Design and Fine-Tuning Redox Potentials of Manganese(II) Complexes with Isoindoline-Based Ligands: H2O2 Oxidation and Oxidative Bleaching Performance in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of [MnII(HLn)Cl2] Complexes
2.2. Catalase-Like Reactivity of [MnII(HL1–7)Cl2] Complexes in Aqueous Solution
2.3. Oxidative Degradation of Morin: [MnII(HL1–6)Cl2] Complexes as Bleaching Catalysts
3. Materials and Methods
3.1. Materials and Methods
3.2. Syntheses and Characterization
3.2.1. 1,3-Bis(2’-imidazolyl)isoindoline (HL3)
3.2.2. [MnII(HL3)Cl2] (3)
3.2.3. [MnII(HL2)Cl2] (2)
3.2.4. [MnII(HL4)Cl2] (4)
3.2.5. [MnII(HL6)Cl2] (6)
3.3. Test Reactions of the Catalase-Like Activity
3.4. Bleaching of Morin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Csonka, R.; Speier, G.; Kaizer, J. Isoindoline-derived ligands and applications. RSC Adv. 2015, 5, 18401–18419. [Google Scholar] [CrossRef]
- Oehlmann, W.; Auling, G. Ribonucleotid reductase (RNR) of Corynebacterium glutamicum ATCC 13032-genetic characterization of a second class IV enzyme. Microbiology 1999, 145, 1595–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, J.K.; Gold, M.H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1985, 242, 329–341. [Google Scholar] [CrossRef]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzym. Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Dal Pozzo, L.; Del Conte, R.; Tien, M. Monitoring the role of oxalate in manganese peroxidase. Biochemistry 1998, 37, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem. 1983, 258, 6015–6019. [Google Scholar] [PubMed]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal structure of manganese catalase from Lactobacillus plantarum. Structure 2001, 9, 725–738. [Google Scholar] [CrossRef]
- Antonyuk, S.V.; Melik-Adman, V.R.; Popov, A.N.; Lamzin, V.S.; Hempstead, P.D.; Harrison, P.M.; Artymyuk, P.J.; Barynin, V.V. Three-dimensional structure of the enzyme dimanganese catalase from Thermus thermophilus at 1 Å resolution. Crystallogr. Rep. 2000, 45, 105–113. [Google Scholar] [CrossRef]
- Barynin, V.V.; Grebenko, A.I. T-catalase is nonheme catalase of the extremely thermophilic bacterium Thermus thermophilus HB8. Dokl. Akad. Nauk SSSR 1986, 286, 461–464. [Google Scholar]
