Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical, Morphological, and Optical Characterizations
2.1.1. Raman Spectra Evaluation
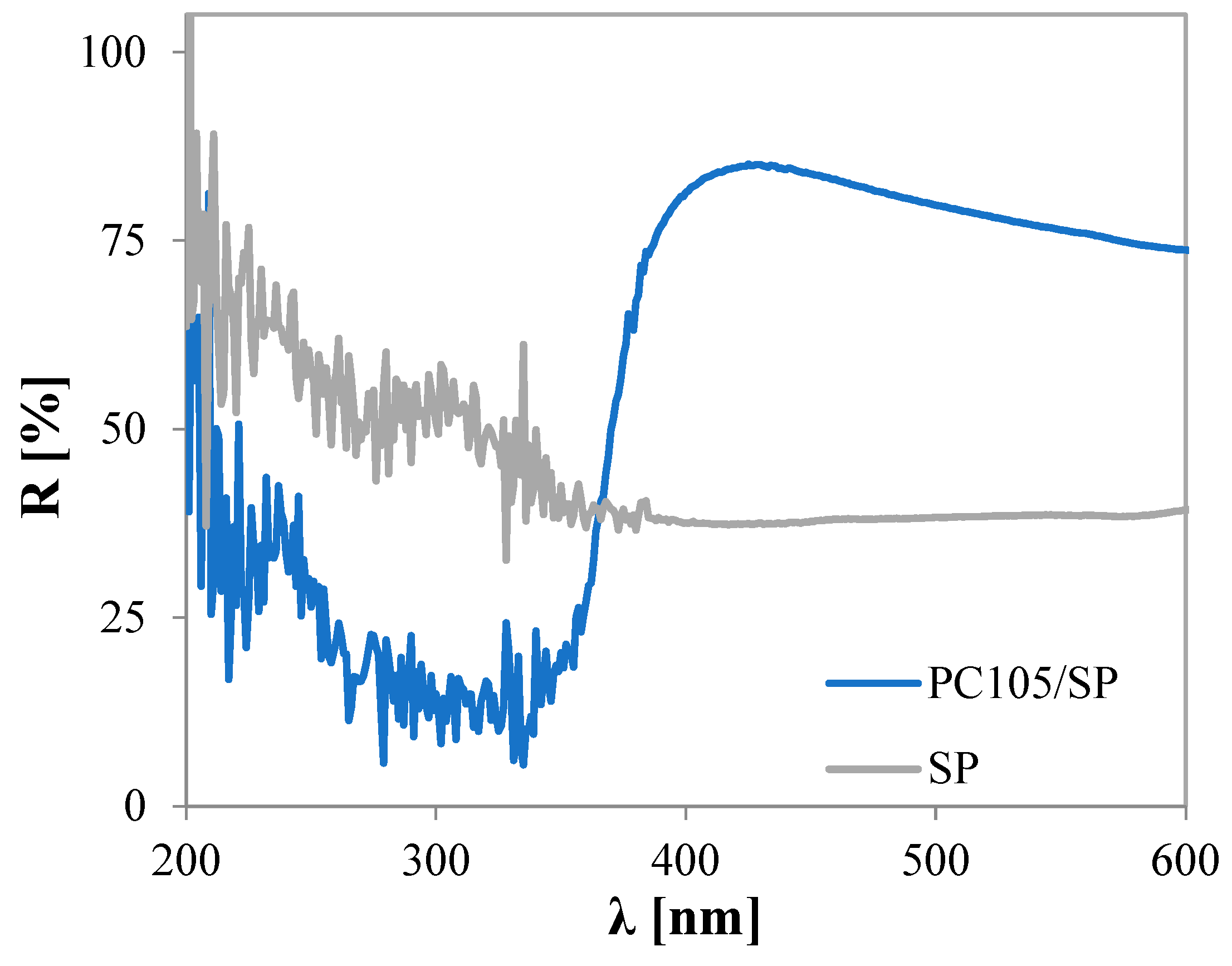
2.1.2. UV–Vis DRS Spectra and Energy Bandgap Calculation
2.2. Photocatalytic Tests
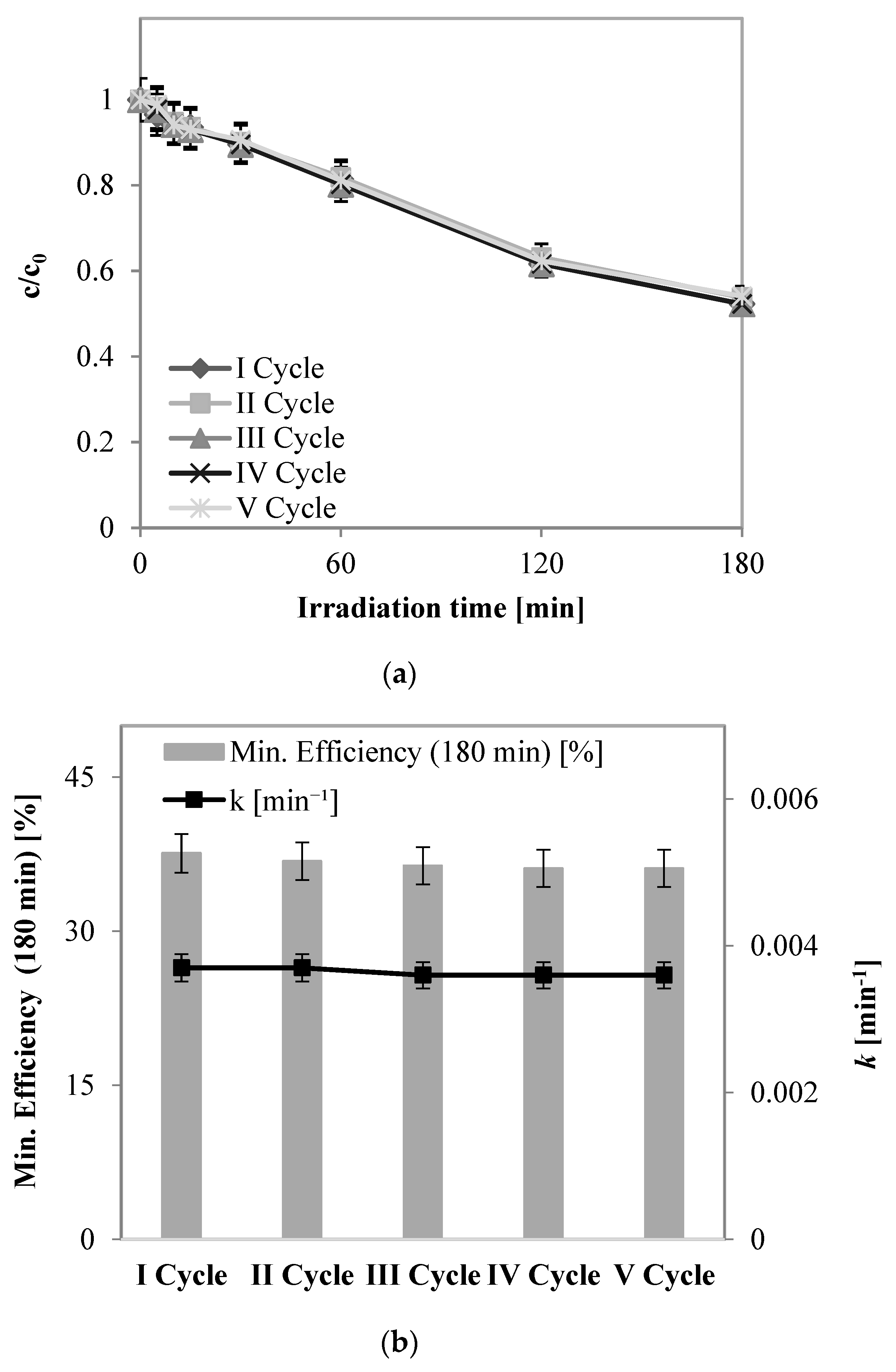
2.2.1. Preliminary Tests: Evaluation of Stability of Structured Photocatalyst
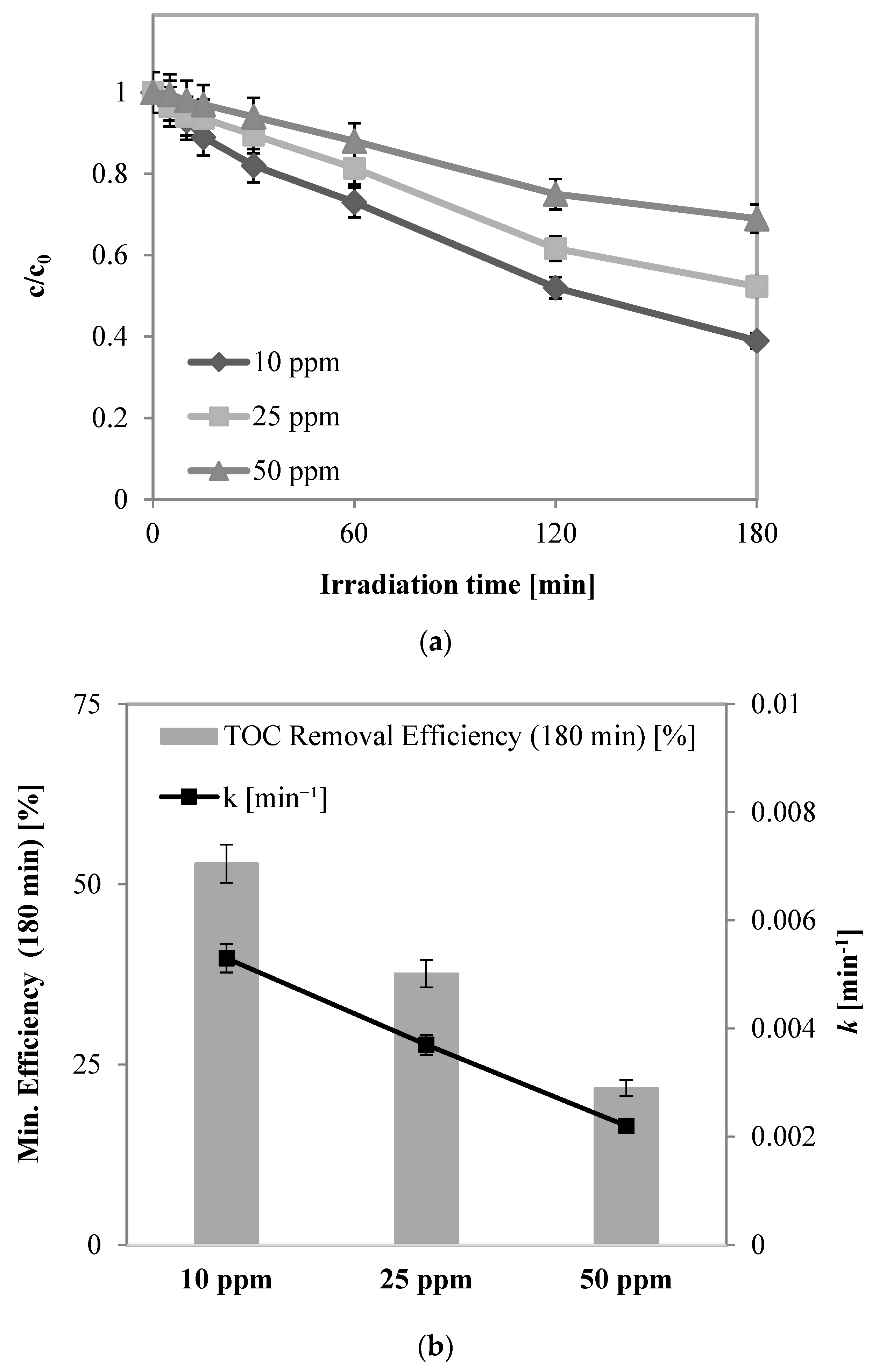
2.2.2. Preliminary Tests: Influence of Initial Phenol Concentration
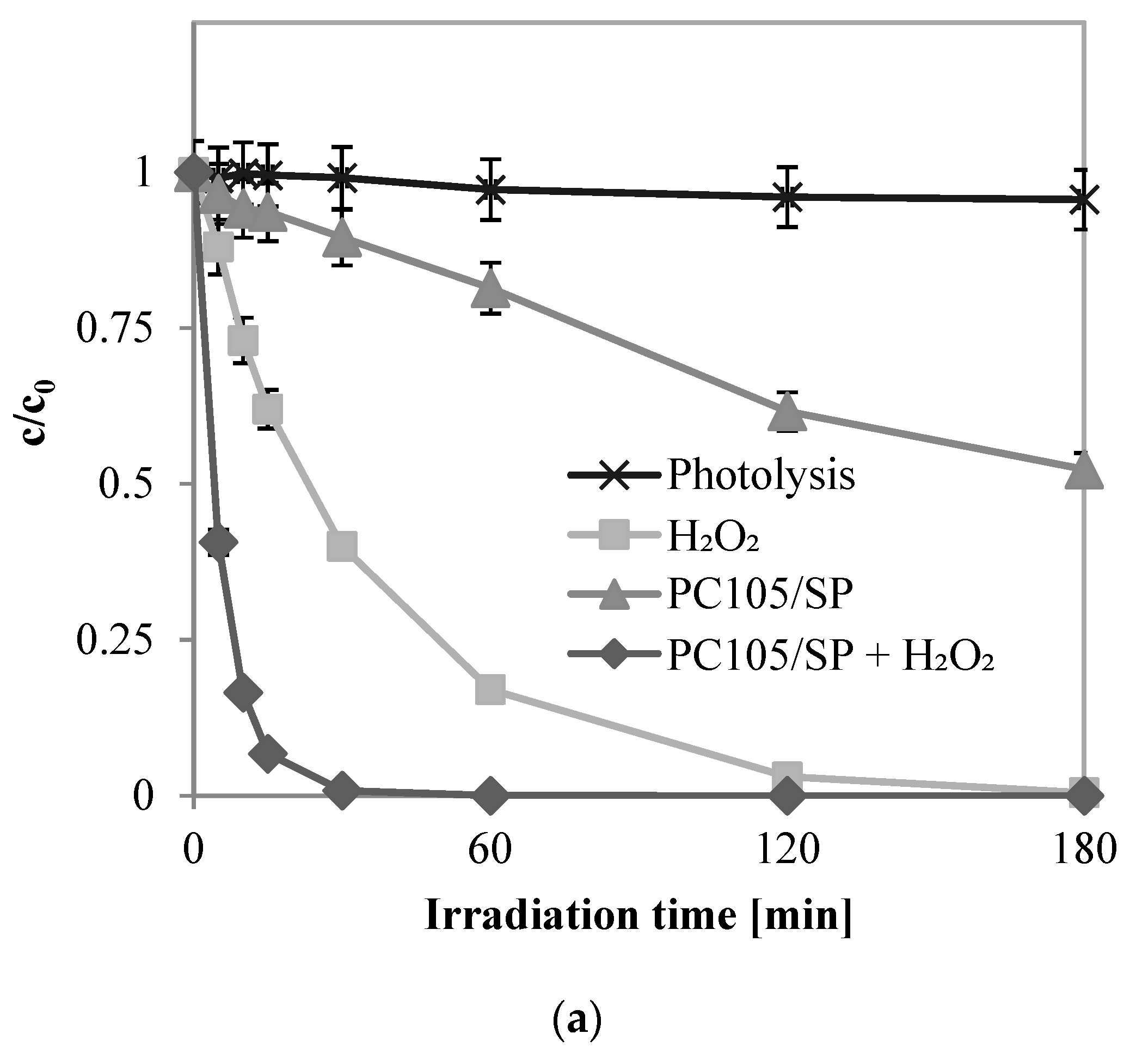
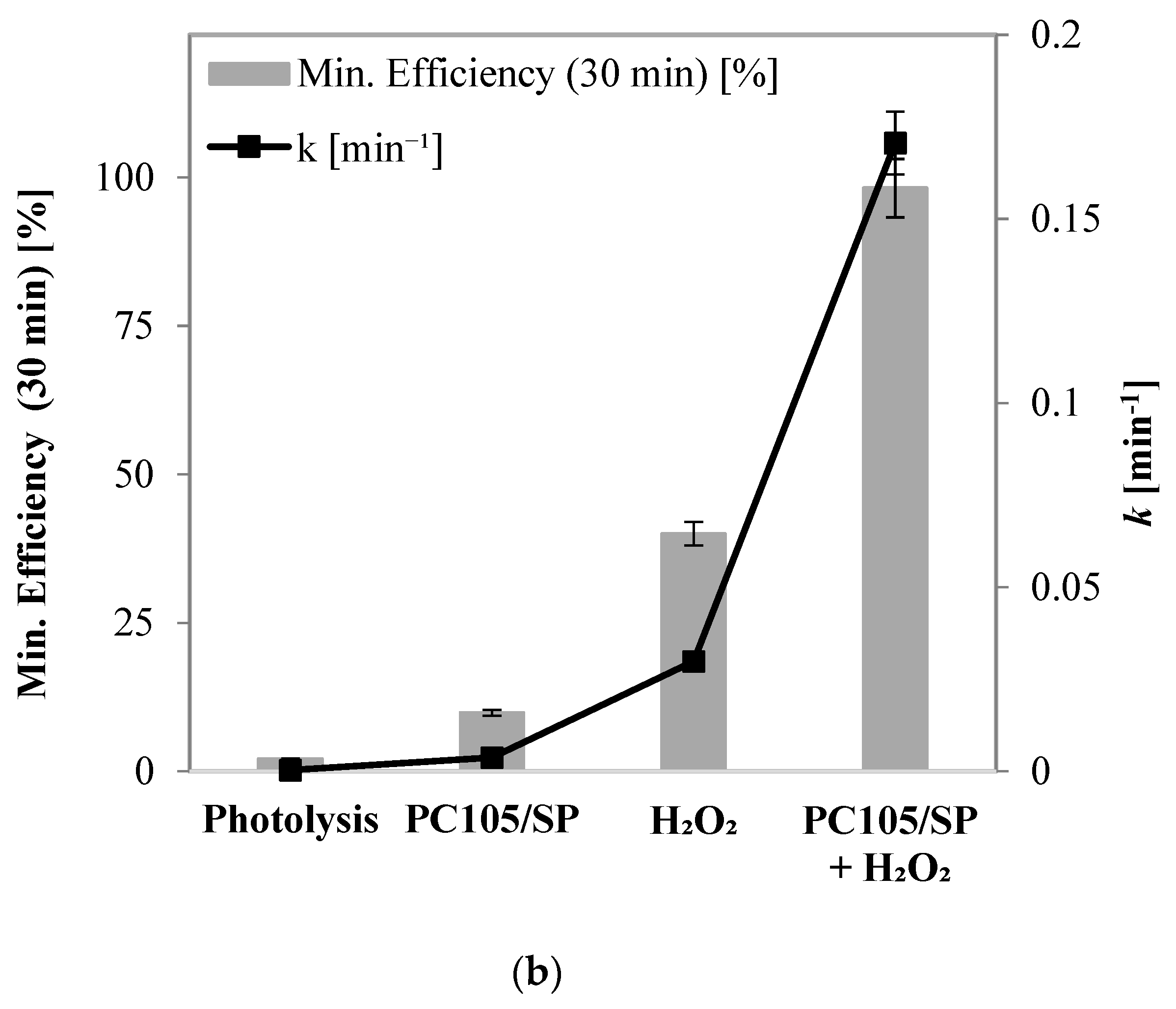
2.2.3. Effects of UV-A, UV-A + H2O2, PC105/SP + UV-A, and PC105/SP + H2O2 + UV-A on Phenol Degradation
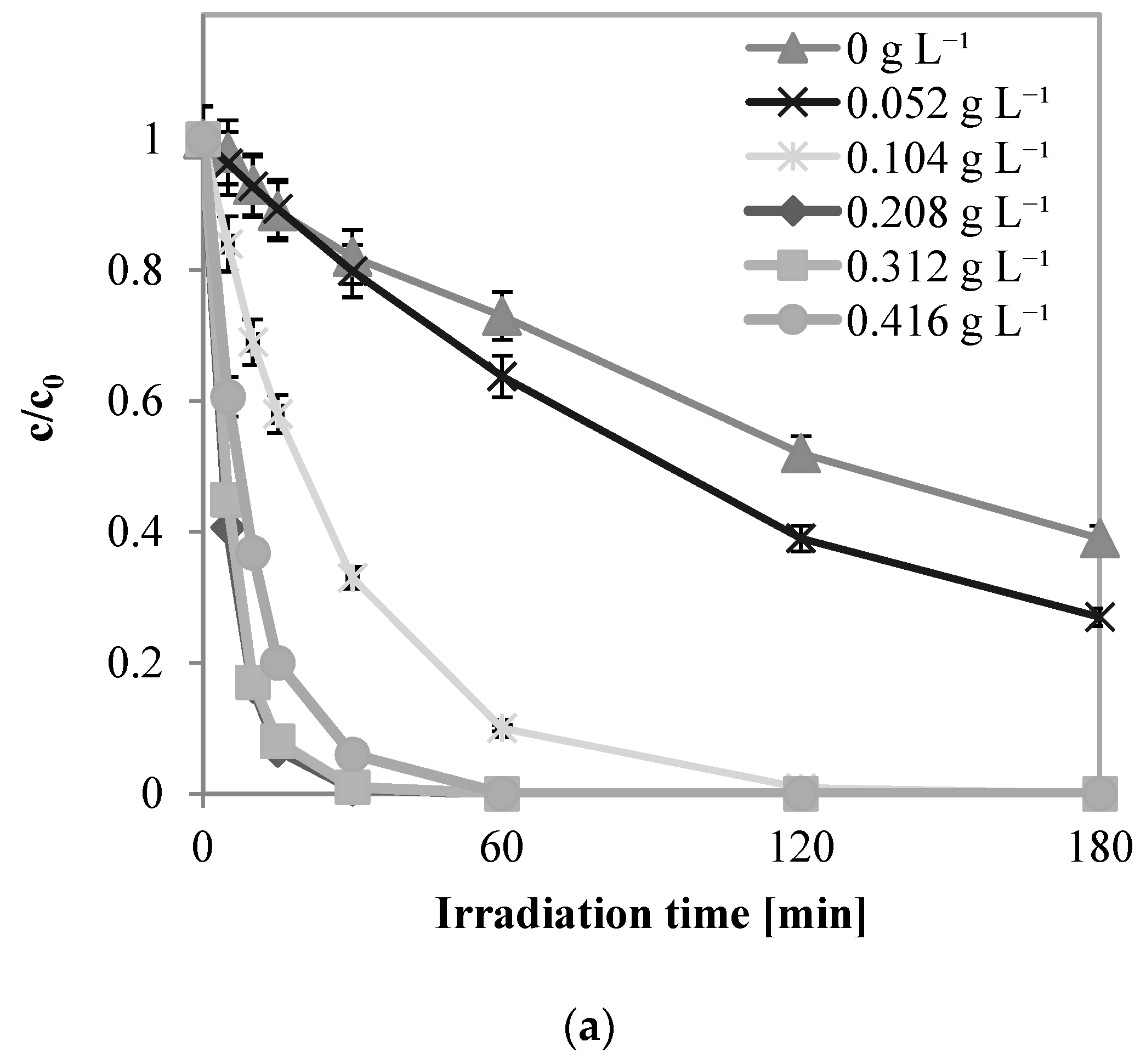
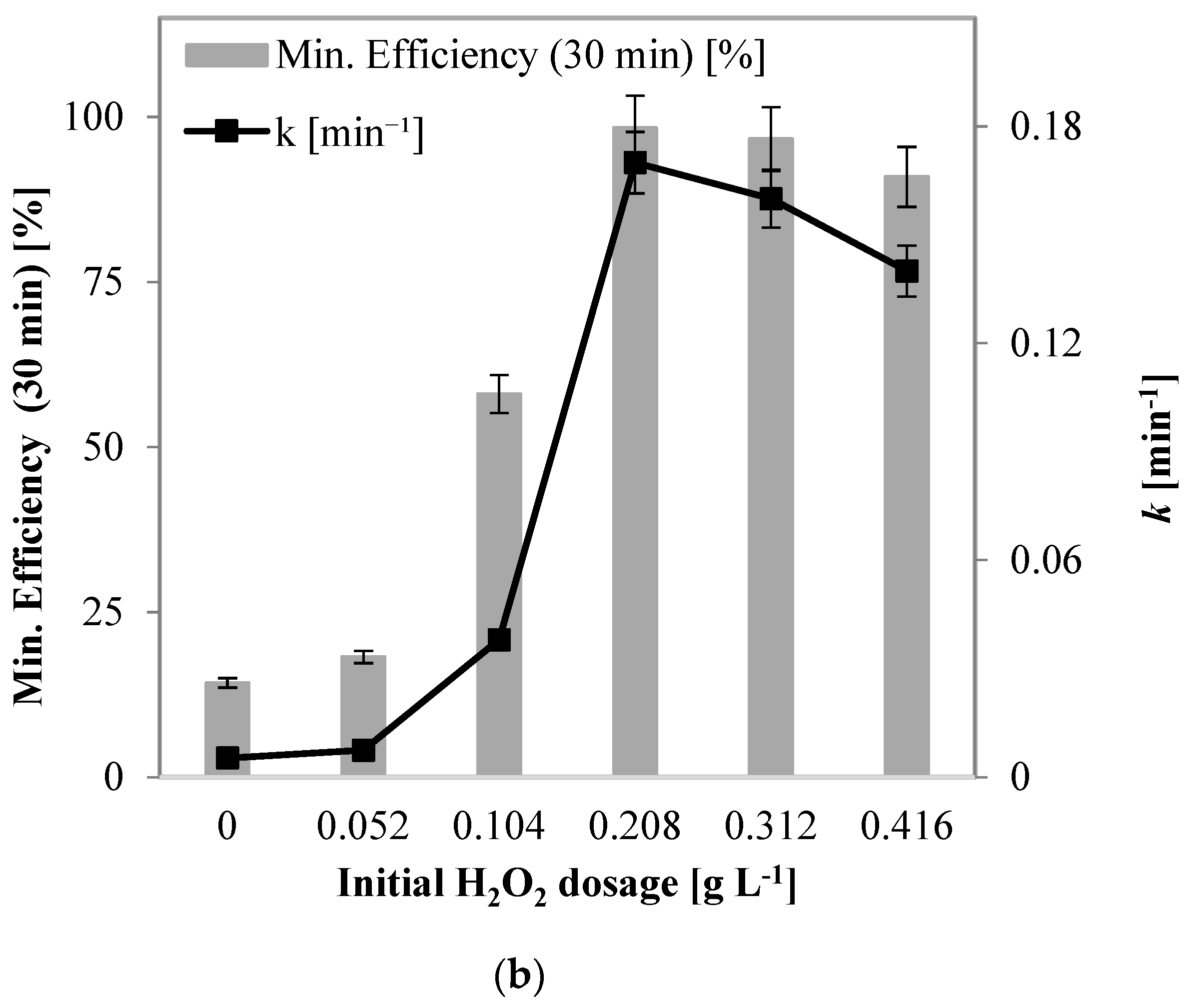
2.2.4. Influence of H2O2 Dosage
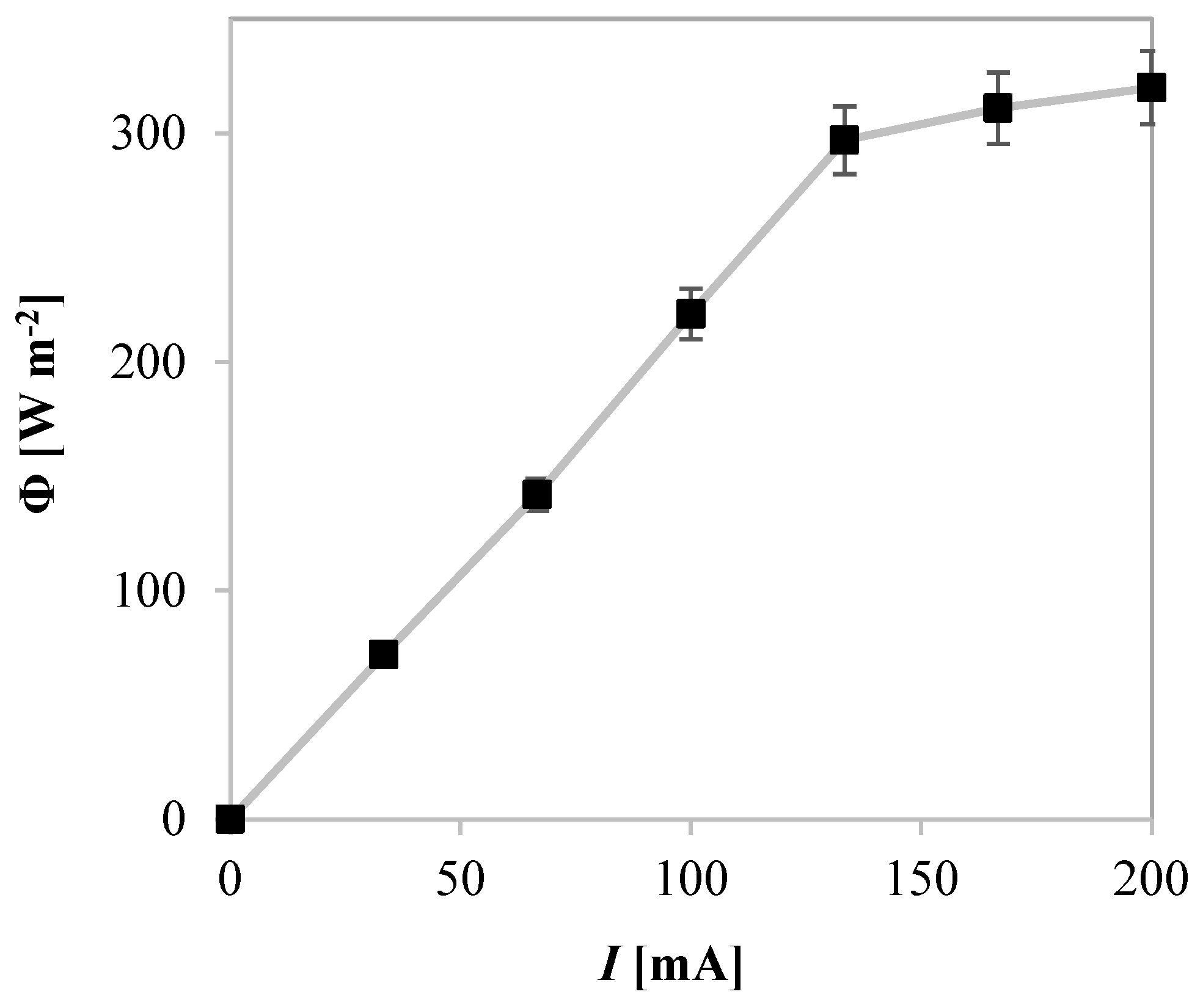
2.2.5. Influence of UV-A Light Modulation
3. Materials and Methods
3.1. Chemicals and Photocatalyst
3.2. Preparation of the Structured Photocatalyst PC105/SP
3.3. Raman Spectra Acquisition
3.4. Determination of Specific Surface Area
3.5. UV–Vis DRS Analysis
3.6. Experimental Setup Used for Phenol Mineralization Tests
3.7. Analytical Techniques Used for the Test Results Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galeas, S.; Valdivieso-Ramírez, C.S.; Pontón, P.I.; Guerrero, V.H.; Goetz, V. Photocatalytic degradation of phenol under visible light irradiation by using ferrous oxalate synthesized from iron-rich mineral sands via a green hydrothermal route. Environ. Technol. Innov. 2023, 32, 103325. [Google Scholar] [CrossRef]
- Bibi, A.; Bibi, S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. Towards sustainable physiochemical and biological techniques for the remediation of phenol from wastewater: A review on current applications and removal mechanisms. J. Clean. Prod. 2023, 417, 137810. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Rabat, N.E.; Mansor, N. Photocatalytic degradation and adsorption of phenol by solvent-controlled TiO2 nanosheets assisted with H2O2 and FeCl3: Kinetic, isotherm and thermodynamic analysis. J. Mol. Liq. 2020, 308, 112941. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Chen, S.M.; Kumar, R.S.; Arivalagan, G.; Firdhouse, M.J.; Hameed, K.S.; Sivakumar, S. Photocatalytic degradation of phenolic pollutants over palladium-tungsten trioxide nanocomposite. Chem. Eng. J. 2024, 489, 151127. [Google Scholar] [CrossRef]
