The Influence of Platinum on the Catalytic Properties of Bifunctional Cobalt Catalysts for the Synthesis of Hydrocarbons from CO and H2
Abstract
:1. Introduction
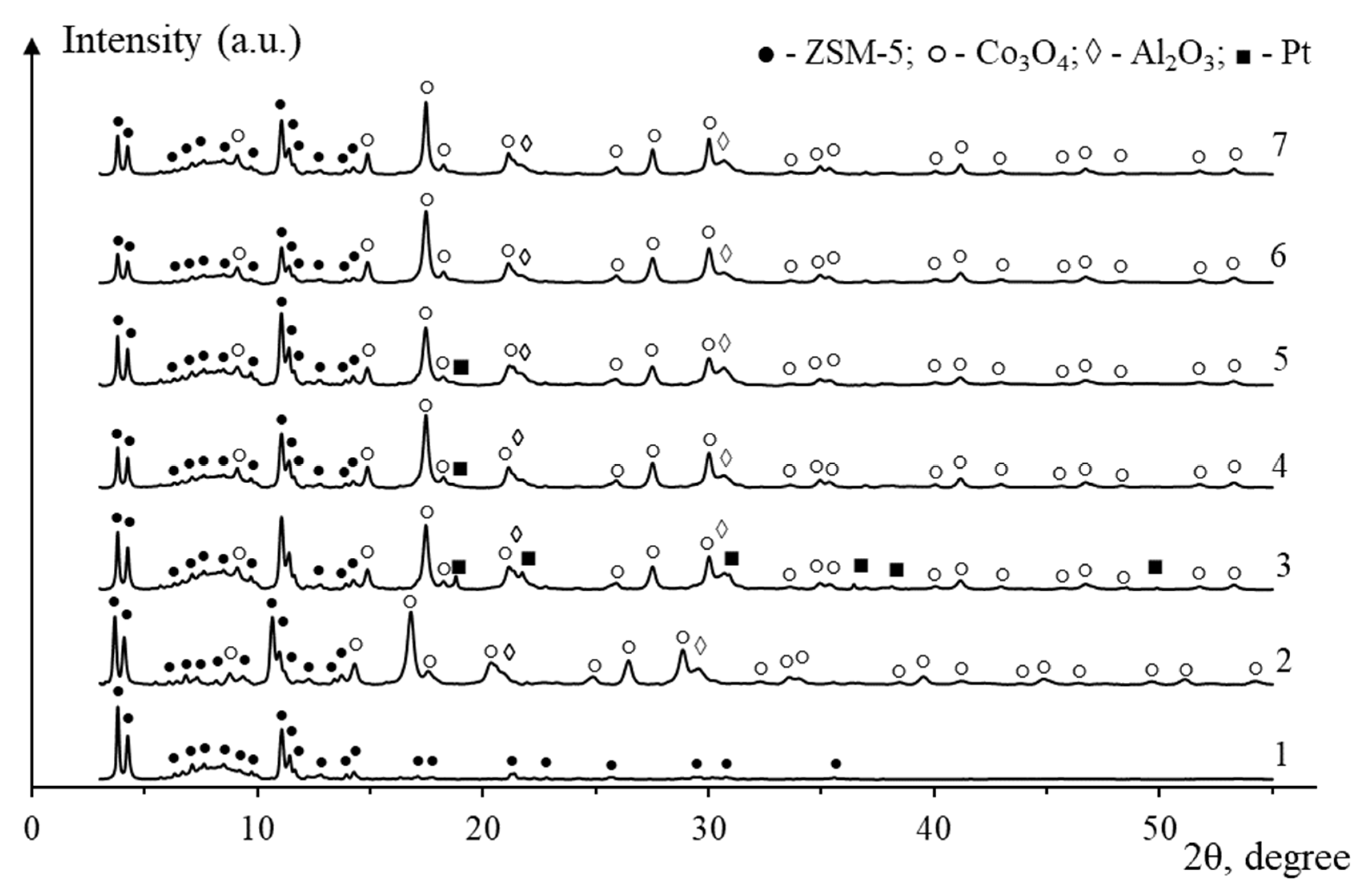
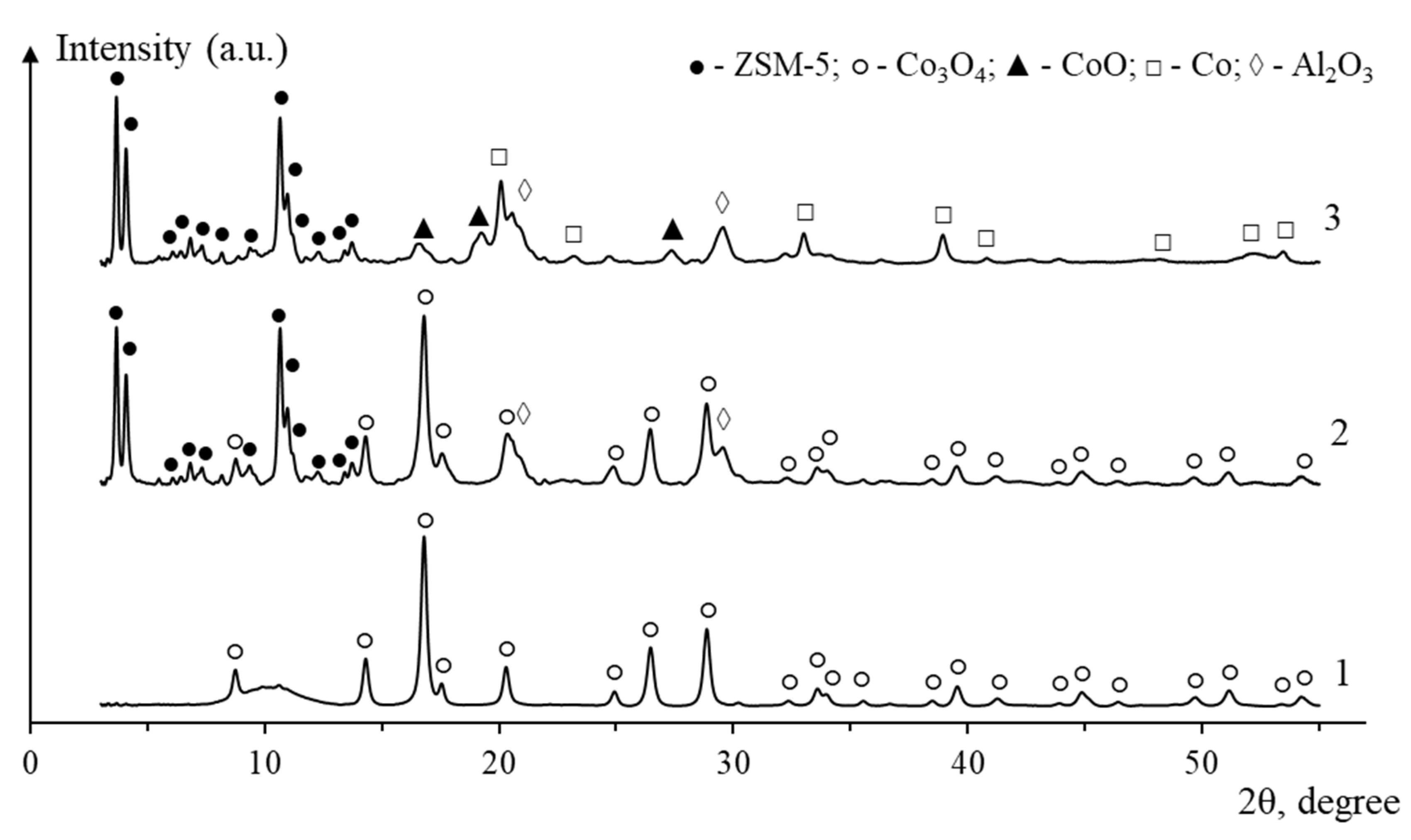
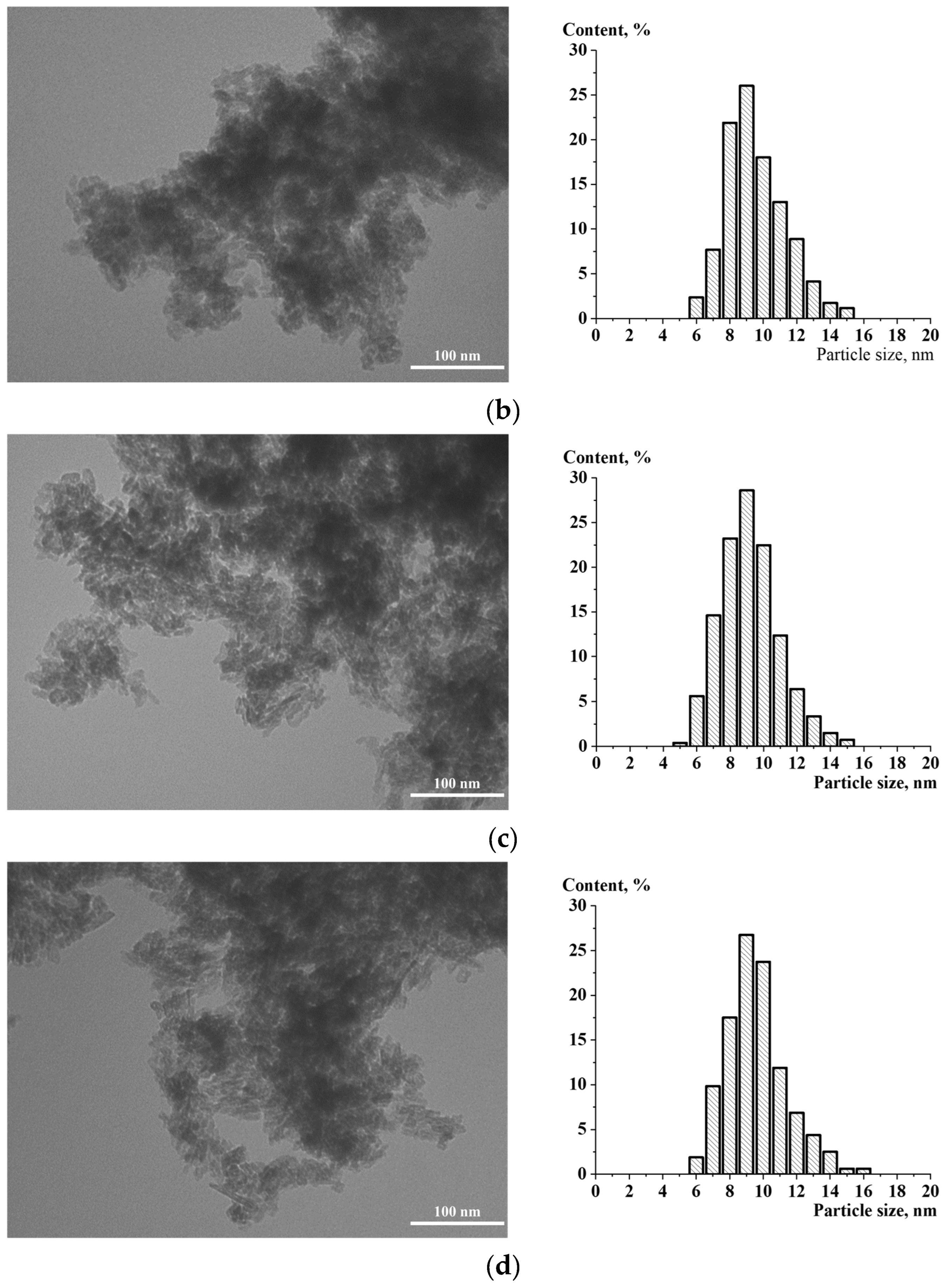
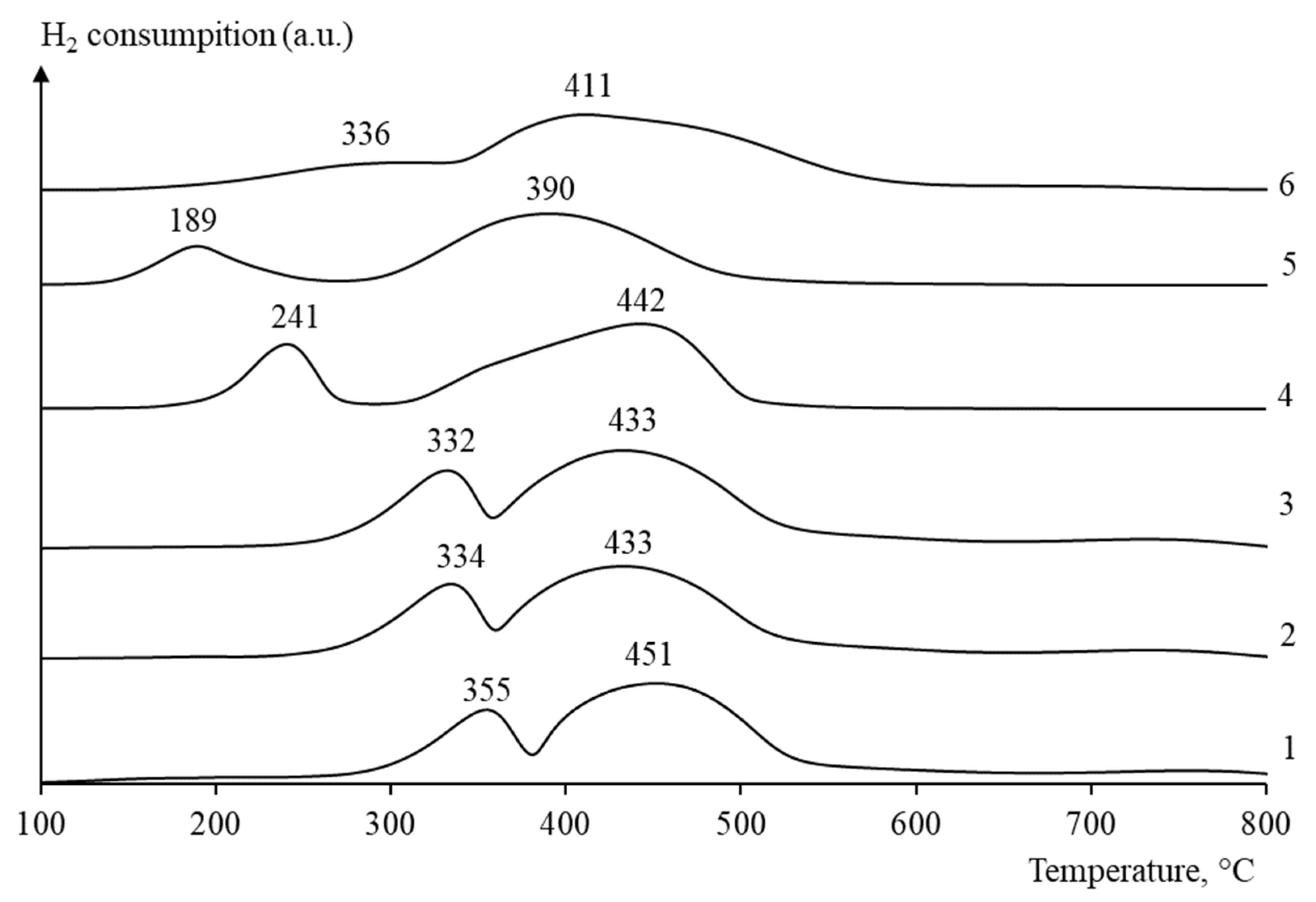
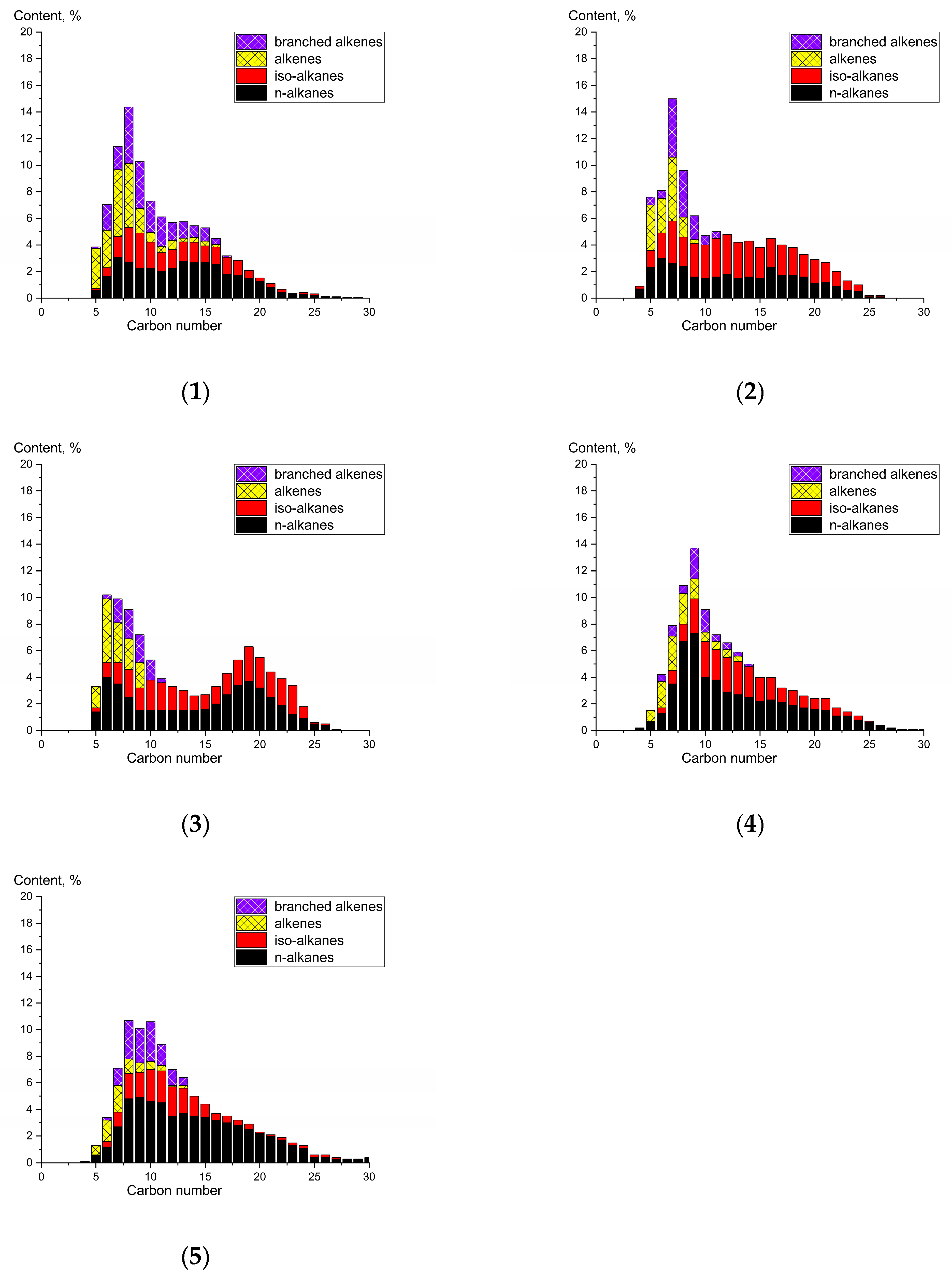
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, P.K.; Kumar, V.; Maity, S. Renewable fuels from different carbonaceous feedstocks: A sustainable route through Fischer–Tropsch synthesis. J. Chem. Technol. Biotechnol. 2021, 96, 853–868. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, K.; Wang, Y.; Liu, Z.; Gao, X.; Zhang, J.; Ma, Q.; Fan, S.; Zhao, T.-S.; Yao, M. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway. J. CO2 Util. 2023, 67, 102321. [Google Scholar] [CrossRef]
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An overview of Fischer-Tropsch Synthesis: XtL processes, catalysts and reactors. Appl. Catal. A Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- Gavrilović, L.; Brandin, J.; Holmen, A.; Venvik, H.J.; Myrstad, R.; Blekkan, E.A. Fischer-Tropsch synthesis—Investigation of the deactivation of a Co catalyst by exposure to aerosol particles of potassium salt. Appl. Catal. B Environ. 2018, 230, 203–209. [Google Scholar] [CrossRef]
- Shiba, N.C.; Yao, Y.; Liu, X.; Hildebrandt, D. Recent developments in catalyst pretreatment technologies for cobalt based Fisher–Tropsch synthesis. Rev. Chem. Eng. 2022, 38, 503–538. [Google Scholar] [CrossRef]
- Sulima, S.I.; Bakun, V.G.; Chistyakova, N.S.; Larina, M.V.; Yakovenko, R.E.; Savost’yanov, A.P. Prospects for technologies in the production of synthetic base stocks for engine oils (a review). Pet. Chem. 2021, 61, 1178–1189. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Liu, X.; Lu, X.; Moyo, M.; Hildebrandt, D. Cobalt hybrid catalysts in Fischer-Tropsch synthesis. Rev. Chem. Eng. 2020, 36, 437–457. [Google Scholar] [CrossRef]
- Sartipi, S.; Van Dijk, J.E.; Gascon, J.; Kapteijn, F. Toward bifunctional catalysts for the direct conversion of syngas to gasoline range hydrocarbons: H-ZSM-5 coated Co versus H-ZSM-5 supported Co. Appl. Catal. A Gen. 2013, 456, 11–22. [Google Scholar] [CrossRef]
- Park, G.; Ahn, C.; Park, S.; Lee, Y.; Kwak, G.; Kim, S.K. Diffusion-dependent upgrading of hydrocarbons synthesized by Co/zeolite bifunctional Fischer–Tropsch catalysts. Appl. Catal. A Gen. 2020, 607, 117840. [Google Scholar] [CrossRef]
