Shining a Light on Sewage Treatment: Building a High-Activity and Long-Lasting Photocatalytic Reactor with the Elegance of a “Kongming Lantern”
Abstract
:1. Introduction
2. Results
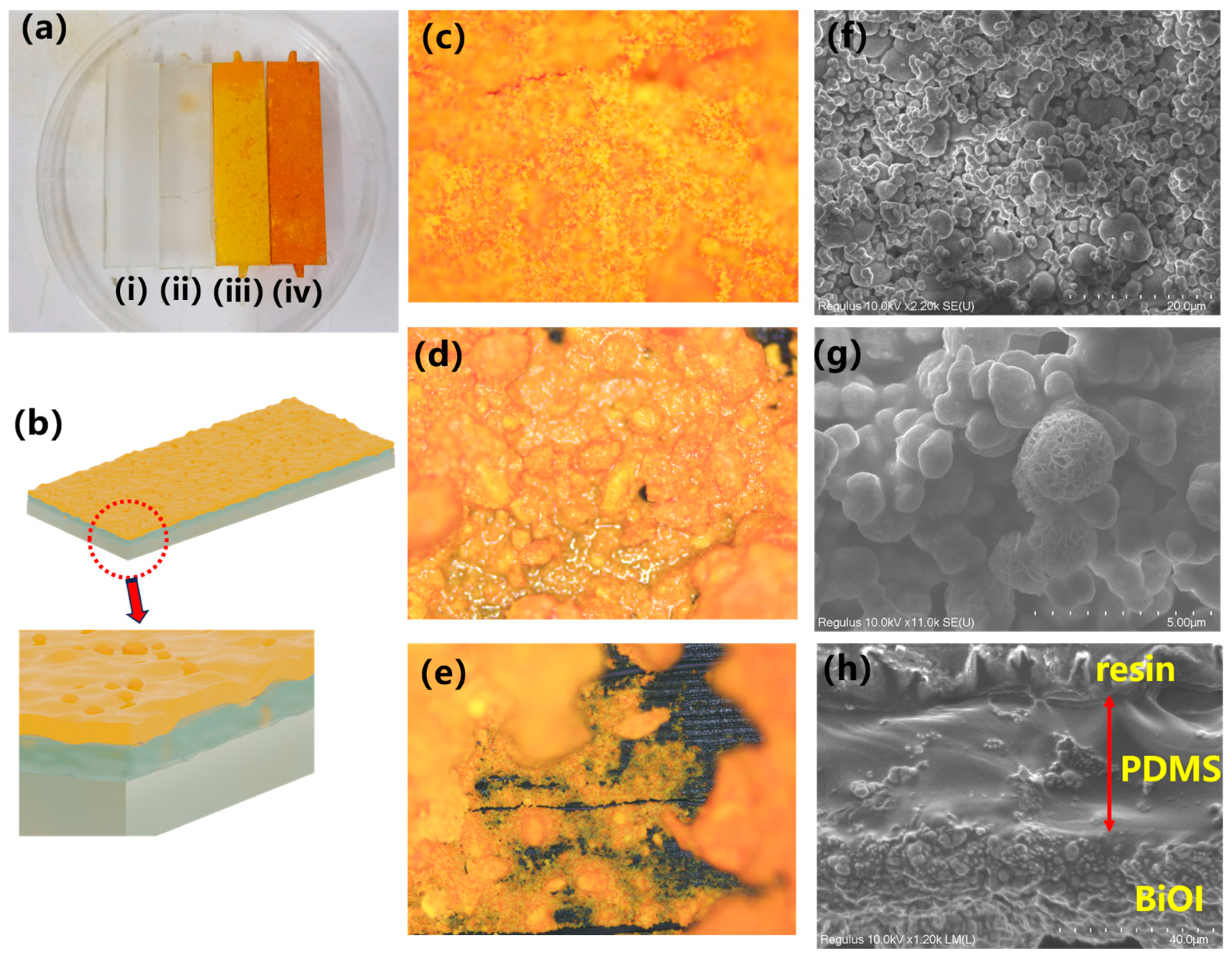
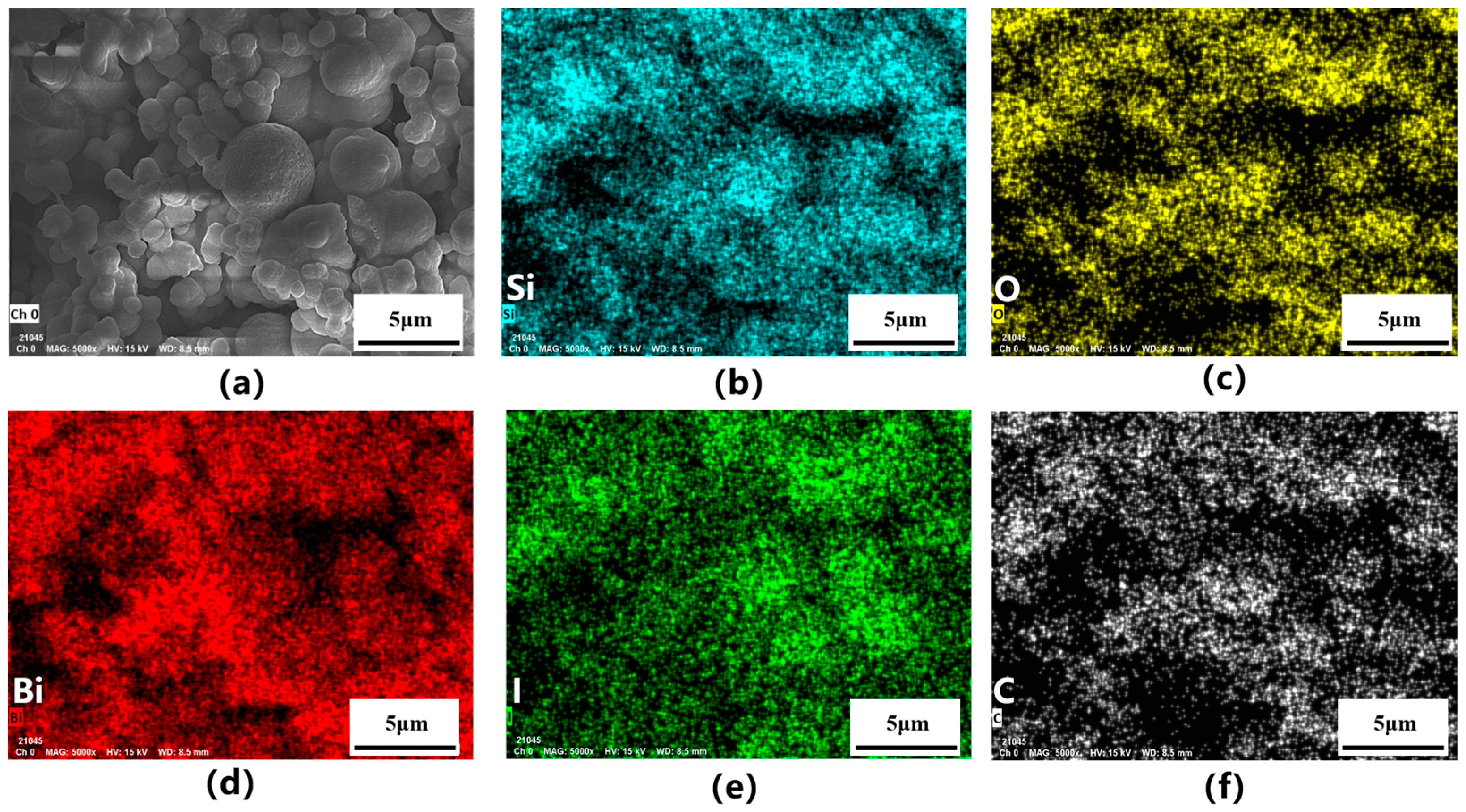
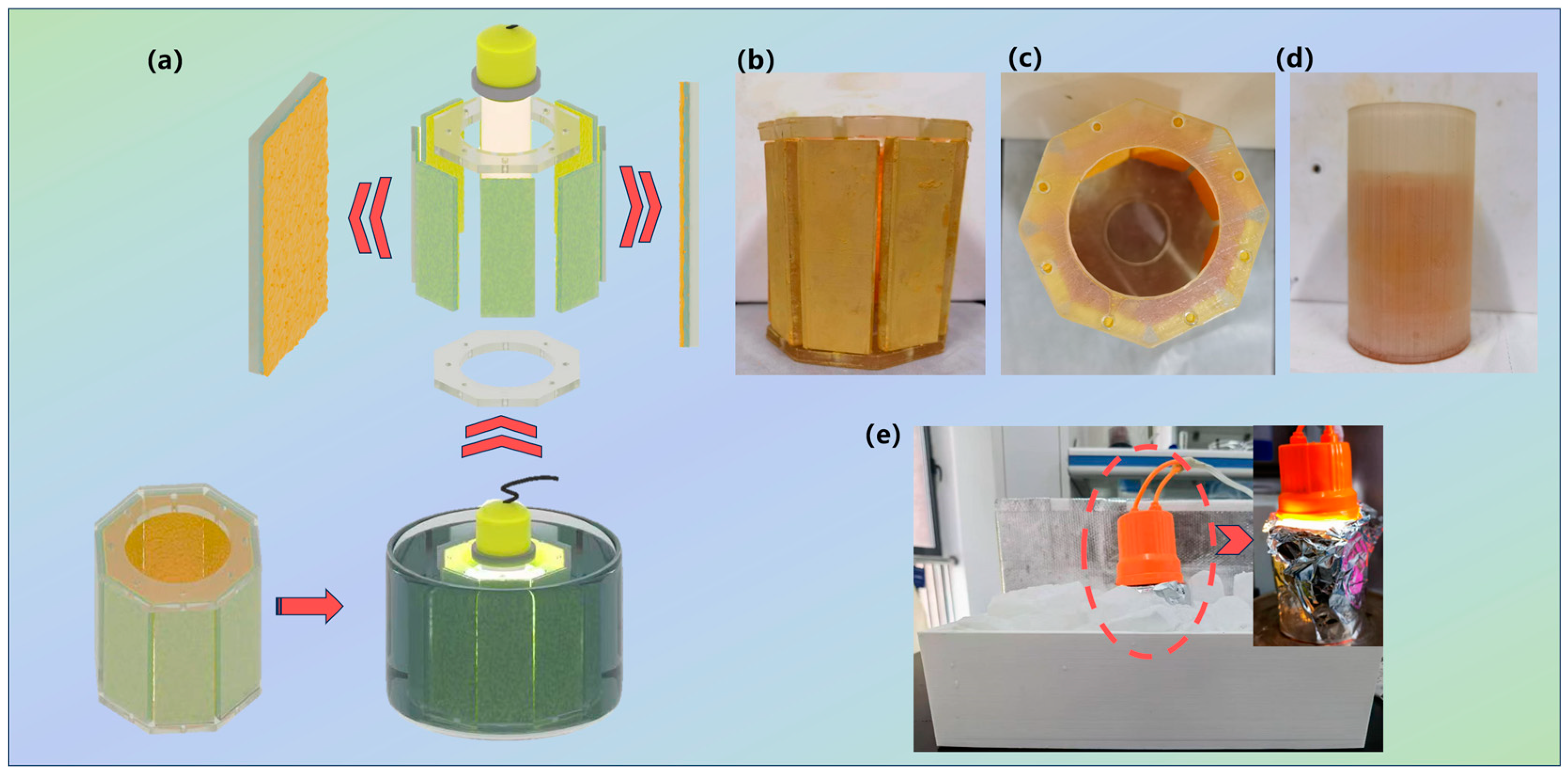
2.1. The Characterization of the Reactor
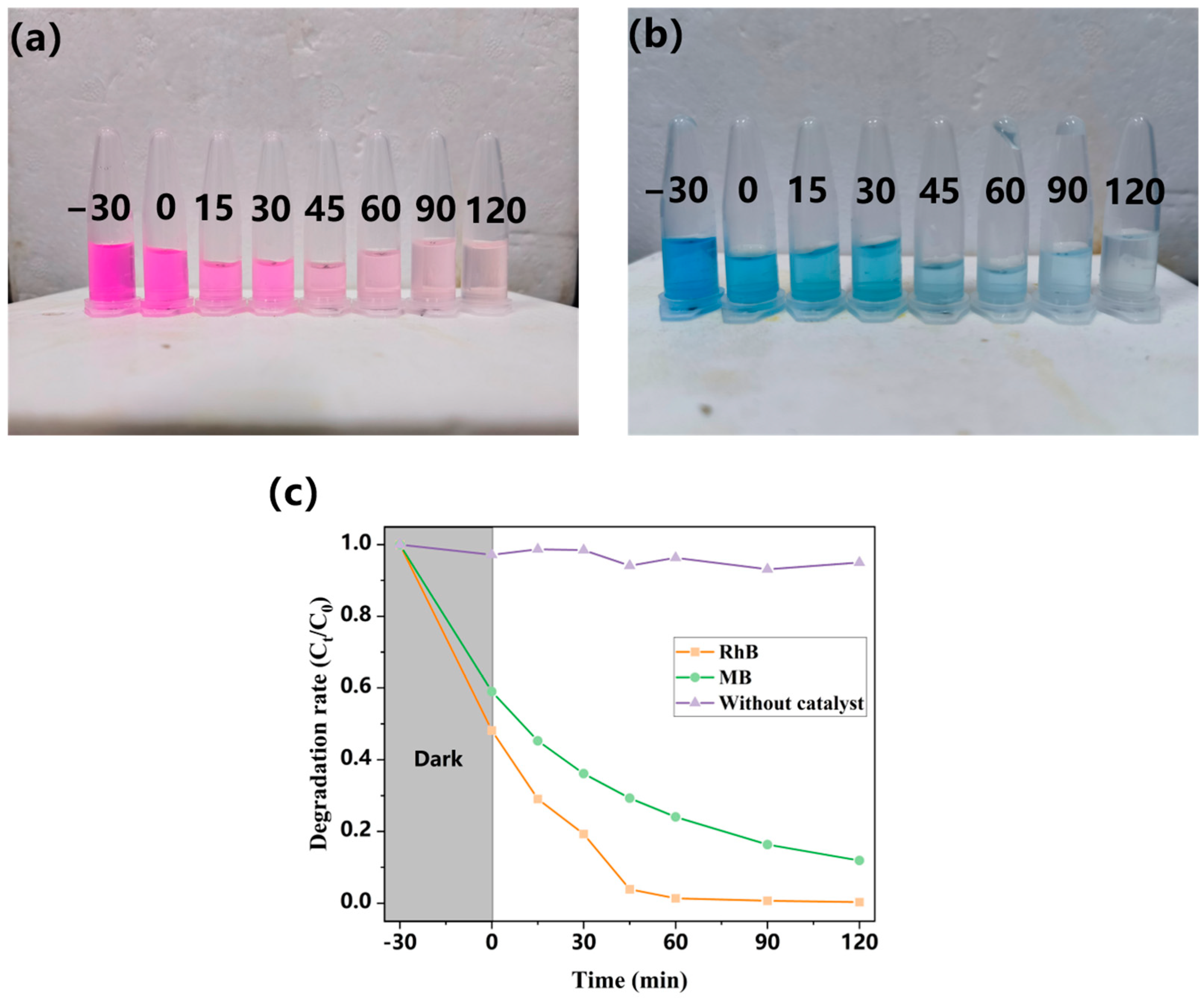
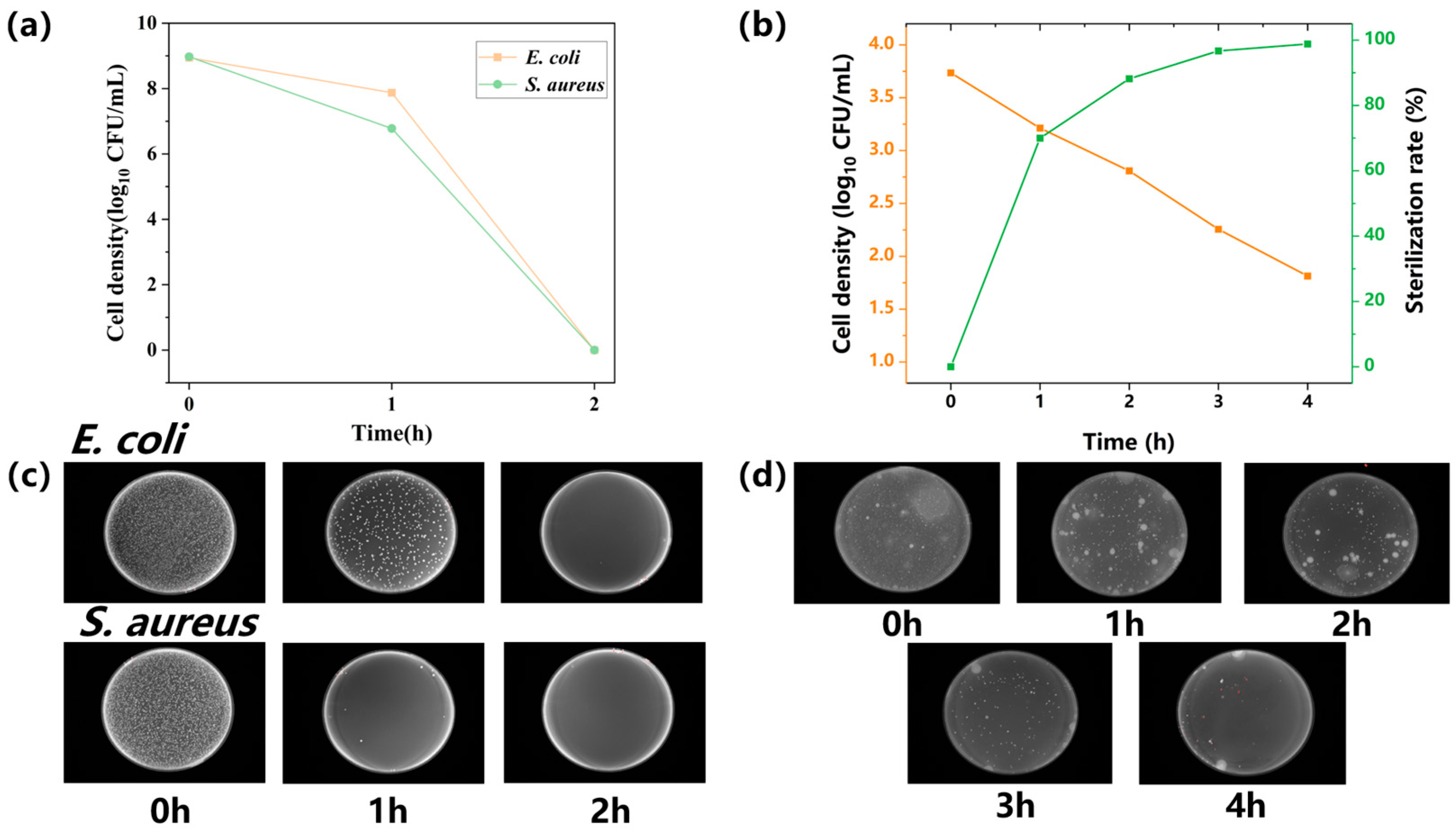
2.2. An Evaluation of the Photocatalytic Performance of the Water Purification Reactor Device
2.3. Load Stability of Photocatalytic Water Purification Reactor
2.3.1. The Recycling Stability of the Reactor
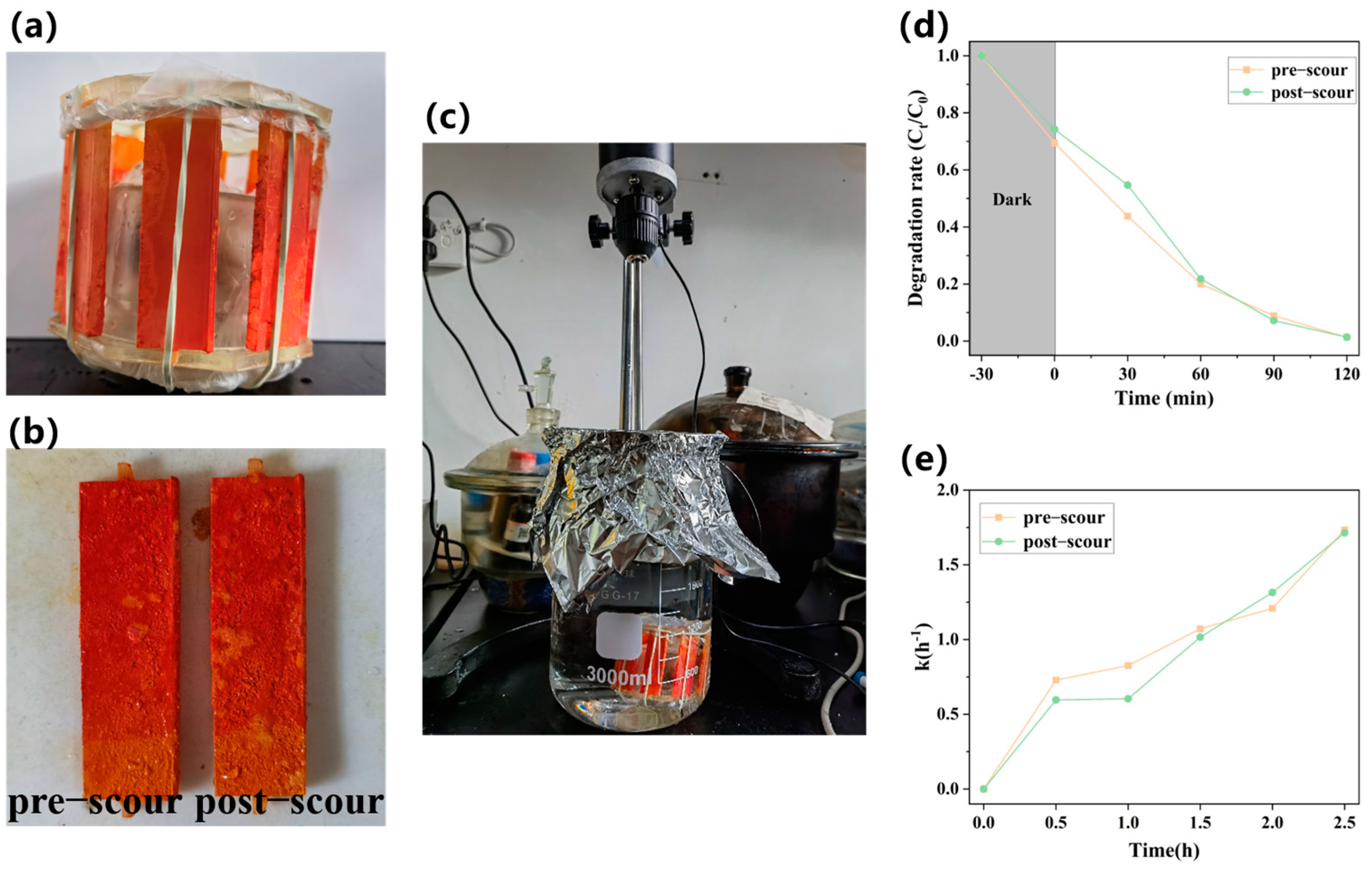
2.3.2. Simulated Scour Stability of Reactor
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of BiOI
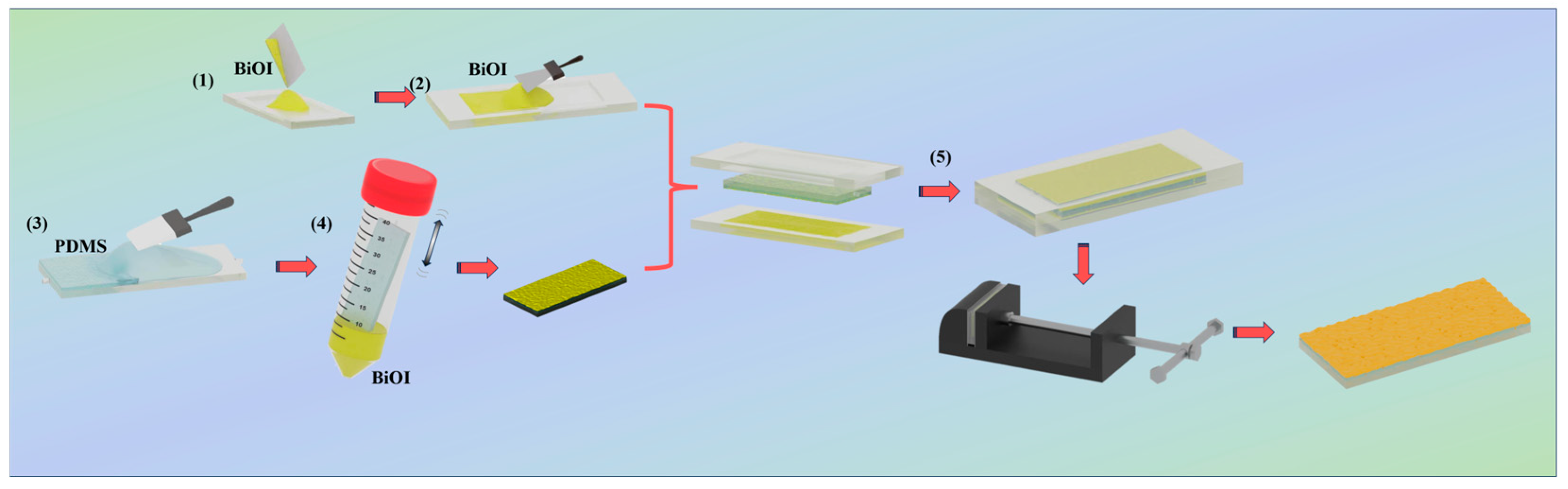
3.3. The Preparation and Characterization of the Photocatalytic Active Layer
3.4. The Design of the Photocatalytic Reactor Based on the “Kongming Lantern” Standard
3.5. Evaluation of Photocatalytic Activity
3.5.1. Photocatalytic Degradation of Different Colored Organic Dyes
3.5.2. Bactericidal Activity against Conventional Gram-Negative and Gram-Positive Bacteria
3.5.3. Photocatalytic Microbial Killing Activity in Environmental Water Samples
3.6. Stability Test of Photocatalytic Active Layer Loading