- Allgood, G.S.; Perry, J.J. Characterization of a manganese-containing catalase from the obligate thermophile Thermoleophilum album. J. Bacteriol. 1986, 168, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Beyer, W.F.; Fridovich, I. Catalases-with and without heme. Basic Life Sci. 1988, 49, 651–661. [Google Scholar] [PubMed]
- Nicholls, P.; Fita, I.; Loewen, P.C. Enzymology and structure of catalases. Adv. Inorg. Chem. 2001, 51, 51–106. [Google Scholar]
- Call, H.P.; Mijcke, I. History, overview and application of mediated lignolytic systems, especially laccase-mediator-systems (LignozymR-process). J. Biotechnol. 1997, 53, 163–202. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates, an expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally designed mimics of antioxidant manganoenzymes: Role of structural features in the quest for catalysts with catalaseand superoxide dismutase activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- Wu, A.J.; Penner-Hahn, J.E.; Pecoraro, V.L. Structural, spectroscopic, and reactivity models for the manganese catalases. Chem. Rev. 2004, 104, 903–938. [Google Scholar] [CrossRef]
- Boelrijk, A.E.M.; Dismukes, G.C. Mechanism of hydrogen peroxide dismutation by a dimanganese catalase mimic: Dominant role of an intramolecular base on substrate binding affinity and rate acceleration. Inorg. Chem. 2000, 39, 3020. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Maia, C.G.C.; Weitner, T.; Carballal, S.; Sampaio, R.S.; Lieb, D.; Ghazaryan, R.; Ivanovic-Burmazovic, I.; Radi, R.; Reboucas, J.S.; et al. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free Radic. Biol. Med. 2015, 86, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Ember, E.; Gazzaz, H.A.; Rothbart, S.; Puchta, R.; van Eldik, R. MnII–A fascinating oxidation catalyst: Mechanistic insight into the catalyzed oxidative degradation of organic dyes by H2O2. Appl. Catal. B Environ. 2010, 95, 179–191. [Google Scholar] [CrossRef]
- Hage, R.; Lienke, A. Bleach and oxidation catalysis by manganese-1,4,7-triazacyclononane complexes and hydrogen peroxide. J. Mol. Catal. A Chem. 2006, 251, 150–158. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Kudrik, E.V. Phthalocyanine metal complexes: Versatile catalysts for selective oxidation and bleaching. Catal. Today 2011, 159, 37–46. [Google Scholar] [CrossRef]
