Clin. Case Rep. 2017, 5, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Kahlberg, A.; Rinaldi, E.; Piffaretti, G.; Speziale, F.; Trimarchi, S.; Bonardelli, S.; Melissano, G.; Chiesa, R. Results from the Multicenter Study on Aortoenteric Fistulization After Stent Grafting of the Abdominal Aorta (MAEFISTO). J. Vasc. Surg. 2016, 64, 313–320.e311. [Google Scholar] [CrossRef]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to Interpret and Pursue an Abnormal Prothrombin Time, Activated Partial Thromboplastin Time, and Bleeding Time in Adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Ferrell, C.; Chandler, W.L. Comparing the prothrombin time INR versus the APTT to evaluate the coagulopathy of acute trauma. Thromb. Res. 2007, 120, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; James, M.T. Acute Kidney Injury. Ann. Intern. Med. 2017, 167, ITC66–ITC80. [Google Scholar] [CrossRef]
- Eriksson, M.; Brattström, O.; Mårtensson, J.; Larsson, E.; Oldner, A. Acute kidney injury following severe trauma: Risk factors and long-term outcome. J. Trauma Acute Care Surg. 2015, 79, 407–412. [Google Scholar] [CrossRef]
- Perkins, Z.B.; Haines, R.W.; Prowle, J.R. Trauma-associated acute kidney injury. Curr. Opin. Crit. Care 2019, 25, 565–572. [Google Scholar] [CrossRef]

| Variable | Total n = 257 | Derivation Cohort n = 155 | Validation Cohort n = 102 | p Value |
|---|---|---|---|---|
| Age (years) | 59.3 11.5 | 59.2 11.6 | 59.4 11.3 | 0.873 |
| Male | 249 (96.9) | 151 (97.4) | 98 (96.1) | 0.716 |
| BMI (kg/m2) | 20.6 3.6 | 20.6 3.5 | 20.8 3.8 | 0.707 |
| ECOG-PS | 0.698 | |||
| 0 | 1 (0.4) | 0 (0) | 1 (1.0) | |
| 1 | 188 (73.2) | 113 (72.9) | 75 (73.5) | |
| 2 | 46 (17.9) | 30 (19.4) | 16 (15.7) | |
| 3 | 18 (7.0) | 10 (6.5) | 8 (7.8) | |
| 4 | 4 (1.6) | 2 (1.3) | 2 (2.0) | |
| Initial Vital Signs | ||||
| SBP (mmHg) | 123.4 29.0 | 120.7 28.5 | 127.4 29.5 | 0.072 |
| DBP (mmHg) | 76.3 15.9 | 76.0 16.6 | 76.7 15.0 | 0.700 |
| Heart rate (beats/min) | 105.6 21.6 | 104.4 22.1 | 107.5 20.8 | 0.266 |