3.6.1. Cyclic Stability Test of Reactor’s Photocatalytic Activity
3.6.2. Stability Test of Material Loading in Simulated Oceanic Current Erosion Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Kong, L.; Ouyang, Z. Characteristics and Driving Mechanism of Regional Ecosystem Assets Change in the Process of Rapid Urbanization—A Case Study of the Beijing–Tianjin–Hebei Urban Agglomeration. Remote Sens. 2022, 14, 5747. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, Q.; Li, C.; Huang, Y. Characteristics and Spatial–Temporal Differences of Urban “Production, Living and Ecological” Environmental Quality in China. Int. J. Environ. Res. Public Health 2022, 19, 15320. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yu, B.; Zheng, R.; Wang, X.; Liu, J.; Wu, X.; Qin, L. Applications of Advanced Oxidation Technologies for Sludge Treatment. IOP Conf. Series Earth Environ. Sci. 2019, 252, 042044. [Google Scholar] [CrossRef]
- Kadam, R.; Khanthong, K.; Park, B.; Jun, H.; Park, J. Realizable wastewater treatment process for carbon neutrality and energy sustainability: A review. J. Environ. Manag. 2023, 328, 116927. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Z.; Yang, Y.; Liu, H.; Fang, S.; Liu, S. Research Status and Development Trend of Wastewater Treatment Technology and Its Low Carbonization. Appl. Sci. 2023, 13, 1400. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Prakash, M.; Kavitha, H.P.; Abinaya, S.; Vennila, J.P.; Lohita, D. Green synthesis of bismuth based nanoparticles and its applications—A review. Sustain. Chem. Pharm. 2022, 25, 100547. [Google Scholar] [CrossRef]
- Ke, S.; Min, X.; Liu, Y.; Mi, R.; Wu, X.; Huang, Z.; Fang, M. Tungsten-Based Nanocatalysts: Research Progress and Future Prospects. Molecules 2022, 27, 4751. [Google Scholar] [CrossRef]
- Wang, L.; Tong, Y.; Feng, J.; Hou, J.; Li, J.; Hou, X.; Liang, J. G-C3N4-based films: A rising star for photoelectrochemical water splitting. Sustain. Mater. Technol. 2019, 19, e00089. [Google Scholar] [CrossRef]
- Du, Y.; Ma, R.; Wang, L.; Qian, J.; Wang, Q. 2D/1D BiOI/g-C3N4 nanotubes heterostructure for photoelectrochemical overall water splitting. Sci. Total. Environ. 2022, 838, 156166. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, T.-T.; Ren, H.-T.; Zhang, X.; Shen, B.; Lin, J.-H.; Lou, C.-W. Construction of BiOI/TiO2 flexible and hierarchical S-scheme heterojunction nanofibers membranes for visible-light-driven photocatalytic pollutants degradation. Sci. Total. Environ. 2022, 806, 150698. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, A.; Zhang, Q.; Mei, Y.; Wang, C.; Wang, Y.; Xiang, J.; Su, S.; Zhang, X.; Tan, Z. Facile synthesis of ternary AgBr/BiOI/Bi2O2CO3 heterostructures via BiOI self-sacrifice for efficient photocatalytic removal of gaseous mercury. Sep. Purif. Technol. 