- Tan, L.; Feng, H.; Li, L.; Lin, H.; Xiong, J. Phenol degradation by a combined hydrogenation and photocatalytic oxidation over the bifunctional Rh/WO3 catalyst. J. Environ. Chem. Eng. 2024, 12, 112020. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Tabassum, S. Based on nanocomposites for degradation of phenolic compounds from aqueous environments by advanced oxidation processes: A review. J. Water Process Eng. 2024, 61, 105286. [Google Scholar] [CrossRef]
- Yadav, G.; Ahmaruzzaman, M. Recent development of novel nanocomposites for photocatalysis mediated remediation of phenolic derivatives: A comprehensive review. J. Ind. Eng. Chem. 2023, 127, 18–35. [Google Scholar] [CrossRef]
- Firoozi, M.; Hashemi, M.; Narooie, M.R.; Daraei, H. Evaluation of phenol degradation rate using advanced oxidation/reduction process (ao/rp) in the presence of sulfite and zinc oxide under uv. Optik 2023, 279, 170787. [Google Scholar] [CrossRef]
- Andries, D.M.; Garrido, A.; De Stefano, L. The evolution of the eu drinking water policy towards a source-to-mouth approach. Int. J. Water Resour. Dev. 2024, 40, 723–745. [Google Scholar] [CrossRef]
- Naguib, D.M.; Badawy, N.M. Phenol removal from wastewater using waste products. J. Environ. Chem. Eng. 2020, 8, 103592. [Google Scholar] [CrossRef]
- Mumtaz, F.; Li, B.; Al Shehhi, M.R.; Feng, X.; Wang, K. Treatment of phenolic-wastewater by hybrid technologies: A review. J. Water Process Eng. 2024, 57, 104695. [Google Scholar] [CrossRef]
- Aslam, Z.; Alam, P.; Islam, R.; Khan, A.H.; Samaraweera, H.; Hussain, A.; Zargar, T.I. Recent developments in moving bed biofilm reactor (mbbr) for the treatment of phenolic wastewater—A review. J. Taiwan Inst. Chem. Eng. 2024, 105517. [Google Scholar] [CrossRef]