| Respiratory rate (breaths/min) | 19.5 2.9 | 19.6 2.9 | 19.4 3.0 | 0.596 |
| Personal Habits | ||||
| Smoking history | 211 (82.1) | 126 (81.3) | 85 (83.3) | 0.741 |
| Betel nut chewer | 130 (50.6) | 78 (50.3) | 52 (51.0) | 1.000 |
| Alcohol consumption | 191 (74.3) | 116 (74.8) | 75 (73.5) | 0.884 |
| Comorbidity | ||||
| Hypertension | 77 (30.0) | 42 (27.1) | 35 (34.3) | 0.266 |
| Diabetes mellitus | 39 (15.2) | 21 (13.5) | 18 (17.6) | 0.380 |
| Coronary artery disease | 12 (4.7) | 7 (4.5) | 5 (4.9) | 1.000 |
| Congestive heart failure | 5 (1.9) | 3 (1.9) | 2 (2.0) | 1.000 |
| Gastroesophageal reflux disease | 135 (52.5) | 87 (56.1) | 48 (47.1) | 0.162 |
| Chronic kidney disease | 19 (7.4) | 11 (7.1) | 8 (7.8) | 1.000 |
| Prior cerebrovascular accident | 12 (4.7) | 2 (1.3) | 10 (9.8) | 0.002* |
| Liver cirrhosis | 44 (17.1) | 27 (17.4) | 17 (16.7) | 1.000 |
| Chronic obstructive pulmonary disease | 7 (2.7) | 5 (3.2) | 2 (2.0) | 0.706 |
| Other malignancy | 58 (22.6) | 33 (21.3) | 25 (24.5) | 0.648 |
| Charlson comorbidity index | 6.92 2.83 | 6.89 2.84 | 6.97 2.82 | 0.824 |
| Current Medication | ||||
| Use of NSAIDs | 24 (9.3) | 12 (7.7) | 12 (11.8) | 0.381 |
| Use of Anti-platelets Agent〒 | 14 (5.4) | 7 (4.5) | 7 (6.9) | 0.576 |
| Use of Anti-coagulant Agent† | 1 (0.4) | 1 (0.6) | 0 (0) | 1.000 |
| Use of PPI or H2-receptor antagonist | 89 (34.6) | 54 (34.8) | 35 (34.3) | 1.000 |
| Initial Presenting symptoms | ||||
| Hematemesis | 176 (68.5) | 108 (69.7) | 68 (66.7) | 0.681 |
| Melena | 81 (31.5) | 47 (30.3) | 34 (33.3) | 0.681 |
| Active bleeding | 64 (24.9) | 38 (24.5) | 26 (25.5) | 0.884 |
| Arterial–esophageal fistula | 36 (14.0) | 22 (14.2) | 14 (13.7) | 1.000 |
| Initial Laboratory data | ||||
| WBC (103/) | 11.4 9.5 | 11.7 10.4 | 11.2 8.2 | 0.700 |
| Hb (g/dL) | 9.5 2.5 | 9.6 2.5 | 9.3 2.4 | 0.317 |
| PLT (103/) | 253 138 | 243 127 | 269 154 | 0.140 |
| PT (s) | 12.9 6.1 | 13.1 7.6 | 12.6 2.7 | 0.544 |
| Creatinine (mg/dL) | 1.05 0.75 | 1.02 0.53 | 1.10 1.00 | 0.364 |
| Variable | Total n = 257 | Derivation Cohort n = 155 | Validation Cohort n = 102 | p Value |