2022, 299, 121722. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, P.; Liu, Y.; Wang, N.; Li, W.; Cheng, F.; Yang, B.; Liang, J.; Zhang, Y.; Lai, B. Strongly enhanced Fenton-like oxidation (Fe/peroxydisulfate) by BiOI under visible light irradiation: A novel and green strategy for Fe(III) reduction. J. Hazard. Mater. 2022, 428, 128202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, D.; Jiao, S.; Xu, Z.; Liu, Y.; Zhao, C.; Pan, J.; Liu, D.; Liu, G.; Jiang, B.; et al. TiO2-X mesoporous nanospheres/BiOI nanosheets S-scheme heterostructure for high efficiency, stable and unbiased photocatalytic hydrogen production. Chem. Eng. J. 2022, 446, 137138. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Y.; Zhang, D. Facile in Situ Growth of High Strong BiOI Network Films on Metal Wire Meshes with Photocatalytic Activity. ACS Sustain. Chem. Eng. 2017, 5, 2454–2462. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, D. A novel strategy of hydrothermal in-situ grown bismuth based film on epoxy resin as recyclable photocatalyst for photodegrading antibiotics and sterilizing microorganism. Sep. Purif. Technol. 2022, 290, 120842. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, D.; Wang, J.; Yang, Z. In situ growth of photocatalytic Ag-decorated β-Bi2O3/Bi2O2.7 heterostructure film on PVC polymer matrices with self-cleaning and antibacterial properties. Chem. Eng. J. 2022, 429, 131058. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Y.; Yang, Z.; Zhang, D. A novel ion-exchange strategy for the fabrication of high strong BiOI/BiOBr heterostructure film coated metal wire mesh with tunable visible-light-driven photocatalytic reactivity. J. Hazard. Mater. 2018, 351, 11–19. [Google Scholar] [CrossRef]
- Liu, G.; Xia, H.; Zhang, W.; Song, L.; Chen, Q.; Niu, Y. Improvement mechanism of NO photocatalytic degradation performance of self-cleaning synergistic photocatalytic coating under high humidity. J. Hazard. Mater. 2021, 418, 126337. [Google Scholar] [CrossRef]
- Valenzuela, L.; Iglesias, A.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial surfaces with self-cleaning properties functionalized by photocatalytic ZnO electrosprayed coatings. J. Hazard. Mater. 2019, 369, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, J.; Feng, X.; Li, H. Thermal-Sprayed Photocatalytic Coatings for Biocidal Applications: A Review. J. Therm. Spray Technol. 