Hydrogen-Ignited-Methanol Catalytic Co-Combustion of Aromatic Volatile Organic Compounds over PdPt/Al2O3 Bimetallic Catalyst
Abstract
:1. Introduction
2. Results
2.1. Structure Evolution of PdPt/Al2O3 Catalyst
2.1.1. X-ray Diffraction
2.1.2. BET Analysis
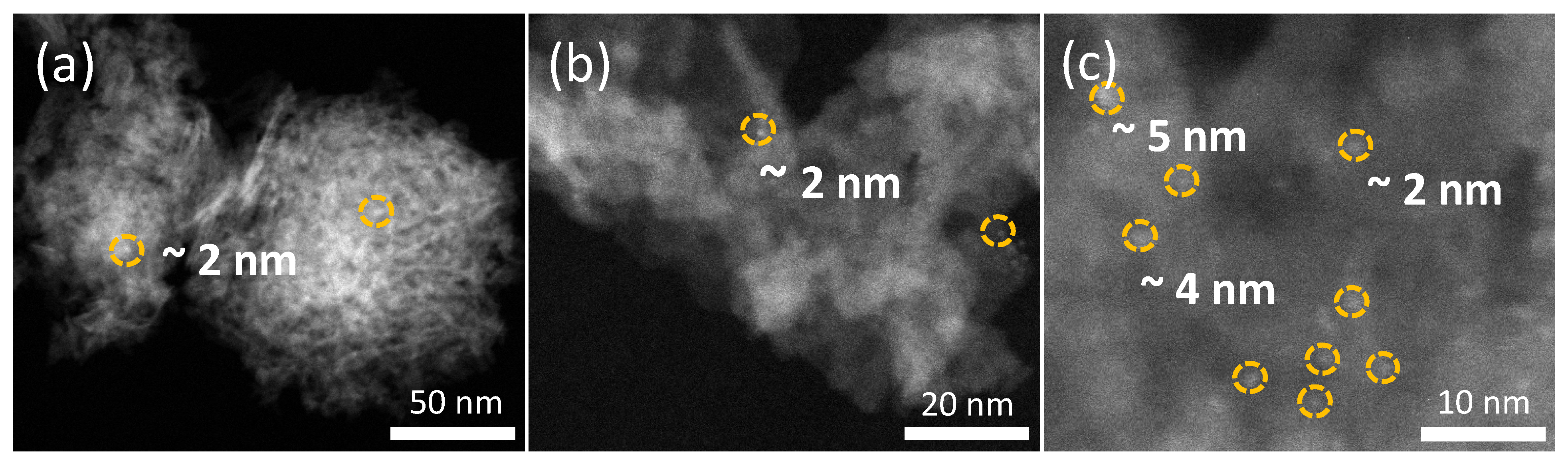
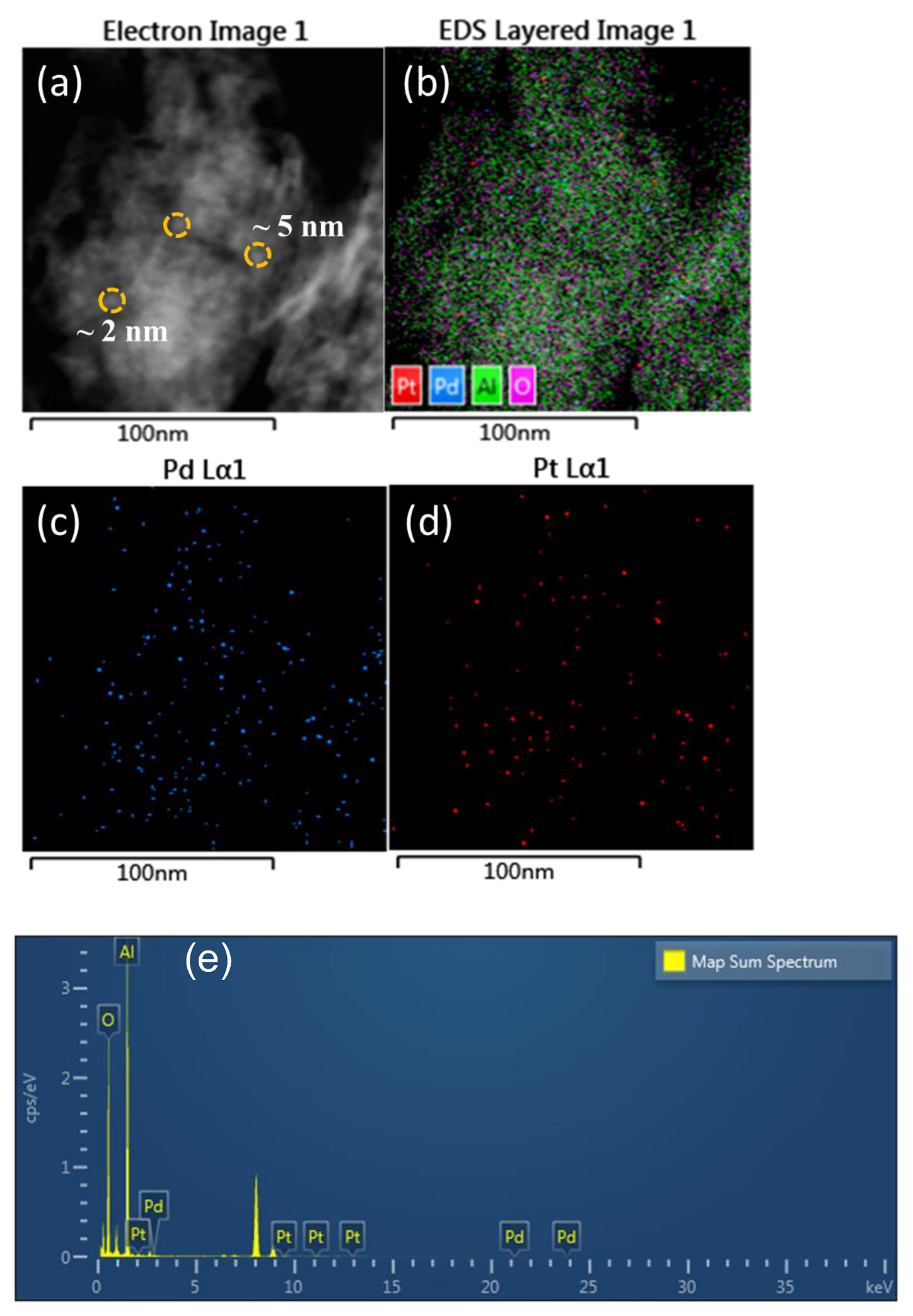
2.1.3. STEM Analysis
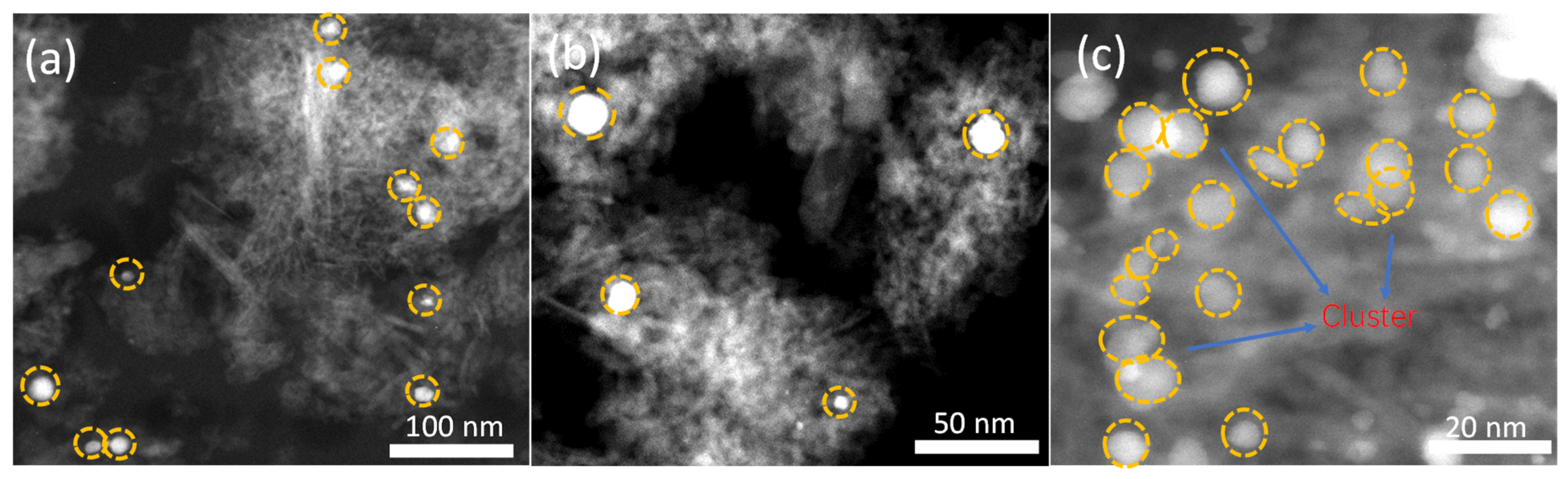
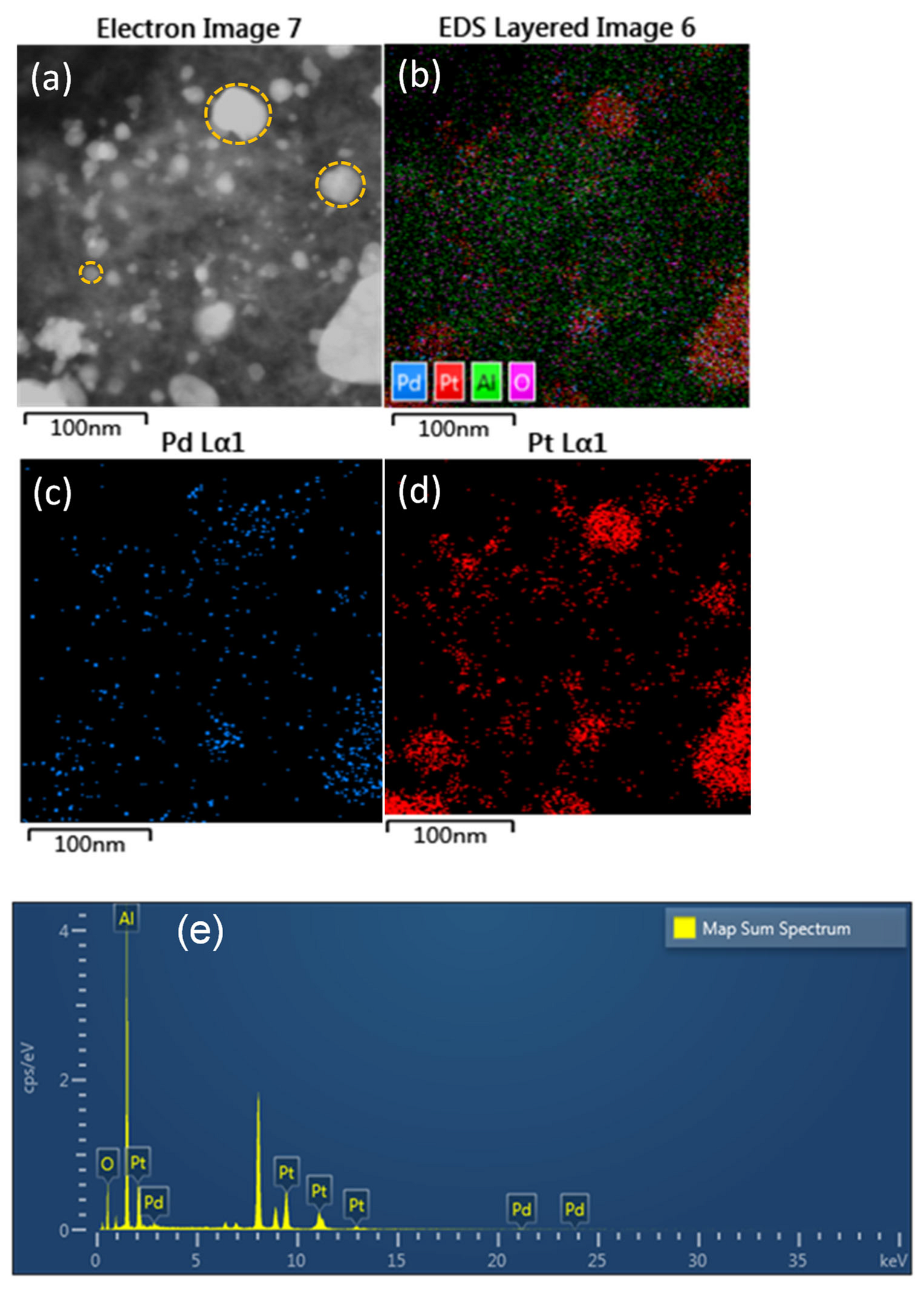
2.1.4. ICP Analysis
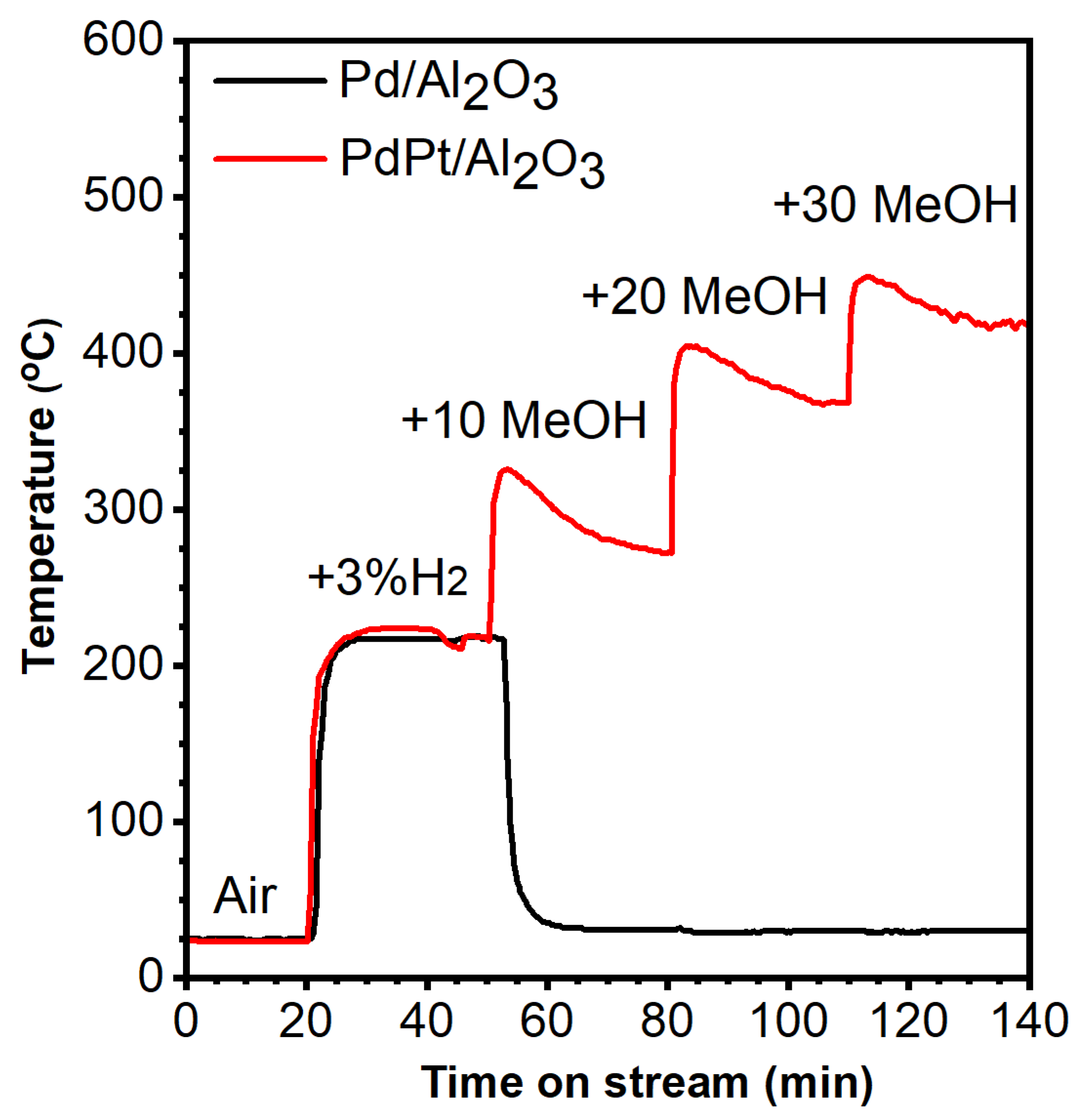
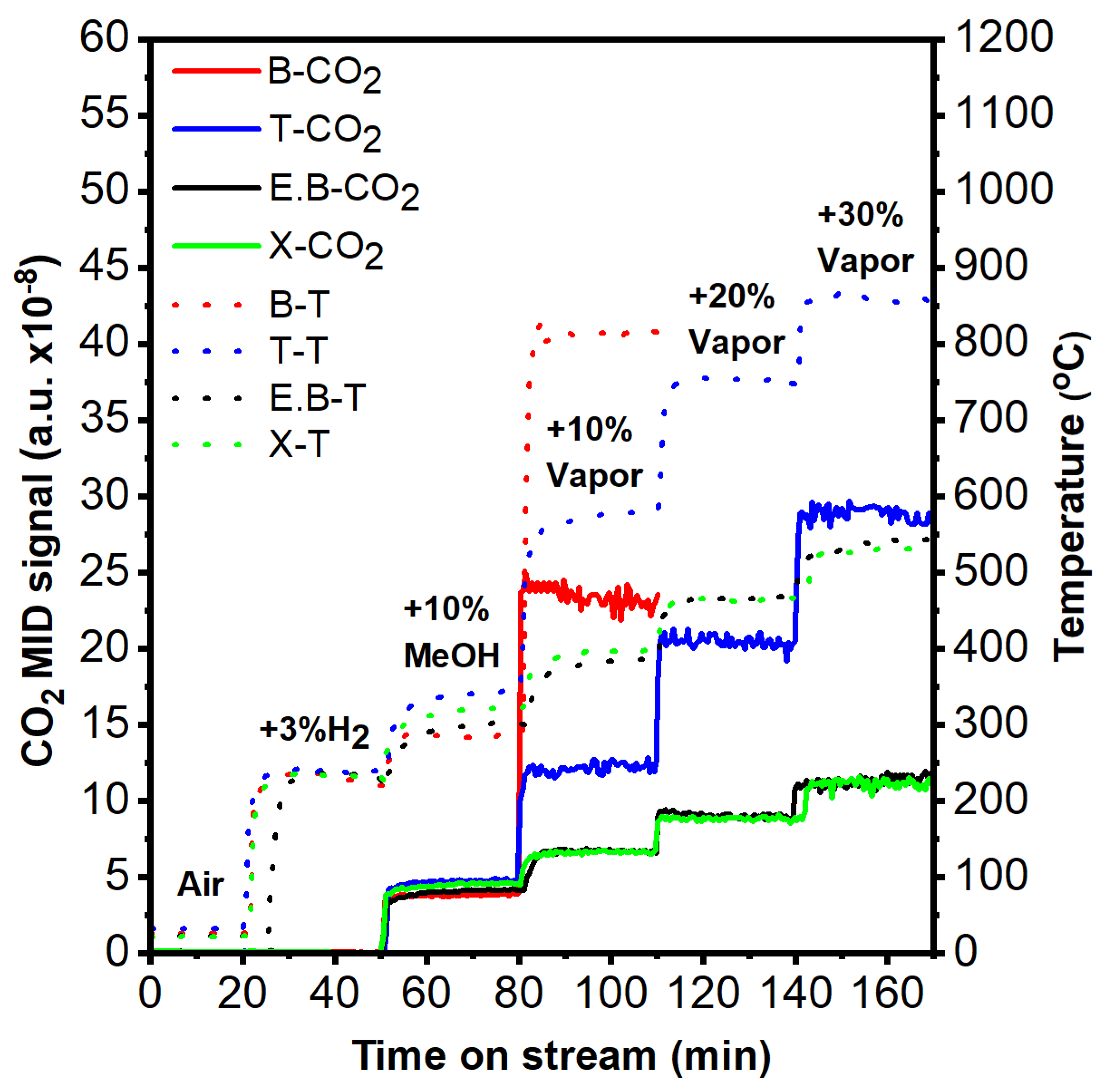
2.2. Catalytic Performance Assessment
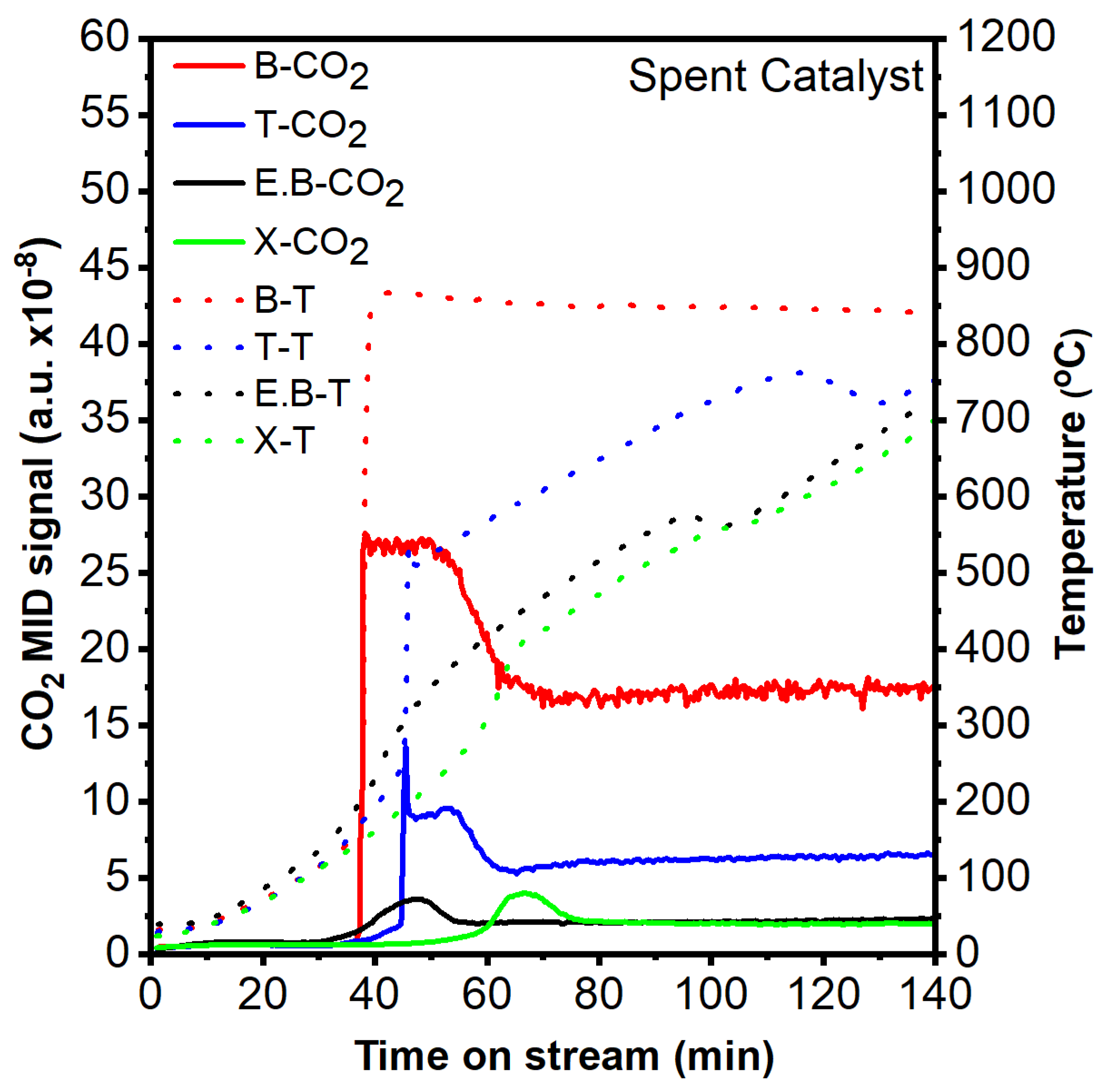
2.3. Performance of Spent Catalyst
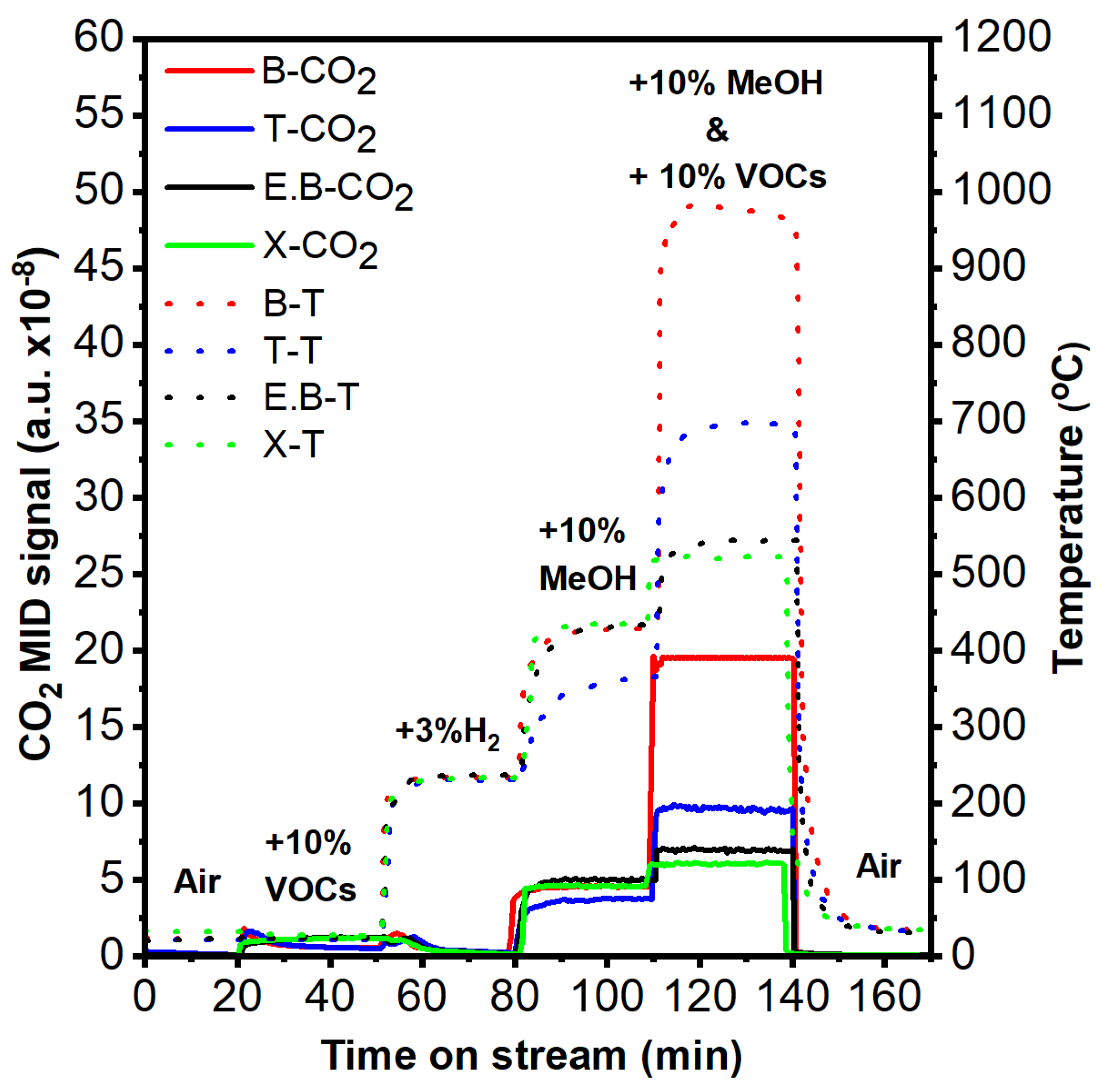
2.4. Hydrogen-Ignited-Methanol Effect
3. Discussion
3.1. Heating Technology Comparison: Electric vs. Co-Combustion
3.2. Catalytic Advantages of Onsite Heating with H2-Ignited Methanol Combustion
4. Experimental Section
4.1. Preparation of Catalyst
4.2. Catalyst Characterizations
4.3. Reactor Setup and Performance Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, Y.; Yang, J.; Pan, C. The Surface/Interface Modulation of Platinum Group Metal (PGM)-Free Catalysts for VOCs and CO Catalytic Oxidation. ACS Appl. Mater. Interfaces 2024, 16, 37379–37389. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; He, H.; Liu, F.; Wang, C. Effect of Doping Metals on OMS-2/γ-Al2O3 Catalysts for Plasma-Catalytic Removal of o-Xylene. J. Phys. Chem. C 2016, 120, 6136–6144. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.; He, H. Complete oxidation of o-xylene over Pd/Al2O3 catalyst at low temperature. Catal. Today 2008, 139, 15–23. [Google Scholar] [CrossRef]