|---|---|---|---|---|
| Tumor site (Esophagus) | 0.283 | |||
| Upper third | 50 (19.5) | 34 (21.9) | 16 (15.7) | |
| Middle third | 95 (40.0) | 52 (33.5) | 43 (42.2) | |
| Lower third | 112 (43.6) | 69 (44.5) | 43 (42.2) | |
| Tumor length (cm) | 7.11 3.30 | 6.88 3.05 | 8.24 4.21 | 0.119 |
| Tumor pathology | 0.283 | |||
| Squamous cell carcinoma | 236 (91.8) | 142 (91.6) | 94 (92.2) | |
| Adenocarcinoma | 13 (5.0) | 8 (5.2) | 5 (4.9) | |
| Small cell carcinoma | 1 (0.4) | 0 (0) | 1 (1.0) | |
| Melanoma | 2 (0.8) | 1 (0.6) | 1 (1.0) | |
| Neuroendocrine | 1 (0.4) | 0 (0) | 1 (1.0) | |
| Unknown | 4 (1.6) | 4 (2.6) | 0 (0) | |
| T stage | 0.378 | |||
| T1 | 7 (3.9) | 6 (3.9) | 1 (1.0) | |
| T2 | 25 (9.7) | 17 (11) | 8 (7.8) | |
| T3 | 116 (45.1) | 64 (41.3) | 52 (51.0) | |
| T4 | 105 (40.9) | 66 (42.6) | 39 (38.2) | |
| Unknown | 4 (1.6) | 2 (1.3) | 2 (2.0) | |
| N stage | 0.701 | |||
| N0 | 40 (15.6) | 22 (14.2) | 18 (17.6) | |
| N+ | 215 (83.7) | 132 (85.2) | 83 (81.4) | |
| Unknown | 2 (0.8) | 1 (0.6) | 1 (1.0) | |
| M stage | 0.752 | |||
| M0 | 155 (60.3) | 90 (58.1) | 65 (63.7) | |
| M1 | 99 (38.5) | 63 (40.6) | 36 (35.3) | |
| Unknown | 3 (1.2) | 2 (1.3) | 1 (1.0) | |
| Cancer stage | 0.728 | |||
| I | 6 (2.3) | 5 (3.2) | 1 (1.0) | |
| II | 17 (6.6) | 10 (6.5) | 7 (6.9) | |
| III | 59 (23.0) | 34 (21.9) | 25 (24.5) | |
| IV | 175 (68.1) | 106 (68.4) | 69 (67.6) | |
| Initial cancer treatment | ||||
| Surgical resection | 17 (6.6) | 7 (4.5) | 10 (9.8) | 0.124 |
| Chemoradiation | 170 (66.1) | 98 (63.3) | 72 (70.6) | 0.229 |
| Stent implantation | 38 (14.8) | 20 (12.9) | 18 (17.6) | 0.369 |
| Local recurrence | 74 (28.8) | 45 (29.0) | 29 (28.4) | 1.000 |
| Emergent examination | ||||
| Endoscopy | 222 (86.4) | 133 (85.8) | 89 (87.3) | 0.853 |
| Emergent CTA | 53 (20.6) | 32 (20.6) | 21 (20.6) | 1.000 |
| Initial medication | ||||
| Proton pump inhibitor | 235 (91.3) | 142 (91.6) | 93 (91.2) | 1.000 |
| Tranexamic acid | 188 (73.2) | 116 (74.8) | 72 (70.6) | 0.474 |