- Wieprecht, T.; Xia, J.; Heinz, U.; Dannacher, J.; Schlingloff, G. Novel terpyridine-manganese(II) complexes and their potential to activate hydrogen peroxide. J. Mol. Catal. A Chem. 2003, 203, 113–128. [Google Scholar] [CrossRef]
- Sen, P.; Yildiz, S.Z. The investigation of oxidative bleaching performance of peripherally Schiff base substituted tri-nuclear cobalt-phthalocyanine complexes. Inorg. Chim. Acta 2017, 462, 30–39. [Google Scholar] [CrossRef]
- Sen, P.; Yildirim, E.; Yildiz, S.Z. New alkaline media-soluble functional zinc(II) phthalocyanines bearing poly(hydroxylmethyl)iminomethane Schiff base complexes in catalytic bleaching. Synth. Met. 2016, 215, 41–49. [Google Scholar] [CrossRef]
- Kripli, B.; Baráth, G.; Balogh-Hergovich, É.; Giorgi, M.; Simaan, A.J.; Párkányi, L.; Pap, J.S.; Kaizer, J.; Speier, G. Correlation between the SOD-like activity of hexacoordinate iron(II) complexes and their Fe3+/Fe2+ redox potentials. Inorg. Chem. Commun. 2011, 14, 205–209. [Google Scholar] [CrossRef]
- Kaizer, J.; Baráth, G.; Speier, G.; Réglier, M.; Giorgi, M. Synthesis, structure and catalase mimics of novel homoleptic manganese(II) complexes of 1,3-bis(2 ’-pyridylimino)isoindoline, Mn(4R-ind)2 (R = H, Me). Inorg. Chem. Commun. 2007, 10, 292–294. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Váradi, T.; Giorgi, M.; Kaizer, J.; Speier, G. Comparison of the SOD-like activity of hexacoordinate Mn(II), Fe(II) and Ni(II) complexes having isoindoline-based ligands. J. Inorg. Biochem. 2011, 105, 911–918. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Giorgi, M.; Kaizer, J.; Speier, G. Redox properties of cobalt(II) complexes with isoindoline-based ligands. Transit. Met. Chem. 2011, 36, 481–487. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Bányai, V.; Giorgi, M.; Korecz, L.; Gajda, T.; Árus, D.; Kaizer, J.; Speier, G. Tetra-, penta- and hexacoordinate copper(II) complexes with N3 donor isoindoline-based ligands: Characterization and SOD-like activity. Inorg. Chim. Acta 2011, 376, 158–169. [Google Scholar] [CrossRef]
- Váradi, T.; Pap, J.S.; Giorgi, M.; Párkányi, L.; Csay, T.; Speier, G.; Kaizer, J. Iron(III) complexes with meridional ligands as functional models of intradiol-cleaving catechol dioxygenases. Inorg. Chem. 2013, 52, 1559–1569. [Google Scholar] [CrossRef]