- Ghogia, A.C.; Nzihou, A.; Serp, P.; Soulantica, K.; Minh, D.P. Cobalt catalysts on carbon-based materials for Fischer-Tropsch synthesis: A review. Appl. Catal. A Gen. 2021, 609, 117906. [Google Scholar] [CrossRef]
- Sineva, L.; Mordkovich, V.; Asalieva, E.; Smirnova, V. Zeolite-Containing Co Catalysts for Fischer–Tropsch Synthesis with Tailor-Made Molecular-Weight Distribution of Hydrocarbons. Reactions 2023, 4, 359–380. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Mazurova, K.; Miyassarova, A.; Eliseev, O.; Stytsenko, V.; Glotov, A.; Stavitskaya, A. Fischer–Tropsch Synthesis Catalysts for Selective Production of Diesel Fraction. Catalysts 2023, 13, 1215. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Yang, C.; Wang, S.; Gao, P.; Sun, Y. Hierarchical ZSM-5 Supported CoMn Catalyst for the Production of Middle Distillate from Syngas. Ind. Eng. Chem. Res. 2021, 60, 5783–5791. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Savost’yanov, A.P.; Narochniy, G.B.; Soromotin, V.N.; Zubkov, I.N.; Papeta, O.P.; Svetogorov, R.D.; Mitchenko, S.A. Preliminary evaluation of a commercially viable Co-based hybrid catalyst system in Fischer–Tropsch synthesis combined with hydroprocessing. Catal. Sci. Technol. 2020, 10, 7613–7629. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Bakun, V.G.; Sulima, S.I.; Narochnyi, G.B.; Mitchenko, S.A.; Zubkov, I.N.; Savost’yanov, A.P. Cobalt Supported and Polyfunctional Hybrid Catalysts for Selective Fischer–Tropsch Synthesis: A Review. Catal. Ind. 2023, 15, 6–20. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; De Jong, K.P.; De Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zecevic, J.; Vanbutsele, G.; de Jong, K.P.; Martens, J.A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 2015, 528, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, W.; Du, Y.; Li, J. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review. Appl. Catal. A Gen. 2021, 611, 117916. [Google Scholar] [CrossRef]
- Liuzzi, D.; Pérez-Alonso, F.J.; Rojas, S. Ru-M (M = Fe or Co) Catalysts with high Ru surface concentration for Fischer-Tropsch synthesis. Fuel 2021, 293, 120435. [Google Scholar] [CrossRef]
- Al-Dossary, M.; Fierro, J.L.G. Effect of high-temperature pre-reduction in Fischer–Tropsch synthesis on Fe/ZrO2 catalysts. Appl. Catal. A Gen. 2015, 499, 109–117. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the development of novel cobalt Fischer−Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of novel catalysts for Fischer–Tropsch synthesis: Tuning the product selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer–Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A Gen. 