| Terlipressin | 32 (12.5) | 16 (10.3) | 16 (15.7) | 0.247 |
| Bleeding treatment | 0.687 | |||
| Endoscopic treatment † | 17 (6.6) | 10 (6.5) | 7 (6.9) | |
| Surgical repair/Stent implantation | 26 (10.1) | 13 (8.4) | 13 (12.7) | |
| Arterial embolization | 7 (2.7) | 4 (2.6) | 3 (3.0) | |
| Blood transfusion | 148 (57.6) | 86 (55.5) | 62 (60.8) | 0.440 |
| Intubation | 34 (13.2) | 22 (14.2) | 12 (11.8) | 0.707 |
| Inotropic agents support | 11 (4.3) | 6 (3.9) | 5 (4.9) | 0.758 |
| ICU admission | 29 (11.3) | 17 (11.0) | 12 (11.8) | 1.000 |
| Hospice care | 81 (31.5) | 49 (31.6) | 32 (31.4) | 1.000 |
| Rebleeding event | 160 (62.3) | 92 (59.4) | 68 (66.7) | 0.293 |
| Number of Patients | Univariate | Multivariate | -Regression Coefficient | Point † | |||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p value | ||||
| Age > 65 | 39 | 0.60 (0.19, 1.89) | 0.382 | ||||
| Male | 151 | 0.49 (0.48, 4.88) | 0.539 | ||||
| BMI¶ | |||||||
| Non-underweight | 104 | Reference | |||||
| Underweight〒 | 41 | 1.72 (0.65, 4.56) | 0.274 | ||||
| ECOG-PS > 2 | 11 | 2.18 (0.54, 8.76) | 0.274 | ||||
| Body temperature > 38℃ | 6 | 3.23 (0.55, 18.8) | 0.193 | ||||
| Heart rate > 110 (beats/min) | 59 | 2.56 (1.02, 6.42) | 0.046 * | 0.94 (0.21, 4.20) | 0.933 | −0.064 | - |
| RR > 22 (breaths/min) | 14 | 5.17 (1.63, 16.4) | 0.005 * | 4.18 (0.68, 25.7) | 0.123 | 1.430 | - |
| SBP < 90 (mmHg) | 22 | 1.77 (0.58, 5.35) | 0.316 | ||||
| Underlying disease | |||||||
| Hypertension | 41 | 0.76 (0.26, 2.22) | 0.619 | ||||
| Diabetes mellitus | 20 | 1.01 (0.27, 3.76) | 0.990 | ||||
| Coronary artery disease | 7 | 1.01 (0.12, 8.80) | 0.994 | ||||
| Congestive heart failure | 3 | 0.00 (0.00, 0.00) | 0.999 | ||||
| Chronic kidney disease | 11 | 0.00 (0.00, 0.00) | 0.999 | ||||
| Prior cerebrovascular accident | 2 | 0.00 (0.00, 0.00) | 0.999 | ||||
| Liver cirrhosis | 26 | 0.72 (0.20, 2.62) | 0.615 | ||||
| Gastroesophageal reflux disease | 86 | 0.49 (0.20, 1.22) | 0.125 | ||||
| Initial presentation | |||||||
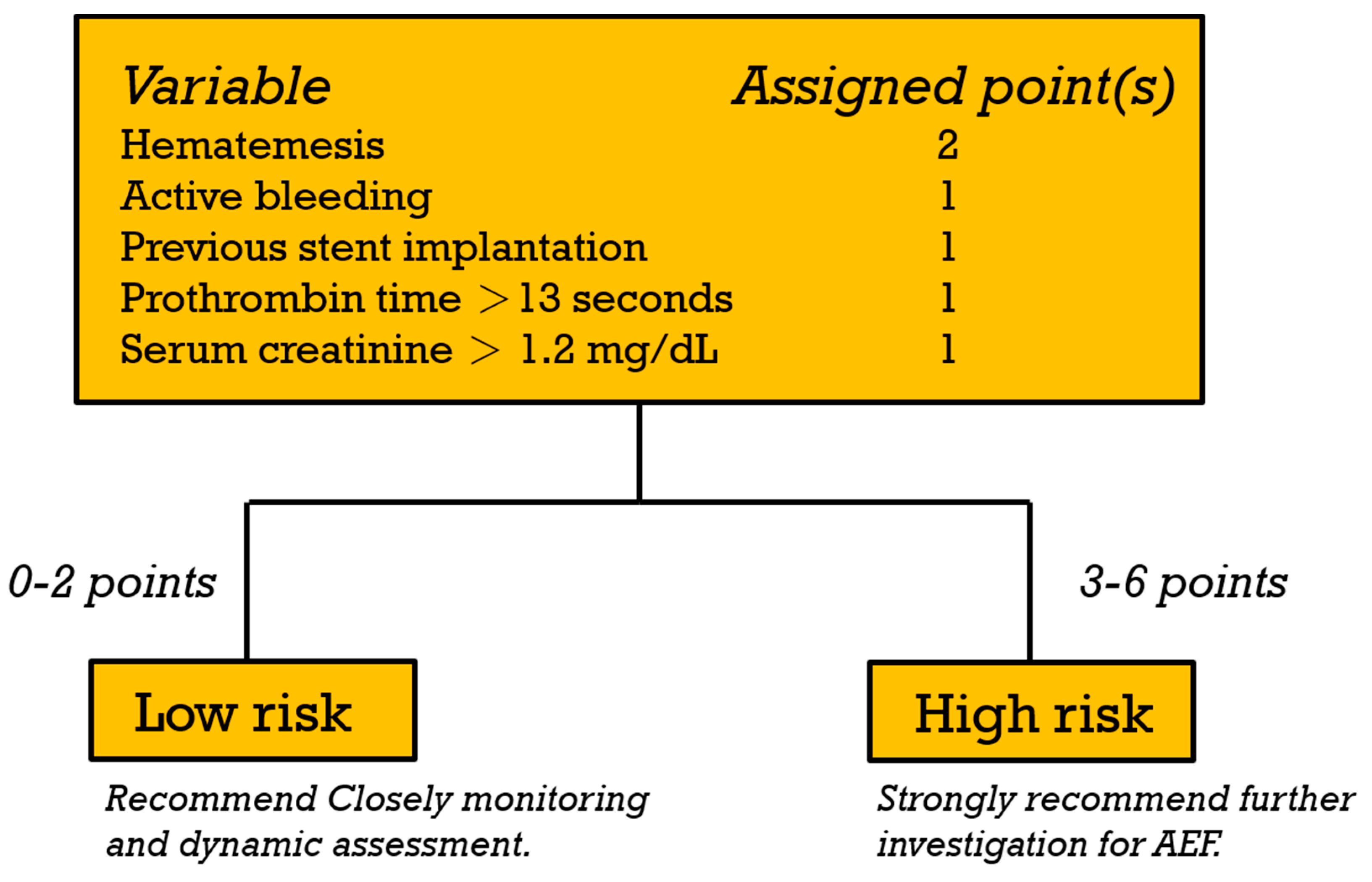