- Ren, Y.; Lei, X.; Wang, H.; Xiao, J.; Qu, Z. Enhanced Catalytic Performance of La-Doped CoMn2O4 Catalysts by Regulating Oxygen Species Activity for VOCs Oxidation. ACS Catal. 2023, 13, 8293–8306. [Google Scholar] [CrossRef]
- Gil-Barbarin, A.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R.; de Rivas, B. Promotion of Cobalt Oxide Catalysts by Acid-Etching and Ruthenium Incorporation for Chlorinated VOC Oxidation. Ind. Eng. Chem. Res. 2024, 63, 3003–3017. [Google Scholar] [CrossRef]
- Shan, C.; Wang, Y.; Li, J.; Zhao, Q.; Han, R.; Liu, C.; Liu, Q. Recent Advances of VOCs Catalytic Oxidation over Spinel Oxides: Catalyst Design and Reaction Mechanism. Environ. Sci. Technol. 2023, 57, 9495–9514. [Google Scholar] [CrossRef]
- Zha, K.; Wu, S.; Zheng, Z.; Huang, Z.; Xu, H.; Shen, W. Insights into Boosting SO2 Tolerance for Catalytic Oxidation of Propane over Fe2O3-Promoted Co3O4/Halloysite Catalysts. Ind. Eng. Chem. Res. 2022, 61, 12482–12492. [Google Scholar] [CrossRef]
- Sun, N.; Wang, L.; Zhang, Y.; Cao, Z.; Sun, J. Co2Cu1CeyOx Mixed Metal Oxide Nanoparticles with Oxygen Vacancies as Catalysts for Toluene Oxidation. ACS Appl. Nano Mater. 2023, 6, 18823–18836. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, S.; Yu, J.; Jin, X.; Ye, D.; Yang, S.; Qiu, Y. Effect of Absorbed Sulfate Poisoning on the Performance of Catalytic Oxidation of VOCs over MnO2. ACS Appl. Mater. Interfaces 2020, 12, 50566–50572. [Google Scholar] [CrossRef]
- Žumbar, T.; Arčon, I.; Djinović, P.; Aquilanti, G.; Žerjav, G.; Pintar, A.; Ristić, A.; Dražić, G.; Volavšek, J.; Mali, G.; et al. Winning Combination of Cu and Fe Oxide Clusters with an Alumina Support for Low-Temperature Catalytic Oxidation of Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2023, 15, 28747–28762. [Google Scholar] [CrossRef]
- Obraztsov, N.V.; Subbotin, D.I.; Pavlova, E.A.; Frolov, V.Y.; Belyaev, V.L. Synthesis of the nickel-containing catalyst on the aluminum oxide supporter produced by glycine-nitrate synthesis. Mater. Today Proc. 2020, 30, 528–531. [Google Scholar] [CrossRef]
- Žumbar, T.; Ristić, A.; Dražić, G.; Lazarova, H.; Volavšek, J.; Pintar, A.; Zabukovec Logar, N.; Tušar, N.N. Influence of Alumina Precursor Properties on Cu-Fe Alumina Supported Catalysts for Total Toluene Oxidation as a Model Volatile Organic Air Pollutant. Catalysts 2021, 11, 252. [Google Scholar] [CrossRef]
- Li, A.; Wang, P.; Yi, J.; Farhan, S.M.; Zhang, L.; Zhao, L.; Lei, L. Influence of M-Doped (M = Ba, Zr, La, and Ce) for Enhanced CO and C3H6 Catalytic Oxidation over Pd/Al2O3 Catalysts. J. Phys. Chem. C 2024, 128, 14638–14648. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and physicochemical characterizations of nanostructured Pt/Al2O3–CeO2 catalysts for total oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Yang, P.; Cheng, Z.; Li, J.; Zuo, S. Ce-modified mesoporous N3-Al2O3 supported Pd-Pt nanoparticle catalysts and their structure-function relationship in complete benzene oxidation. Chem. Eng. J. 2019, 356, 255–261. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Y.; Yao, W.; Wu, Z. One-step synthesis of bimetallic Pt-Pd/MCM-41 mesoporous materials with superior catalytic performance for toluene oxidation. Catal. Commun. 2016, 83, 22–26. [Google Scholar] [CrossRef]
- Maione, A.; Andre, F.; Ruiz, P. Structured bimetallic Pd-Pt/γ-Al2O3 catalysts on FeCrAlloy fibers for total combustion of methane. Appl. Catal. B Environ. 2007, 75, 59–70. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.-K.; Nah, J.W. Property of a highly active bimetallic catalyst based on a supported manganese oxide for the complete oxidation of toluene. Powder Technol. 2014, 266, 292–298. [Google Scholar] [CrossRef]
- Fan, X.; Wang, F.; Zhu, T.; He, H. Effects of Ce on catalytic combustion of methane over Pd-Pt/Al2O3 catalyst. J. Environ. Sci. 2012, 24, 507–511. [Google Scholar] [CrossRef]
- Sultana, S.; Vandenbroucke, A.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs with Alternate Adsorption and Plasma-Assisted Regeneration: A Review. Catalysts 2015, 5, 718–746. [Google Scholar] [CrossRef]