- Kaizer, J.; Kripli, B.; Speier, G.; Párkányi, L. Synthesis, structure, and catalase-like activity of a novel manganese(II) complex: Dichloro[1,3-bis(2’-benzimidazolylimino)isoindoline]manganese(II). Polyhedron 2009, 28, 933–936. [Google Scholar] [CrossRef]
- Kaizer, J.; Csay, T.; Kővári, P.; Speier, G.; Párkányi, L. Catalase mimics of a manganese(II) complex: The effect of axial ligands and pH. J. Mol. Catal. A Chem. 2008, 280, 203–209. [Google Scholar] [CrossRef]
- Kaizer, J.; Baráth, G.; Csonka, R.; Speier, G.; Korecz, L.; Rockenbauer, A.; Párkányi, L. Catechol oxidase and phenoxazinone synthase activity of a manganese(II) isoindoline complex. J. Inorg. Biochem. 2008, 102, 773–780. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S.; Chock, P.B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 1990, 87, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Kripli, B.; Garda, Z.; Sólyom, B.; Tircsó, G.; Kaizer, J. Formation, stability and catalase-like activity of mononuclear manganese(II) and oxomanganese(IV) complexes in protic and aprotic solvents. New J. Chem. 2020, 44, 5545–5555. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins–From superoxide dismutation to HO-driven pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Sueta, G.; Batinic-Haberle, I.; Spasojevic, I.; Fridovich, I.; Radi, R. Catalytic scavenging of peroxynitrite by isomeric Mn(III) N-methylpyridylporphyrins in the presence of reductants. Chem. Res. Toxicol. 1999, 12, 442–449. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Quijano, C.; Alvarez, B.; Radi, R. Reactions of manganese porphyrins and manganese-superoxide dismutase with peroxynitrite. Methods Enzymol. 2002, 349, 23–37. [Google Scholar] [PubMed]
- Ferrer-Sueta, G.; Vitturi, D.; Batinic-Haberle, I.; Fridovich, I.; Goldstein, S.; Czapski, G.; Radi, R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem. 2003, 278, 27432–27438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pap, J.S.; Kripli, B.; Bors, I.; Bogáth, D.; Giorgi, M.; Kaizer, J.; Speier, G. Transition metal complexes bearing flexible N3 or N3O donor ligands: Reactivity toward superoxide radical anion and hydrogen peroxide. J. Inorg. Biochem. 2012, 117, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hage, R.; Lienke, A. Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew. Chem. Int. Ed. 2005, 45, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, J.; Baráth, G.; Pap, J.; Speier, G.; Giorgi, M.; Réglier, M. Manganese and iron flavonolates as flavonol 2,4-dioxygenase mimics. Chem. Commun. 2007, 48, 5235–5237. [Google Scholar] [CrossRef] [PubMed]