| Hematemesis | 108 | 11.1 (1.45, 85.2) | 0.021 * | 16.7 (1.49, 187.1) | 0.022* | 2.816 | 2 |
| Active bleeding | 38 | 13.5 (4.73, 38.2) | <0.001 * | 5.45 (1.37, 21.7) | 0.016* | 1.695 | 1 |
| Anti-platelets agents use | 7 | 2.56 (0.47, 14.1) | 0.280 | ||||
| Anti-coagulant agents use | 1 | 0.00 (0.00, 0.00) | 1.000 | ||||
| Tumor location | |||||||
| Upper third | 34 | 1.41 (0.50, 3.93) | 0.515 | ||||
| Middle third | 52 | 1.81 (0.72, 4.51) | 0.206 | ||||
| Lower third | 69 | 0.42 (0.15, 1.13) | 0.086 | ||||
| Cancer stage¶ | |||||||
| T stage > 2 | 130 | 1.07 (0.29, 3.98) | 0.918 | ||||
| N stage > 0 | 132 | 1.68 (0.36, 7.79) | 0.506 | ||||
| M stage > 0 | 63 | 1.36 (0.54, 3.41) | 0.519 | ||||
| Cancer treatment | |||||||
| Surgical resection | 7 | 0.01 (0.12, 8.80) | 0.994 | ||||
| Chemoradiation | 98 | 2.98 (0.96, 9.30) | 0.060 | ||||
| Stent implantation | 20 | 5.76 (2.01, 16.5) | 0.001 * | 8.75 (1.78, 42.9) | 0.008 * | 2.169 | 1 |
| Local recurrence | 45 | 2.33 (0.93, 5.88) | 0.072 | ||||
| Initial Laboratory data | |||||||
| WBC > 11.0 (103/μL) | 62 | 1.61 (0.65, 3.98) | 0.304 | ||||
| Hb < 8.0 (g/dL) | 38 | 3.93 (1.54, 10.0) | 0.004 * | 2.76 (0.60, 12.7) | 0.195 | 1.013 | - |
| PLT < 150 (103/μL) | 37 | 0.93 (0.317, 2.72) | 0.892 | ||||
| PT > 13 (s)¶ | 51 | 3.31 (1.30, 8.39) | 0.012 * | 5.09 (1.15, 22.6) | 0.032 * | 1.627 | 1 |
| Cr > 1.2 (mg/dL)¶ | 33 | 3.95 (1.52, 10.2) | 0.005 * | 9.76 (2.37, 40.2) | 0.002 * | 2.278 | 1 |
| Risk Score (6-Point Scoring System) | Risk Classification | ||||||
|---|---|---|---|---|---|---|---|
| Total Points | Patients (n = 146) * | AEF | Rate of AEF, % | Risk Category | Patients | AEF | Rate of AEF, % (95% CI) |
| 0 | 15 | 0 | 0.0 | Low risk | 81 | 1 | 1.2 (0–6.7) |
| 1 | 19 | 1 | 5.3 | ||||
| 2 | 47 | 0 | 0.0 | ||||
| 3 | 36 | 5 | 13.9 | High risk | 65 | 21 | 32.3 (21.2–45.0) |
| 4 | 20 | 7 | 35.0 | ||||
| 5 | 9 | 9 | 100.0 | ||||
| 6 | 0 | 0 | NA | ||||
| Risk Score (6-Point Scoring System) | Risk Classification | ||||||
|---|---|---|---|---|---|---|---|