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Nabaho, D.; Niemantsverdriet, J.H.; Claeys, M.; van Steen, E. Hydrogen spillover in the Fischer–Tropsch synthesis: An analysis of platinum as a promoter for cobalt–alumina catalysts. Catal. Today 2016, 261, 17–27. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J.; Wu, L. Effect of Zr, Ca and Mn as promoters on the Co/SiC catalysts for the Fischer–Tropsch synthesis. React. Kinet. Mech. Catal. 2017, 122, 887–900. [Google Scholar] [CrossRef]
- Jermwongratanachai, T.; Jacobs, G.; Shafer, W.D.; Pendyala, V.R.R.; Ma, W.; Gnanamani, M.K.; Hopps, S.; Thomas, G.A.; Kitiyanan, B.; Khalid, S.; et al. Fischer–Tropsch synthesis: TPR and XANES analysis of the impact of simulated regeneration cycles on the reducibility of Co/alumina catalysts with different promoters (Pt, Ru, Re, Ag, Au, Rh, Ir). Catal. Today 2014, 228, 15–21. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.J.; Karandikar, P.R.; Jun, K.W.; Bae, J.W.; Ha, K.S. Ru promoted cobalt catalyst on γ-Al2O3 support: Influence of pre-synthesized nanoparticles on Fischer–Tropsch reaction. J. Mol. Catal. A Chem. 2011, 344, 153–160. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Zubkov, I.N.; Narochniy, G.B.; Papeta, O.P.; Denisov, O.D.; Savost’yanov, A.P. Effect of Re and Al2O3 Promotion on the Working Stability of Cobalt Catalysts for the Fischer–Tropsch Synthesis. Kinet. Catal. 2020, 61, 310–317. [Google Scholar] [CrossRef]
- Shiba, N.C.; Liu, X.; Mao, H.; Qian, X.; Hildebrandt, D.; Yao, Y. Effect of Ru-promotion on the catalytic performance of a cobalt-based Fischer-Tropsch catalyst activated in syngas or H2. Fuel 2022, 320, 123939. [Google Scholar] [CrossRef]
- Guo, S.; Ma, Z.; Wang, J.; Hou, B.; Jia, L.; Wang, B.; Li, D. Effect of Ba on the catalytic performance of Co-Ru/Al2O3 catalyst for Fischer-Tropsch synthesis. Fuel 2021, 292, 120398. [Google Scholar] [CrossRef]
- Martínez-Vargas, D.X.; Sandoval-Rangel, L.; Campuzano-Calderon, O.; Romero-Flores, M.; Lozano, F.J.; Nigam, K.D.P.; Mendoza, A.; Montesinos-Castellanos, A. Recent advances in bifunctional catalysts for the Fischer–Tropsch process: One-stage production of liquid hydrocarbons from syngas. Ind. Eng. Chem. Res. 2019, 58, 15872–15901. [Google Scholar] [CrossRef]