- Barhács, L.; Kaizer, J.; Speier, G. Kinetics and mechanism of the oxygenation of potassium flavonolate. Evidence for an electron transfer mechanism. J. Org. Chem. 2000, 65, 3449–3452. [Google Scholar] [CrossRef]
- Pap, J.S.; Kaizer, J.; Speier, G. Model systems for the CO-releasing flavonol 2,4-dioxygenase enzyme. Coord. Chem. Rev. 2010, 254, 781–793. [Google Scholar] [CrossRef]
- Kaizer, J.; Balogh-Hergovich, É.; Czaun, M.; Csay, T.; Speier, G. Redox and nonredox metal assisted model systems with relevance to flavonol and 3-hydroxyquinolin-4(1H)-one 2,4-dioxygenase. Coord. Chem. Rev. 2006, 250, 2222–2233. [Google Scholar] [CrossRef]


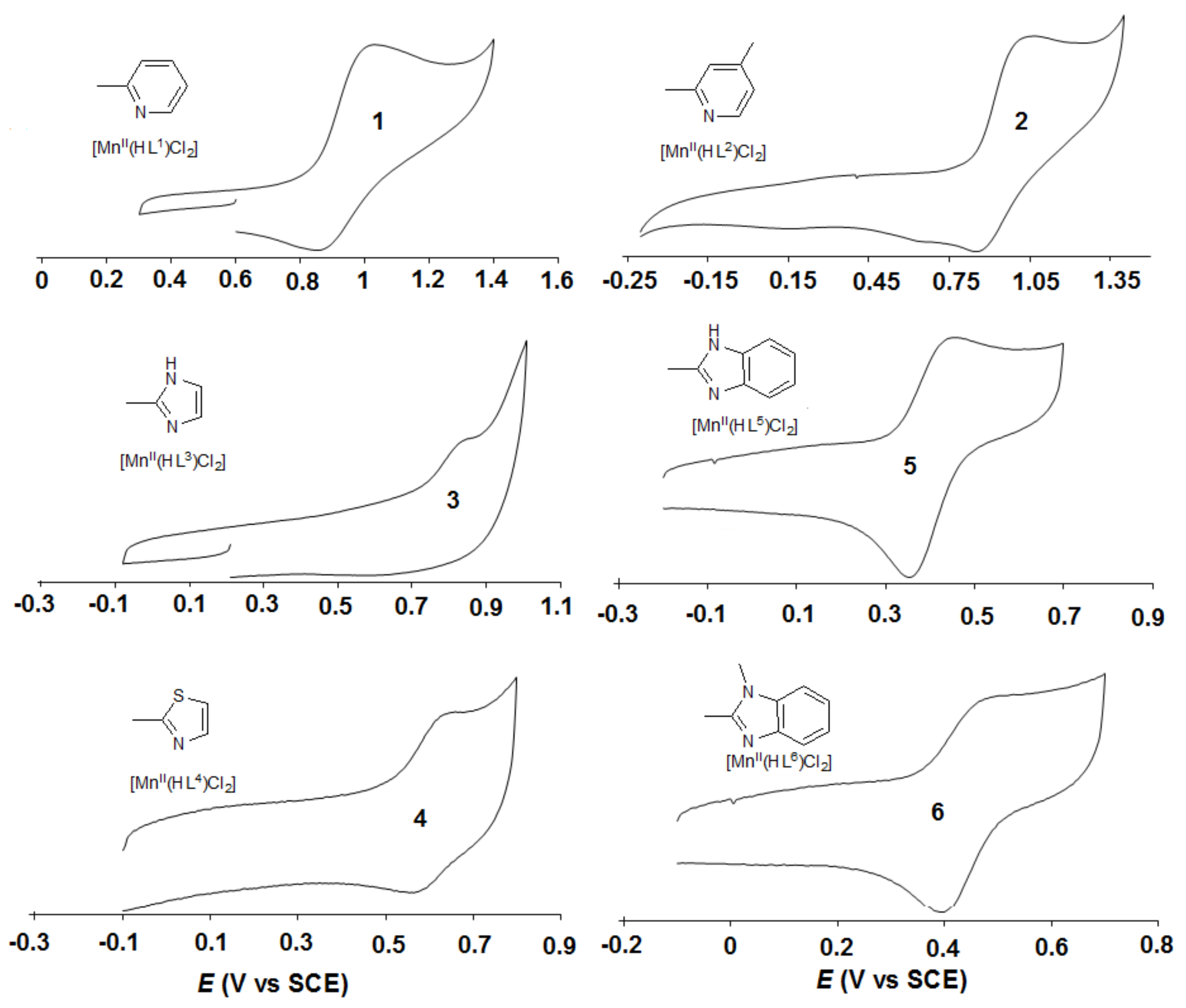

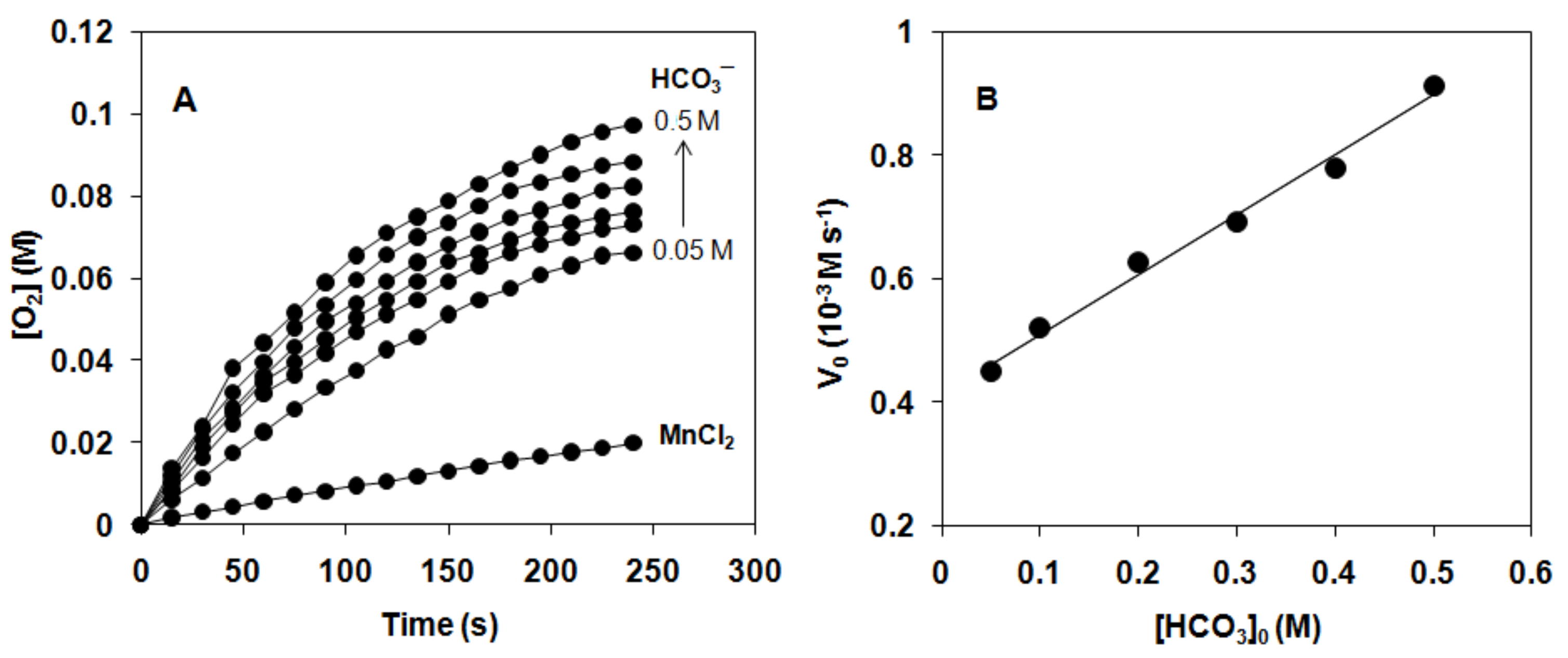








| Catalyst | Epa (mV) | Epc (mV) | E01/2 (mV vs SCE) | ΔEp = Epa-Epc (mV) | λmax (π–π*)1 (nm) | ν1 (λmax−1) 1 (104cm−1) | ν2 (λmax−1) 2 (104cm−1) |
|---|---|---|---|---|---|---|---|
| [Mn(HL1)Cl2] (1) | 987 | 865 | 926 | 122 | 386 | 2.591 | 2.174 |
| [Mn(HL2)Cl2] (2) | 1016 | 880 | 948 | 136 | 366 | 2.73 | 2.188 |
| [Mn(HL3)Cl2] (3) | 816 | - | - | 131 | 391 | 2.56 | 2.128 |
| [Mn(HL4)Cl2] (4) | 625 | 573 | 600 | 52 | 419 | 2.39 | 2.028 |
| [Mn(HL5)Cl2] (5) | 421 | 354 | 388 | 67 | 455 | 2.20 | 1.859 |
| [Mn(HL6)Cl2] (6) | 455 | 395 | 425 | 60 | 448 | 2.23 | 1.894 |
| Catalyst 1 | E0pa (mV) | E0pc (mV) | E01/2 vs SCE (mV) | Yield (%) | TON | V0 (10−3Ms−1) | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| [Mn(HL1)Cl2] (1) | 987 | 865 | 926 | 32.6 | 692 | 0.569 | 19416 |
| [Mn(HL2)Cl2] (2) | 1016 | 880 | 948 | 36.6 | 775 | 0.682 | 23272 |
| [Mn(HL3)Cl2] (3) | 816 | 685 | 750 | 27.1 | 574 | 0.422 | 13820 |
| [Mn(HL4)Cl2] (4) | 625 | 573 | 600 | 21.8 | 463 | 0.328 | 12012 |
| [Mn(HL5)Cl2] (5) | 421 | 354 | 388 | 16.9 | 320 | 0.187 | 6382 |
| [Mn(HL6)Cl2] (6) | 455 | 395 | 425 | 15.13 | 357 | 0.204 | 6962 |
| MnCl2/HCO3− | - | - | - | 8.88 | 188 | 0.081 | 1382 |
| Catalyst (1) (10−6M) | [H2O2] (10−3M) | [HCO3] (10−3M) | [Morin] (10−3M) | kobs (10−3s−1) | kox (106M−3s−1) |