- Zhong, B.-j.; Yang, F. Characteristics of hydrogen-assisted catalytic ignition of n-butane/air mixtures. Int. J. Hydrogen Energy 2012, 37, 8716–8723. [Google Scholar] [CrossRef]
- Zhong, B.-J.; Yang, Q.-T.; Yang, F. Hydrogen-assisted catalytic ignition characteristics of different fuels. Combust. Flame 2010, 157, 2005–2007. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Wang, R.; Lu, W.; Liu, W.; Zhang, Y. Investigation on the effects of blending hydrogen-rich gas in the spark-ignition engine. Energy Rep. 2022, 8, 797–803. [Google Scholar] [CrossRef]
- Verhelst, S.; Turner, J.W.G.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Jang, E.J.; Lee, J.; Oh, D.G.; Kwak, J.H. CH4 oxidation activity in pd and Pt–Pd bimetallic catalysts: Correlation with surface PdO x quantified from the DRIFTS study. ACS Catal. 2021, 11, 5894–5905. [Google Scholar] [CrossRef]
- Ho, P.H.; Shao, J.; Yao, D.; Ilmasani, R.F.; Salam, M.A.; Creaser, D.; Olsson, L. The effect of Pt/Pd ratio on the oxidation activity and resistance to sulfur poisoning for Pt-Pd/BEA diesel oxidation catalysts with high siliceous content. J. Environ. Chem. Eng. 2022, 10, 108217. [Google Scholar] [CrossRef]
- Kim, S.-I.; Im, M.; Cho, E.; Jang, H.; Jang, S.Y.; Kim, D.W.; Kim, K.W.; Heo, I.; Kim, Y.J.; Lee, J.H. Effects of thermal aging on the electronic and structural properties of Pt-Pd and toluene oxidation activity. Sci. Total Environ. 2022, 847, 157482. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Zhao, L.; Wang, Y.; He, Z.; Xing, W.; Bai, P.; Mintova, S.; Yan, Z. Formation of PdO on Au–Pd bimetallic catalysts and the effect on benzyl alcohol oxidation. J. Catal. 2019, 375, 32–43. [Google Scholar] [CrossRef]
- Ho, P.H.; Woo, J.W.; Feizie Ilmasani, R.; Han, J.; Olsson, L. The role of Pd–Pt interactions in the oxidation and sulfur resistance of bimetallic Pd–Pt/γ-Al2O3 diesel oxidation catalysts. Ind. Eng. Chem. Res. 2021, 60, 6596–6612. [Google Scholar] [CrossRef]
- Mussio, A.; Danielis, M.; Divins, N.r.J.; Llorca, J.; Colussi, S.; Trovarelli, A. Structural evolution of bimetallic PtPd/CeO2 methane oxidation catalysts prepared by dry milling. ACS Appl. Mater. Interfaces 2021, 13, 31614–31623. [Google Scholar] [CrossRef]
- Narui, K.; Yata, H.; Furuta, K.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Effects of addition of Pt to PdO/Al2O3 catalyst on catalytic activity for methane combustion and TEM observations of supported particles. Appl. Catal. A Gen. 1999, 179, 165–173. [Google Scholar] [CrossRef]
- Song, M.; Zeng, W.; Li, L.; Wu, X.; Li, G.; Hu, C. Effect of the Zr/Al Molar Ratio on the Performance of Cu/ZrO2–Al2O3 Catalysts for Methanol Steam Reforming. Ind. Eng. Chem. Res. 2023, 62, 3898–3908. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yu, X.; Ge, M. Exploration of strong metal-support interaction in zirconia supported catalysts for toluene oxidation. Appl. Catal. B Environ. 2020, 263, 118355. [Google Scholar] [CrossRef]