- Oenema, J.; Harmel, J.; Vélez, R.P.; Meijerink, M.J.; Eijsvogel, W.; Poursaeidesfahani, A.; Vlugt, T.J.H.; Zečević, J.; De Jong, K.P. Influence of nanoscale intimacy and zeolite micropore size on the performance of bifunctional catalysts for n-heptane hydroisomerization. ACS Catal. 2020, 10, 14245–14257. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Feng, Y.L.; Liu, F.; Kang, C.L.; Zhou, X.L.; Xu, L.Y.; Yu, G.X. Effect of variations in pore structure and acidity of alkali treated ZSM-5 on the isomerization performance. J. Mol. Catal. A Chem. 2009, 310, 130–137. [Google Scholar] [CrossRef]
- Tomasek, S.; Lonyi, F.; Valyon, J.; Wollmann, A.; Hancsok, J. Fuel production from Fischer-Tropsch paraffin mixtures. Chem. Eng. Trans. 2018, 70, 667–672. [Google Scholar]
- Hu, S.; Chen, J.; Zhang, Q.; Liu, J.; Meng, J.; Ye, G.; Zhou, X.; Yuan, W. Crystal-size–dependent external surface diffusion barriers in Pt/ZSM-5 catalyzed n-pentane isomerization. AIChE J. 2022, 68, e17677. [Google Scholar] [CrossRef]
- Tomasek, S.; Lonyi, F.; Valyon, J.; Wollmann, A.; Hancsók, J. Hydrocracking of Fischer–Tropsch paraffin mixtures over strong acid bifunctional catalysts to engine fuels. ACS Omega 2020, 5, 26413–26420. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Cheng, K.; Zhang, L.; Zhang, Q.; Ding, J.; Hua, W.; Lou, Y.; Zhai, Q.; Wang, Y. Mesoporous zeolite-supported ruthenium nanoparticles as highly selective Fischer–Tropsch catalysts for the production of C5–C11 isoparaffins. Angew. Chem. Int. Ed. 2011, 50, 5200–5203. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, J.; Huang, S.; You, Z.; Zhang, Q.; Ding, J.; Hua, W.; Lou, Y.; Deng, W.; Wang, Y. Mesoporous beta zeolite-supported ruthenium nanoparticles for selective conversion of synthesis gas to C5–C11 isoparaffins. ACS Catal. 2012, 2, 441–449. [Google Scholar] [CrossRef]
- Horáček, J. Fischer-Tropsch synthesis, the effect of promoters, catalyst support, and reaction conditions selection. Monatshefte FÜR Chem.-Chem. Mon. 2020, 151, 649–675. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Deactivation and regeneration of commercial type Fischer-Tropsch Co-catalysts—A mini-review. Catalysts 2015, 5, 478–499. [Google Scholar] [CrossRef]
- Kibby, C.; Jothimurugesan, K.; Das, T.; Lacheen, H.S.; Rea, T.; Saxton, R.J. Chevron’s gas conversion catalysis-hybrid catalysts for wax-free Fischer–Tropsch synthesis. Catal. Today 2020, 215, 131–141. [Google Scholar] [CrossRef]
- Sulima, S.I.; Bakun, V.G.; Zubkov, I.N.; Savost’yanov, A.P.; Yakovenko, R.E. Effects of Ruthenium on the Catalytic Performance of Cobalt-Based Hybrid Catalysts for Synthesis of Hydrocarbons from CO and H2. Pet. Chem. 2023, 63, 746–758. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Zubkov, I.N.; Bakun, V.G.; Savost’yanov, A.P. Combined synthesis and hydroprocessing of hydrocarbons over Co/SiO2+ZSM-5+Al2O3 catalysts promoted by nickel. Pet. Chem. 2021, 61, 516–526. [Google Scholar] [CrossRef]