- Melo, J.; Lütke, S.; Igansi, A.; Franco, D.; Vicenti, J.; Dotto, G.; Pinto, L.; Cadaval, T.C., Jr.; Felipe, C. Mass transfer and equilibrium modelings of phenol adsorption on activated carbon from olive stone. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132628. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, J. Investigation of the extraction ability and mechanism of environmentally friendly ionic liquids for phenol in the model oil. Fuel 2023, 341, 127673. [Google Scholar] [CrossRef]
- Kavian, N.; Asadollahfardi, G.; Hasanbeigi, A.; Delnavaz, M.; Samadi, A. Degradation of phenol in wastewater through an integrated dielectric barrier discharge and fenton/photo-fenton process. Ecotoxicol. Environ. Saf. 2024, 271, 115937. [Google Scholar] [CrossRef]
- Cheng, A.; He, Y.; Liu, X.; He, C. Honeycomb-like biochar framework coupled with Fe3O4/FeS nanoparticles as efficient heterogeneous fenton catalyst for phenol degradation. J. Environ. Sci. 2024, 136, 390–399. [Google Scholar] [CrossRef]
- Fan, Y.; Wan, C.; Zhao, X.; Li, M.; Wu, C. Ce-doped lamno3-δ perovskite as an efficient ozonation catalyst for the degradation of high-concentration phenol wastewater. J. Environ. Chem. Eng. 2024, 12, 112761. [Google Scholar] [CrossRef]
- Song, Z.; Wu, X.; He, Q.; Xiang, P.; Ma, P.; Miao, Z. Degradation of phenol in high-salt wastewater by three-dimensional fecu co-doped materials: Interaction of FeCu with g-C3N4–rGO and synergistic catalytic mechanism. J. Environ. Chem. Eng. 2024, 12, 112164. [Google Scholar] [CrossRef]
- Mandal, R.; Mandal, A.; Mukherjee, M.; Pandit, N.; Mukherjee, B. Mechanochemically synthesized MnO2-gcn nanocomposite for photocatalytic dye and phenol degradation: A combined experiment and dft study. Micro Nano Eng. 2024, 22, 100240. [Google Scholar] [CrossRef]
- Chen, Y.; Long, L.; Luo, Y.; Zeng, B.; Liu, Z.; Shao, Q.; Wu, F.; Xie, P.; Ma, J. Insights into persulfate activation by feo for phenol removal: The production and effect of Fe (iv). Chem. Eng. J. 2023, 468, 143842. [Google Scholar] [CrossRef]
- You, Y.; He, Z. Phenol degradation in iron-based advanced oxidation processes through ferric reduction assisted by molybdenum disulfide. Chemosphere 2023, 312, 137278. [Google Scholar] [CrossRef]