2020, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xia, H.; Niu, Y.; Zhao, X.; Zhang, G.; Song, L.; Chen, H. Fabrication of self-cleaning photocatalytic durable building coating based on WO3-TNs/PDMS and NO degradation performance. Chem. Eng. J. 2021, 409, 128187. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Abdelwahab, N.A.; Mohamed, W.A.; Taha, N.A.; Abdel-Mottaleb, M. Solar photocatalytic treatment of industrial wastewater utilizing recycled polymeric disposals as TiO2 supports. J. Clean. Prod. 2020, 249, 119430. [Google Scholar] [CrossRef]
- Kanth, N.; Xu, W.; Prasad, U.; Ravichandran, D.; Kannan, A.M.; Song, K. PMMA-TiO2 Fibers for the Photocatalytic Degradation of Water Pollutants. Nanomaterials 2020, 10, 1279. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Choe, J.W.; Kim, E.-J.; Chung, Y.C.; Cho, K.; Lee, C.; Hong, S.W. Highly reusable TiO2 nanoparticle photocatalyst by direct immobilization on steel mesh via PVDF coating, electrospraying, and thermal fixation. Chem. Eng. J. 2016, 306, 344–351. [Google Scholar] [CrossRef]
- Wang, J.-C.; Li, Y.; Li, H.; Cui, Z.-H.; Hou, Y.; Shi, W.; Jiang, K.; Qu, L.; Zhang, Y.-P. A novel synthesis of oleophylic Fe2O3/polystyrene fibers by γ-Ray irradiation for the enhanced photocatalysis of 4-chlorophenol and 4-nitrophenol degradation. J. Hazard. Mater. 2019, 379, 120806. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, T.; Que, W. Fabrication of Nanoparticle/Polymer Composite Photocatalytic Membrane for Domestic Sewage In Situ Treatment. Materials 2022, 15, 2466. [Google Scholar] [CrossRef]