- Narochnyi, G.B.; Zubkov, I.N.; Savost’yanov, A.P.; Allaguzin, I.K.; Lavrenov, S.A.; Yakovenko, R.E. A bifunctional cobalt catalyst for the synthesis of waxy diesel fuel by the Fischer–Tropsch method—From the development to implementation. Part 3. The experience of industrial implementation of the preparation technology. Katal. V Promyshlennosti 2024, 24, 34–43. (In Russian) [Google Scholar] [CrossRef]
- Barbosa, A.S.; Barbosa, A.S.; Freire Rodrigues, M.G.; Grau, J.M. Preparation and Characterization: The Effect of Incorporation by Ion Exchange of Pt, Ni and Ru on a H-ZSM5 Zeolite. Mater. Sci. Forum 2022, 727, 32–37. [Google Scholar] [CrossRef]
- PDF-2. The Powder Diffraction File TM. International Center for Diffraction Data (ICDD), PDF-2 Release 2012. 2014. Available online: www.icdd.com (accessed on 19 January 2024).
- Sousa, B.V.; Brito, K.D.; Alves, J.J.; Rodrigues, M.G.; Yoshioka, C.M.; Cardoso, D. n-Hexane isomerization on Pt/HMOR: Effect of platinum content. React. Kinet. Mech. Catal. 2019, 102, 473–485. [Google Scholar] [CrossRef]
- Schmutzler, F.; Zschiesche, C.; Titus, J.; Poppitz, D.; Freiding, J.; Rakoczy, R.; Reitzmann, A.; Gläser, R. Hydroisomerization of Renewable and Fossil n-Alkanes over Bifunctional Dealuminated ZSM-5 Catalysts. Chem. Ing. Tech. 2021, 93, 981–989. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Tsybulya, S.V. In situ XRD investigation of Co3O4 reduction. Zeitschrift Fur Kristallographie 2009, 30, 329–334. [Google Scholar] [CrossRef]
- Liu, Z.W.; Li, X.; Asami, K.; Fujimoto, K. High performance Pd/beta catalyst for the production of gasoline-range iso-paraffins via a modified Fischer–Tropsch reaction. Appl. Catal. A Gen. 2006, 300, 162–169. [Google Scholar] [CrossRef]
- Aghdam, N.C.; Ejtemaei, M.; Sharafi, S.M.; Babaluo, A.A.; Tavakoli, A.; Bayati, B. Catalytic performance of nanostructured Pt/ZSM-5 catalysts synthesized by extended Charnell’s method in hydroisomerization of n-pentane. Polyhedron 2019, 162, 155–164. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kang, S.H.; Kim, J.H.; Lee, Y.J.; Jun, K.W. Fischer-Tropsch synthesis on Co-Al2O3-(promoter)/ZSM5 hybrid catalysts for the production of gasoline range hydrocarbons. Korean J. Chem. Eng. 2015, 32, 1993–1998. [Google Scholar] [CrossRef]
- Oppmann, N.; Jess, A. Improving the Selectivity to Liquefied Petroleum Gas by Combining Fischer-Tropsch Synthesis with Zeolite Cracking. Chem. Eng. Technol. 2023, 46, 908–917. [Google Scholar] [CrossRef]