- Chen, K.; Wan, J.; Wang, T.; Sun, Q.; Zhou, R. Construction of bimetallic Pt–Pd/CeO2–ZrO2–La2O3 catalysts with different Pt/Pd ratios and its structure–activity correlations for three-way catalytic performance. J. Rare Earths 2023, 41, 896–904. [Google Scholar] [CrossRef]
- Kozhukhova, A.; Du Preez, S.; Shuro, I.; Bessarabov, D. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Schefer, R.; Robben, F.; Cheng, R. Catalyzed combustion of H2/air mixtures in a flat-plate boundary layer: I. Experimental results. Combust. Flame 1980, 38, 51–63. [Google Scholar] [CrossRef]
- Ullah, L.; Munsif, S.; Cao, L.; Zhang, J.-C.; Li, W.-Z. Facile Abatement of Oxygenated Volatile Organic Compounds via Hydrogen Co-Combustion over Pd/Al2O3 Catalyst as Onsite Heating Source. Catalysts 2024, 14, 372. [Google Scholar] [CrossRef]
- Saint-Just, J.; Der Kinderen, J. Catalytic combustion: From reaction mechanism to commercial applications. Catal. Today 1996, 29, 387–395. [Google Scholar] [CrossRef]

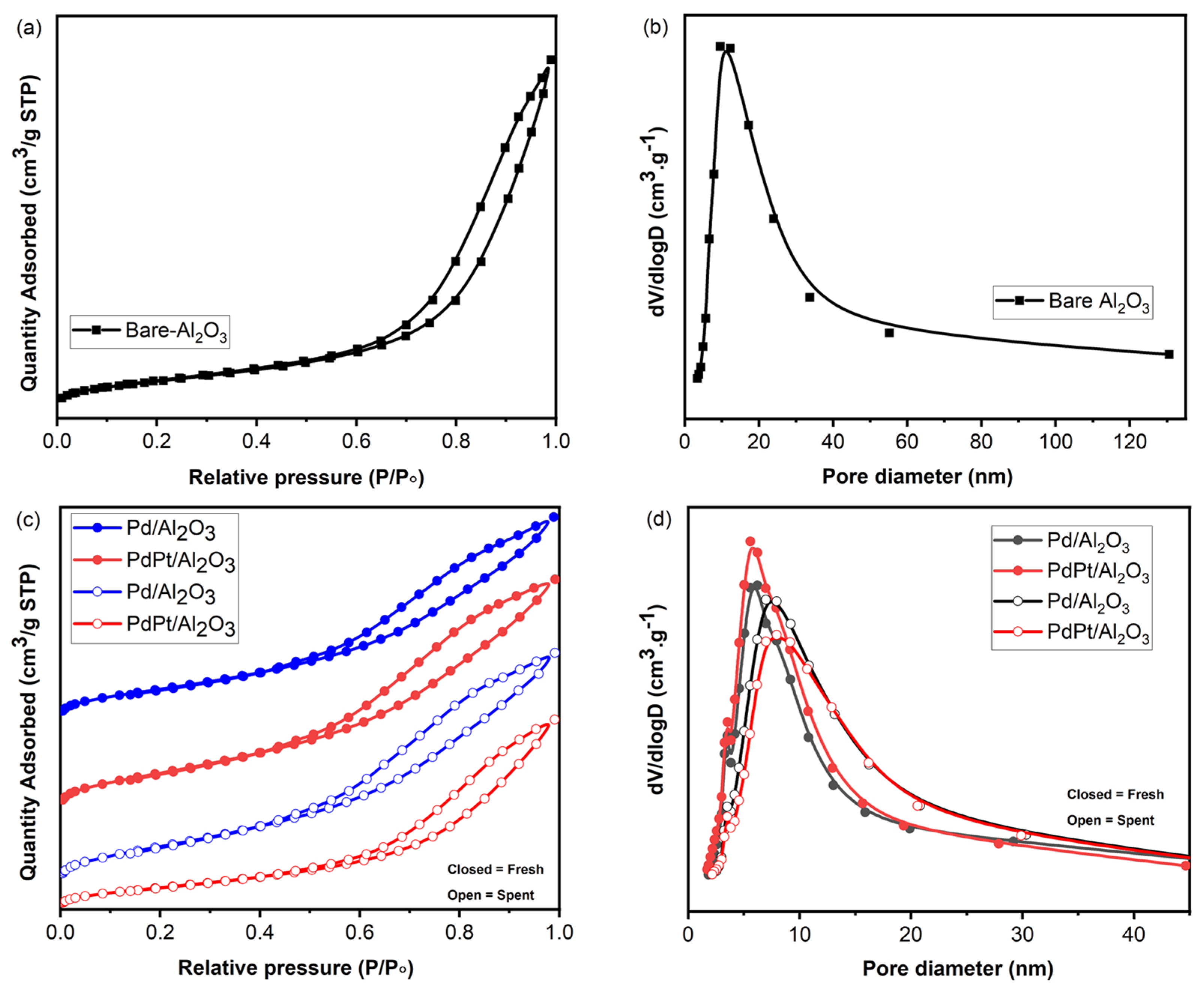


| Sample | SBET (m²/g) | Pore Size (nm) | Pore Volume (cm³/g) |
|---|---|---|---|
| Al2O3 | 115.9 | 4.9 | 2.7 |
| Pd/Al2O3-F * | 208.1 | 6.6 | 0.4 |
| Pd/Al2O3-S # | 164.8 | 8.4 | 0.4 |
| PdPt/Al2O3-F | 251.3 | 6.3 | 0.5 |
| PdPt/Al2O3-S | 143.3 | 8.8 | 0.4 |
| Chemical | Vapor Pressure (20 °C, kPa) | Heat of Combustion (25 °C, kJ/mol) | Nominal Concentration (%) | Calorific Value (kJ/m3) | ||||