- Lin, Y.; Dai, Y.; Zhang, L.; Wu, Q. Efficient degradation of phenol in aqueous solution by Fe2+/H2O2/CAO2 system. Environ. Technol. Innov. 2022, 27, 102320. [Google Scholar] [CrossRef]
- Tammaro, O.; Morante, N.; Marocco, A.; Fontana, M.; Castellino, M.; Barrera, G.; Allia, P.; Tiberto, P.; Arletti, R.; Fantini, R. The beneficial role of nano-sized Fe3O4 entrapped in ultra-stable y zeolite for the complete mineralization of phenol by heterogeneous photo-fenton under solar light. Chemosphere 2023, 345, 140400. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile tio2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Gareso, P.L.; Armynah, B.; Tahir, D. A review of tio2 photocatalyst for organic degradation and sustainable hydrogen energy production. Int. J. Hydrogrn Energy 2023, 55, 984–996. [Google Scholar] [CrossRef]
- Song, C.; Xiao, L.; Chen, Y.; Yang, F.; Meng, H.; Zhang, W.; Zhang, Y.; Wu, Y. TiO2-based catalysts with various structures for photocatalytic application: A review. Catalysts 2024, 14, 366. [Google Scholar] [CrossRef]
- Janson, O.; Unosson, E.; Strømme, M.; Engqvist, H.; Welch, K. Organic degradation potential of a TiO2/H2O2/UV–vis system for dental applications. J. Dent. 2017, 67, 53–57. [Google Scholar] [CrossRef]
- Schwarze, M.; Borchardt, S.; Frisch, M.L.; Collis, J.; Walter, C.; Menezes, P.W.; Strasser, P.; Driess, M.; Tasbihi, M. Degradation of phenol via an advanced oxidation process (aop) with immobilized commercial titanium dioxide (TiO2) photocatalysts. Nanomaterials 2023, 13, 1249. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Akram, S.; Lal, B.; Hassan, S.U.; Ashraf, R.; Kezembayeva, G.; Mushtaq, M.; Chinibayeva, N.; Hosseini-Bandegharaei, A. Advanced photocatalysis as a viable and sustainable wastewater treatment process: A comprehensive review. Environ. Res. 2024, 253, 118947. [Google Scholar] [CrossRef]
- Thaha, S.S.M.; Nalandhiran, P.; Kaliyamoorthy, S.; Mizota, I.; Mangalaraja, R.V.; Sathishkumar, P. Critical review of photocatalytic reactor designs for environmental applications. In Photocatalysis for Energy and Environmental Applications: Current Trends and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–50. [Google Scholar] [CrossRef]