- Boymans, E.; Nijbacker, T.; Slort, D.; Grootjes, S.; Vreugdenhil, B. Jet fuel synthesis from syngas using bifunctional cobalt-based catalysts. Catalysts 2022, 12, 288. [Google Scholar] [CrossRef]
- Savost’yanov, A.P.; Yakovenko, R.E.; Sulima, S.I.; Bakun, V.G.; Narochnyi, G.B.; Chernyshev, V.M.; Mitchenko, S.A. The impact of Al2O3 promoter on an efficiency of C5+ hydrocarbons formation over Co/SiO2 catalysts via Fischer-Tropsch synthesis. Catal. Today 2017, 279, 107–114. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: Oxford, UK, 1995; 298p. [Google Scholar]
- Schanke, D.; Vada, S.; Blekkan, E.A.; Hilmen, A.M.; Hoff, A.; Holmen, A. Study of Pt-promoted cobalt CO hydrogenation catalysts. J. Catal. 1995, 156, 85–95. [Google Scholar] [CrossRef]






| Catalyst | Pt-Impregnated * |
|---|---|
| K | - |
| PtHZ | HZSM-5 |
| Pt(i)HZ | HZSM-5 ** |
| PtK | Composite |
| Pt(Co-Al2O3/SiO2) | Co-Al2O3/SiO2 |
| PtSiO2 | SiO2 |
| Catalyst | Particle Size According to XRD, nm * | D, % | Particle Size According to XRD, nm | D, % | Co0 Particle Size by TEM, nm ** | ||
|---|---|---|---|---|---|---|---|
| Co3O4 | Co0 | Co3O4 | Co0 | ||||
| K | 11.0 ± 0.6 | 8.2 | 11.7 | 14.5 | 10.8 | 8.9 | 8.8 ± 1.3 |
| PtHZ | 13.0 ± 0.7 | 9.7 | 9.9 | 14.2 | 10.6 | 9.0 | - |
| Pt(i)HZ | 13.0 ± 0.7 | 10.2 | 9.4 | 13.7 | 10.3 | 9.3 | 9.4 ± 1.2 |
| PtK | 12.2 ± 0.7 | 9.1 | 10.5 | 12.8 | 9.6 | 10.0 | 8.9 ± 1.2 |
| Pt(Co-Al2O3/SiO2) | 13.1 ± 0.4 | 9.8 | 9.8 | 14.4 | 10.8 | 8.9 | 9.4 ± 1.2 |
| PtSiO2 | 16.7 ± 0.8 | 12.5 | 7.7 | 18.0 | 13.5 | 7.1 | - |
| Catalyst | Peak 1 | Peak 2 | S2/S1 | R, % * | ||
|---|---|---|---|---|---|---|
| Temperature, °C | Area S1, % | Temperature, °C | Area S2, % | |||
| K | 355 | 27.0 | 451 | 73.0 | 2.7 | 50.0 |
| PtHZ | 334 | 26.1 | 433 | 73.9 | 2.8 | - |
| Pt(i)HZ | 332 | 26.2 | 433 | 73.8 | 2.8 | - |
| PtK | 241 | 25.5 | 442 | 74.5 | 2.9 | 54.4 |
| Pt(CoAl2O3/SiO2) | 189 | 21.9 | 390 | 78.7 | 3.6 | 48.0 |
| PtSiO2 | 336 | 22.1 | 411 | 77.9 | 3.5 | 58.0 |
| Catalyst | Conversion CO, % | Selectivity, % | Productivity for Hydrocarbons C5+, kg/(m3cat·h) | |||
|---|---|---|---|---|---|---|
| CH4 | C2–C4 | C5+ | CO2 | |||
| K | 75.6 | 18.7 | 11.9 | 67.1 | 2.3 | 106.0 |
| PtHZ | 66.3 | 17.3 | 11.2 | 68.4 | 3.1 | 101.9 |
| Pt(i)HZ | 84.0 | 14.4 | 8.2 | 74.4 | 3.0 | 135.6 |
| PtK | 74.7 | 18.6 | 12.8 | 65.7 | 2.9 | 105.6 |
| Pt(CoAl2O3/SiO2) | 87.2 | 16.5 | 9.4 | 70.6 | 3.5 | 138.5 |
| PtSiO2 | 61.1 | 20.1 | 16.9 | 60.2 | 2.8 | 84.2 |
| Catalyst | Hydrocarbons | Group Composition of Hydrocarbons C5+, wt.% | Total | iso/n ** | o/p *** | ||