- Yu, H.; Jiao, Z.; Hu, H.; Lu, G.; Ye, J.; Bi, Y. Fabrication of Ag3PO4–PAN composite nanofibers for photocatalytic applications. CrystEngComm 2013, 15, 4802–4805. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Zhu, X.; Wang, H.; Liao, Q.; Zhang, M.-X. Highly-durable optofluidic microreactor for photocatalytic water splitting. Energy 2015, 83, 797–804. [Google Scholar] [CrossRef]
- Gomes, J.; Maniezo, B.; Alves, P.; Ferreira, P.; Martins, R.C. Immobilization of TiO2 onto a polymeric support for photocatalytic oxidation of a paraben’s mixture. J. Water Process. Eng. 2022, 46, 102458. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Yeom, J. A Floatable Piezo-Photocatalytic Platform Based on Semi-Embedded ZnO Nanowire Array for High-Performance Water Decontamination. Nano-Micro Lett. 2019, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Aziz, F.; Jamaludin, N.; Mutalib, M.A.; Ismail, A.; Salleh, W.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Li, M.; Hu, Z.; Liu, D.; Liang, Y.; Liu, S.; Wang, B.; Niu, C.; Xu, D.; Li, J.; Han, B. Efficient antibacterial and microbial corrosion resistant photocatalytic coating: Enhancing performance with S-type heterojunction and Cu synergy. Chem. Eng. J. 2024, 495, 153519. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevée, J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef]