- Molinari, R.; Severino, A.; Lavorato, C.; Argurio, P. Which configuration of photocatalytic membrane reactors has a major potential to be used at an industrial level in tertiary sewage wastewater treatment? Catalysts 2023, 13, 1204. [Google Scholar] [CrossRef]
- Yusuf, A.O.; Mohamed, G.H.; Al-Sakkaf, R.; Rodríguez, J.; Žerjav, G.; Pintar, A.; Palmisano, G. Photocatalytic degradation of diclofenac amide in a fixed-bed reactor using TiO2/β-Bi2O3: Process optimization and stability analysis. J. Photochem. Photobiol. A: Chem. 2024, 450, 115470. [Google Scholar] [CrossRef]
- Soison, P.; Weerapreechachai, S.; Chawengkijwanich, C. A hybrid solar/uv lamp photoreactor using TiO2/clay catalyst beads for enhanced water reclamation of secondary textile wastewater effluent. Sol. Energy 2024, 274, 112606. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Kumar, N.; Al Marzooqi, M.; Siraj, M.; Fatehmulla, A. In-situ synthesis of V2AlC@ V2O5/TiO2 immobilized over honeycomb support with vanadium oxide electron transfer mediator for stimulating selective CO2 photoreduction through bi-reforming in a monolith reactor. Fuel 2024, 370, 131816. [Google Scholar] [CrossRef]
- Palak, B.; Kumari, A.; Parmar, M.S. Textile wastewater treatment through aops with special focus on fixed-bed approach: A review. J. Water Chem. Technol. 2024, 46, 292–301. [Google Scholar] [CrossRef]
- Schneider, O.M.; Liang, R.; Bragg, L.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P.; Servos, M.R.; Zhou, Y.N. Photocatalytic degradation of microcystins by TiO2 using uv-led controlled periodic illumination. Catalysts 2019, 9, 181. [Google Scholar] [CrossRef]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. A comparative study of the activity of TiO2 degussa p25 and millennium pcs in the photocatalytic degradation of bromothymol blue. Int. J. Chem. React. Eng. 2018, 16, 20170014. [Google Scholar] [CrossRef]
- Rosa, R.H.; Silva, R.S.; Nascimento, L.L.; Okura, M.H.; Patrocinio, A.O.T.; Rossignolo, J.A. Photocatalytic and antimicrobial activity of TiO2 films deposited on fiber-cement surfaces. Catalysts 2023, 13, 861. [Google Scholar] [CrossRef]