|---|---|---|---|---|---|---|---|
| C5–C10 | C11–C18 | C19+ | |||||
| K | n-alkanes | 12.5 | 18.4 | 5.2 | 36.1 | 0.8 | 0.7 |
| iso-alkanes | 9.5 | 10.8 | 1.7 | 22.0 | |||
| alkenes | 18.3 | 2.3 | - | 20.6 | |||
| alkenes * | 14.0 | 7.3 | - | 21.3 | |||
| Total | 54.3 | 38.8 | 6.9 | 100.0 | |||
| PtHZ | n-alkanes | 14.1 | 13.6 | 6.1 | 33.8 | 1.2 | 0.3 |
| iso-alkanes | 13.8 | 20.2 | 7.4 | 41.4 | |||
| alkenes | 12.7 | - | - | 12.7 | |||
| alkenes * | 11.6 | 0.5 | - | 12.1 | |||
| Total | 52.2 | 34.3 | 13.5 | 100.0 | |||
| Pt(i)HZ | n-alkanes | 14.4 | 15.7 | 14.5 | 44.6 | 0.7 | 0.3 |
| iso-alkanes | 9.1 | 12.4 | 12.1 | 33.6 | |||
| alkenes | 13.6 | - | - | 13.6 | |||
| alkenes * | 7.9 | 0.3 | - | 8.2 | |||
| Total | 45.1 | 28.4 | 26.5 | 100.0 | |||
| PtK | n-alkanes | 23.8 | 20.5 | 9.2 | 53.5 | 0.5 | 0.2 |
| iso-alkanes | 8.0 | 15.6 | 3.9 | 27.5 | |||
| alkenes | 10.1 | 1.5 | - | 11.6 | |||
| alkenes * | 5.9 | 1.5 | - | 7.4 | |||
| Total | 47.8 | 39.1 | 13.1 | 100.0 | |||
| Pt(CoAl2O3/SiO2) | n-alkanes | 18.8 | 27.6 | 12.8 | 59.2 | 0.5 | 0.3 |
| iso-alkanes | 7.8 | 10.4 | 1.7 | 19.9 | |||
| alkenes | 6.7 | 0.7 | - | 7.4 | |||
| alkenes * | 10.0 | 3.5 | - | 13.5 | |||
| Total | 43.3 | 42.2 | 14.5 | 100.0 | |||
| PtSiO2 | n-alkanes | 16.9 | 19.0 | 8.9 | 44.8 | 0.9 | 0.3 |
| iso-alkanes | 10.6 | 17.8 | 1.7 | 30.1 | |||
| alkenes | 8.4 | 0.1 | - | 8.5 | |||
| alkenes * | 12.5 | 4.1 | - | 16.6 | |||
| Total | 48.4 | 41.0 | 10.6 | 100.0 | |||
| Catalyst | Conversion CO, % | Selectivity, % | Productivity for Hydrocarbons C5+, kg/(m3cat·h) | |||
|---|---|---|---|---|---|---|
| CH4 | C2–C4 | C5+ | CO2 | |||
| K | 85.9 | 15.8 | 8.2 | 72.8 | 3.3 | 132.0 |
| PtHZ | 77.4 | 15.5 | 9.1 | 71.4 | 4.0 | 124.3 |
| Pt(i)HZ | 90.3 | 16.2 | 9.0 | 70.3 | 4.4 | 137.8 |
| PtK | 90.6 | 15.8 | 8.3 | 70.3 | 5.6 | 137.0 |
| Pt(Co-Al2O3/SiO2) | 92.5 | 15.4 | 8.0 | 71.8 | 4.8 | 149.3 |
| Catalyst | Hydrocarbons | Group Composition of Hydrocarbons C5+, wt.% | Total | iso/n ** | o/p *** | ||
|---|---|---|---|---|---|---|---|
| C5–C10 | C11–C18 | C19+ | |||||
| K | n-alkanes | 9.4 | 13.1 | 3.7 | 26.2 | 1.1 | 1.0 |
| iso-alkanes | 11.5 | 10.5 | 2.1 | 24.1 | |||
| alkenes | 20.3 | 2.1 | - | 22.4 | |||
| alkenes * | 20.9 | 6.4 | - | 27.3 | |||
| Total | 62.1 | 32.1 | 5.8 | 100.0 | |||
| PtHZ | n-alkanes | 12.0 | 10.1 | 4.3 | 26.4 | 1.4 | 0.6 |
| iso-alkanes | 11.0 | 19.7 | 5.9 | 36.6 | |||
| alkenes | 15.2 | - | - | 15.2 | |||
| alkenes * | 21.4 | 0.4 | - | 21.8 | |||
| Total | 59.6 | 30.2 | 10.2 | 100.0 | |||
| Pt(i)HZ | n-alkanes | 10.9 | 16.3 | 6.1 | 33.3 | 1.1 | 0.4 |