| Total Points | Patients (n = 95) * | AEF | Rate of AEF (%) | Risk Category | Patients | AEF | Rate of AEF, % (95% CI) |
| 0 | 10 | 0 | 0.0 | Low risk | 46 | 2 | 4.3(0.5–14.8) |
| 1 | 13 | 0 | 0.0 | ||||
| 2 | 23 | 2 | 8.7 | ||||
| 3 | 35 | 6 | 17.1 | High risk | 49 | 12 | 24.5(13.3–38.9) |
| 4 | 11 | 5 | 45.5 | ||||
| 5 | 2 | 1 | 50.0 | ||||
| 6 | 1 | 0 | 0.0 | ||||
| Derivation Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|
| C-Statistic (95% CI): 0.90 (0.82–0.98) | C-Statistic (95% CI): 0.82 (0.72–0.92) | ||||
| Low risk | High risk | Low risk | High risk | ||
| Patients, n (%) | 81(55.5) | 65(44.5) | Patients, n (%) | 46(48.4) | 49(51.6) |
| AEF, n (%) | 1(1.2) | 21(32.3) | AEF, n (%) | 2(4.3) | 12(24.5) |
| Sensitivity, % | 4.5 | 95.5 | Sensitivity, % | 14.3 | 85.7 |
| Specificity, % | 35.5 | 64.5 | Specificity, % | 45.7 | 54.3 |
| PPV, % | 1.2 | 32.3 | PPV, % | 4.3 | 24.5 |
| NPV, % | 67.7 | 98.8 | NPV, % | 75.5 | 95.7 |
| Weighted accuracy, % | 20.0 | 80.0 | Weighted accuracy, % | 30.0 | 70.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.-W.; Niu, K.-Y.; Pai, C.-P.; Lin, S.-H.; Chen, C.-B.; Lo, Y.-T.; Lee, Y.-C.; Seak, C.-J.; Yen, C.-C. Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study. Cancers 2024, 16, 804. https://doi.org/10.3390/cancers16040804
Lu S-W, Niu K-Y, Pai C-P, Lin S-H, Chen C-B, Lo Y-T, Lee Y-C, Seak C-J, Yen C-C. Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study. Cancers. 2024; 16(4):804. https://doi.org/10.3390/cancers16040804
Chicago/Turabian StyleLu, Sz-Wei, Kuang-Yu Niu, Chu-Pin Pai, Shih-Hua Lin, Chen-Bin Chen, Yu-Tai Lo, Yi-Chih Lee, Chen-June Seak, and Chieh-Ching Yen. 2024. "Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study" Cancers 16, no. 4: 804. https://doi.org/10.3390/cancers16040804
APA StyleLu, S. -W., Niu, K. -Y., Pai, C. -P., Lin, S. -H., Chen, C. -B., Lo, Y. -T., Lee, Y. -C., Seak, C. -J., & Yen, C. -C. (2024). Novel Prediction Score for Arterial–Esophageal Fistula in Patients with Esophageal Cancer Bleeding: A Multicenter Study. Cancers, 16(4), 804. https://doi.org/10.3390/cancers16040804