|---|---|---|---|---|---|
| 0.62 | 10 | 50 | 0.16 | 1.63 ± 0.06 | 5.26 ± 0.2 |
| 1.6 | 10 | 50 | 0.16 | 4.19 ± 0.16 | 5.24 ± 0.2 |
| 2.5 | 10 | 50 | 0.16 | 6.73 ± 0.37 | 5.38 ± 0.3 |
| 0.62 | 10 | 50 | 0.16 | 1.63 ± 0.06 | 5.33 ± 0.2 |
| 0.62 | 7.5 | 50 | 0.16 | 1.22 ± 0.05 | 5.25 ± 0.2 |
| 0.62 | 5.0 | 50 | 0.16 | 0.78 ± 0.02 | 5.12 ± 0.1 |
| 0.62 | 2.5 | 50 | 0.16 | 0.41 ± 0.01 | 5.29 ± 0.2 |
| 0.62 | 10 | 50 | 0.16 | 1.63 ± 0.06 | 5.26 ± 0.2 |
| 0.62 | 10 | 100 | 0.16 | 3.02 ± 0.06 | 5.24 ± 0.1 |
| 0.62 | 10 | 200 | 0.16 | 7.01 ± 0.13 | 5.24 ± 0.1 |
| 0.62 | 10 | 300 | 0.16 | 10.5 ± 0.6 | 5.37 ± 0.3 |
| 0.62 | 10 | 50 | 0.16 | 1.63 ± 0.06 | 5.26 ± 0.2 |
| 0.62 | 10 | 50 | 0.12 | 1.65 ± 0.03 | 5.32 ± 0.1 |
| 0.62 | 10 | 50 | 0.08 | 1.70 ± 0.06 | 5.48 ± 0.2 |
| 0.62 | 10 | 50 | 0.04 | 1.73 ± 0.06 | 5.5 ± 0.2 |
| Catalyst 1 | E0pa (mV) | E0pc (mV) | E01/2vs SCE (mV) | kobs (10−3 s−1) | kox (106 M−3 s−1) |
|---|---|---|---|---|---|
| [Mn(HL1)Cl2] (1) | 987 | 865 | 926 | 4.194 ± 0.126 | 5.241 ± 0.161 |
| [Mn(HL2)Cl2] (2) | 1016 | 880 | 948 | 6.230 ± 0.156 | 7.790 ± 0.192 |
| [Mn(HL3)Cl2] (3) | 816 | 685 | 750 | 2.171 ± 0.086 | 2.713 ± 0.108 |
| [Mn(HL4)Cl2] (4) | 625 | 573 | 600 | 1.101 ± 0.022 | 1.376 ± 0.028 |
| [Mn(HL5)Cl2] (5) | 421 | 354 | 388 | 0.541 ± 0.015 | 0.676 ± 0.018 |
| [Mn(HL6)Cl2] (6) | 455 | 395 | 425 | 0.780 ± 0.021 | 0.975 ± 0.026 |
| - | 0.0076 ± 0.0002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meena, B.I.; Kaizer, J. Design and Fine-Tuning Redox Potentials of Manganese(II) Complexes with Isoindoline-Based Ligands: H2O2 Oxidation and Oxidative Bleaching Performance in Aqueous Solution. Catalysts 2020, 10, 404. https://doi.org/10.3390/catal10040404
Meena BI, Kaizer J. Design and Fine-Tuning Redox Potentials of Manganese(II) Complexes with Isoindoline-Based Ligands: H2O2 Oxidation and Oxidative Bleaching Performance in Aqueous Solution. Catalysts. 2020; 10(4):404. https://doi.org/10.3390/catal10040404
Chicago/Turabian StyleMeena, Bashdar I., and József Kaizer. 2020. "Design and Fine-Tuning Redox Potentials of Manganese(II) Complexes with Isoindoline-Based Ligands: H2O2 Oxidation and Oxidative Bleaching Performance in Aqueous Solution" Catalysts 10, no. 4: 404. https://doi.org/10.3390/catal10040404