|---|---|---|---|---|---|---|---|---|
| 10% Vapor | 20% Vapor | 30% Vapor | 10% Vapor | 20% Vapor | 30% Vapor | |||
| Hydrogen | -- | 286 | -- | -- | -- | -- | -- | -- |
| Methanol | 13.0 | 725 | 1.3 | 2.6 | 3.9 | 420.8 | 841.5 | 1262.3 |
| Benzene | 10 | 3263 | 0.99 | 1.98 | 2.97 | −1435.72 | −2884.25 | −4326.27 |
| Toluene | 9 | 3910 | 0.29 | 0.58 | 0.87 | −504.39 | −1012.48 | −1517.19 |
| a E. benzene | 1.33 | 4568 | 0.13 | 0.26 | 0.39 | −269.12 | −530.21 | −795.32 |
| Xylene | 1.33 | 4568 | 0.13 | 0.26 | 0.39 | −269.12 | −530.21 | −795.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munsif, S.; Ullah, L.; Cao, L.; Murthy, P.R.; Zhang, J.-C.; Li, W.-Z. Hydrogen-Ignited-Methanol Catalytic Co-Combustion of Aromatic Volatile Organic Compounds over PdPt/Al2O3 Bimetallic Catalyst. Catalysts 2024, 14, 637. https://doi.org/10.3390/catal14090637
Munsif S, Ullah L, Cao L, Murthy PR, Zhang J-C, Li W-Z. Hydrogen-Ignited-Methanol Catalytic Co-Combustion of Aromatic Volatile Organic Compounds over PdPt/Al2O3 Bimetallic Catalyst. Catalysts. 2024; 14(9):637. https://doi.org/10.3390/catal14090637
Chicago/Turabian StyleMunsif, Sehrish, Lutf Ullah, Long Cao, Palle Ramana Murthy, Jing-Cai Zhang, and Wei-Zhen Li. 2024. "Hydrogen-Ignited-Methanol Catalytic Co-Combustion of Aromatic Volatile Organic Compounds over PdPt/Al2O3 Bimetallic Catalyst" Catalysts 14, no. 9: 637. https://doi.org/10.3390/catal14090637