| iso-alkanes | 12.1 | 21.0 | 4.8 | 37.9 | |||
| alkenes | 12.5 | 1.0 | - | 13.5 | |||
| alkenes * | 12.2 | 3.1 | - | 15.3 | |||
| Total | 47.7 | 41.4 | 10.9 | 100.0 | |||
| PtK | n-alkanes | 22.9 | 14.4 | 6.5 | 43.8 | 0.8 | 0.3 |
| iso-alkanes | 12.9 | 15.2 | 2.9 | 31.0 | |||
| alkenes | 10.5 | 0.7 | - | 11.2 | |||
| alkenes * | 11.8 | 2.2 | - | 14.0 | |||
| Total | 58.1 | 32.5 | 9.4 | 100.0 | |||
| Pt(Co-Al2O3/SiO2) | n-alkanes | 18.4 | 16.0 | 4.8 | 39.2 | 1.1 | 0.5 |
| iso-alkanes | 11.7 | 15.9 | 1.4 | 29.0 | |||
| alkenes | 7.3 | 0.8 | - | 8.1 | |||
| alkenes * | 17.3 | 6.4 | - | 23.7 | |||
| Total | 54.7 | 39.1 | 6.2 | 100.0 | |||
| Catalyst | Loading, wt.% | Impregnated | Reaction Conditions | Catalysis Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| T, °C | P, MPa | H2/CO Ratio | GHSV, h−1 | Conversion CO, % | C5+ Selectivity, % | ||||
| Pt(Co-Al2O3/SiO2) | 7.4Co 0.3Pt | Pt impregnated in Co-Al2O3/SiO2 | 240 | 2.0 | 2.0 | 1000 | 87.2 | 70.6 | this work |
| Pt(Co-Al2O3/SiO2) | 7.4Co 0.3Pt | Pt impregnated in Co-Al2O3/SiO2 | 250 | 2.0 | 2.0 | 1000 | 92.5 | 71.8 | this work |
| Co-Pt/ZSM-5 | 13Co 0.3Pt | Pt with Co impregnated in ZSM-5 | 240 | 2.0 | 2.0 | n.d. | 42.1 | 81.6 | [53] |
| Co-Mn+Pt-ZSM-5 (layer-by-layer loading) | 20.0 Co-3.0 Mn + 0.5 Pt | Pt-impregnated ZSM-5 | 250 | 2.0 | 2.0 | n.d. | n.d. | 58.1 | [54] |
| Co-Ru/Al2O3 (Riogen, USA) | 11.7Co n.d. Ru | n.d. | 240 | 2.0 | 2.0 | n.d. | 55.5 | 68.1 | [42] |
| Co-Ru/ZSM-5 Al2O3 | 7.5Co 0.19Ru | Ru-impregnated ZSM-5+A2O3 | 220 | 1.0 | 2.0 | 1500 | 50.0 | 75.8 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovenko, R.E.; Zubkov, I.N.; Papeta, O.P.; Kataria, Y.V.; Bakun, V.G.; Svetogorov, R.D.; Savost’yanov, A.P. The Influence of Platinum on the Catalytic Properties of Bifunctional Cobalt Catalysts for the Synthesis of Hydrocarbons from CO and H2. Catalysts 2024, 14, 351. https://doi.org/10.3390/catal14060351
Yakovenko RE, Zubkov IN, Papeta OP, Kataria YV, Bakun VG, Svetogorov RD, Savost’yanov AP. The Influence of Platinum on the Catalytic Properties of Bifunctional Cobalt Catalysts for the Synthesis of Hydrocarbons from CO and H2. Catalysts. 2024; 14(6):351. https://doi.org/10.3390/catal14060351
Chicago/Turabian StyleYakovenko, Roman E., Ivan N. Zubkov, Ol’ga P. Papeta, Yash V. Kataria, Vera G. Bakun, Roman D. Svetogorov, and Alexander P. Savost’yanov. 2024. "The Influence of Platinum on the Catalytic Properties of Bifunctional Cobalt Catalysts for the Synthesis of Hydrocarbons from CO and H2" Catalysts 14, no. 6: 351. https://doi.org/10.3390/catal14060351