- Morante, N.; Folliero, V.; Dell’Annunziata, F.; Capuano, N.; Mancuso, A.; Monzillo, K.; Galdiero, M.; Sannino, D.; Franci, G. Characterization and photocatalytic and antibacterial properties of Ag- and TiOx-based (x = 2, 3) composite nanomaterials under UV irradiation. Materials 2024, 17, 2178. [Google Scholar] [CrossRef]
- Al-Oubidy, E.A.; Kadhim, F.J. Photocatalytic activity of anatase titanium dioxide nanostructures prepared by reactive magnetron sputtering technique. Opt. Quantum Electron. 2019, 51, 23. [Google Scholar] [CrossRef]
- Lee, H.; Kannan, P.; Al Shoaibi, A. Srinivasakannan. Phenol degradation catalyzed by metal oxide supported porous carbon matrix under uv irradiation. J. Water Process Eng. 2019, 31, 100869. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Almond shell activated carbon: Adsorbent and catalytic support in the phenol degradation. Environ. Monit. Assess. 2014, 186, 3875–3890. [Google Scholar] [CrossRef] [PubMed]
- Bognár, S.; Jovanović, D.; Despotović, V.; Finčur, N.; Putnik, P.; Merkulov, D.Š. Advancing wastewater treatment: A comparative study of photocatalysis, sonophotolysis, and sonophotocatalysis for organics removal. Processes 2024, 12, 1256. [Google Scholar] [CrossRef]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.-C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B Environ. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- Xu, J.; Shen, J.; Jiang, H.; Yu, X.; Qureshi, W.A.; Maouche, C.; Gao, J.; Yang, J.; Liu, Q. Progress and challenges in full spectrum photocatalysts: Mechanism and photocatalytic applications. J. Ind. Eng. Chem. 2023, 119, 112–129. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A review on heterogeneous photocatalytic materials: Mechanism, perspectives, and environmental and energy sustainability applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Lee, J.K.; Walker, K.L.; Han, H.S.; Kang, J.; Prinz, F.B.; Waymouth, R.M.; Nam, H.G.; Zare, R.N. Spontaneous generation of hydrogen peroxide from aqueous microdroplets. Proc. Natl. Acad. Sci. USA 2019, 116, 19294–19298. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (iupac technical report). Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous photocatalysis: State of the art and present applications in honor of pr. R.L. Burwell jr. (1912–2003), former head of ipatieff laboratories, northwestern university, evanston (ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Morante, N.; Tammaro, O.; Albarano, L.; De Guglielmo, L.; Oliva, N.; Sacco, O.; Mancuso, A.; Castellino, M.; Sannino, D.; Femia, N.; et al. Influence of visible light leds modulation techniques on photocatalytic degradation of ceftriaxone in a flat plate reactor. Chem. Eng. J. 2024, 482, 149175. [Google Scholar] [CrossRef]
- Gayral, B. Leds for lighting: Basic physics and prospects for energy savings. Comptes Rendus. Phys. 2017, 18, 453–461. [Google Scholar] [CrossRef]
- Pei, Y.; Zhu, S.; Yang, H.; Zhao, L.; Yi, X.; Junxi Wang, J.; Li, J. Led modulation characteristics in a visible-light communication system. Opt. Photonics J. 2013, 3, 4. [Google Scholar] [CrossRef]
- Di Capua, G.; Femia, N.; Migliaro, M.; Sacco, O.; Sannino, D.; Stoyka, K.; Vaiano, V. Intensification of a flat-plate photocatalytic reactor performances by innovative visible light modulation techniques: A proof of concept. Chem. Eng. Process. Process Intensif. 2017, 118, 117–123. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Di Capua, G.; Femia, N.; Sannino, D. Use of visible light modulation techniques in urea photocatalytic degradation. Water 2019, 11, 1642. [Google Scholar] [CrossRef]
- Sannino, D.; Morante, N.; Sacco, O.; Mancuso, A.; De Guglielmo, L.; Di Capua, G.; Femia, N.; Vaiano, V. Visible light-driven degradation of acid orange 7 by light modulation techniques. Photochem. Photobiol. Sci. 2023, 22, 185–193. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Sacco, O.; Morante, N.; De Guglielmo, L.; Di Capua, G.; Femia, N. Visible light driven degradation of terephthalic acid: Optimization of energy demand by light modulation techniques. J. Photocatal. 2021, 2, 13. [Google Scholar] [CrossRef]
- Moreno, I.; Avendaño-Alejo, M.; Tzonchev, R.I. Designing light-emitting diode arrays for uniform near-field irradiance. Appl. Opt. 2006, 45, 2265–2272. [Google Scholar] [CrossRef]
- Bhattacharya, P. Semiconductor Optoelectronic Devices, 2nd ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Bahadori, E.; Vaiano, V.; Esposito, S.; Armandi, M.; Sannino, D.; Bonelli, B. Photo-activated degradation of tartrazine by H2O2 as catalyzed by both bare and Fe-doped methyl-imogolite nanotubes. Catal. Today 2018, 304, 199–207. [Google Scholar] [CrossRef]
- Khuzwayo, Z.; Chirwa, E.M.N. Modelling and simulation of photocatalytic oxidation mechanism of chlorohalogenated substituted phenols in batch systems: Langmuir–hinshelwood approach. J. Hazard. Mater. 2015, 300, 459–466. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Di Capua, G.; Femia, N. Enhanced performances of a photocatalytic reactor for wastewater treatment using controlled modulation of leds light. Chem. Eng. Trans. 2017, 57, 6. [Google Scholar]






| Light Modulation | P [kW] | V [L] | k [min−1] | EE/O [kWh m−3] |
|---|---|---|---|---|
| FD | 0.036 | 0.45 | 0.17 | 7.84 |
| S-VD | 0.036 | 0.45 | 0.34 | 3.92 |
| SW-VD | 0.036 | 0.45 | 0.36 | 3.70 |
| T-VD | 0.036 | 0.45 | 0.39 | 3.42 |
| ST-VD | 0.036 | 0.45 | 0.32 | 4.17 |
| P-VD | 0.036 | 0.45 | 0.28 | 4.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morante, N.; De Guglielmo, L.; Oliva, N.; Monzillo, K.; Femia, N.; Di Capua, G.; Vaiano, V.; Sannino, D. Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2. Catalysts 2024, 14, 544. https://doi.org/10.3390/catal14080544
Morante N, De Guglielmo L, Oliva N, Monzillo K, Femia N, Di Capua G, Vaiano V, Sannino D. Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2. Catalysts. 2024; 14(8):544. https://doi.org/10.3390/catal14080544
Chicago/Turabian StyleMorante, Nicola, Luca De Guglielmo, Nunzio Oliva, Katia Monzillo, Nicola Femia, Giulia Di Capua, Vincenzo Vaiano, and Diana Sannino. 2024. "Influence of UV-A Light Modulation on Phenol Mineralization by TiO2 Photocatalytic Process Coadjuvated with H2O2" Catalysts 14, no. 8: 544. https://doi.org/10.3390/catal14080544