- Son, S.; Jung, P.-H.; Park, J.; Chae, D.; Huh, D.; Byun, M.; Ju, S.; Lee, H. Customizable 3D-printed architecture with ZnO-based hierarchical structures for enhanced photocatalytic performance. Nanoscale 2018, 10, 21696–21702. [Google Scholar] [CrossRef]
- Zheng, Q.; Aiello, A.; Choi, Y.S.; Tarr, K.; Shen, H.; Durkin, D.P.; Shuai, D. 3D printed photoreactor with immobilized graphitic carbon nitride: A sustainable platform for solar water purification. J. Hazard. Mater. 2020, 399, 123097. [Google Scholar] [CrossRef]
- Zhou, R.; Han, R.; Bingham, M.; O’rourke, C.; Mills, A. 3D printed, plastic photocatalytic flow reactors for water purification. Photochem. Photobiol. Sci. 2022, 21, 1585–1600. [Google Scholar] [CrossRef]
- Borrello, J.; Nasser, P.; Iatridis, J.C.; Costa, K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit. Manuf. 2018, 23, 374–380. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Sim, J.-H.; Lee, S.H.; Yang, J.-Y.; Lee, W.-C.; Mun, C.; Lee, S.; Park, S.-G.; Cho, Y.-R. Plasmonic hotspot engineering of Ag-coated polymer substrates with high reproducibility and photothermal stability. Sens. Actuators B Chem. 2022, 354, 131110. [Google Scholar] [CrossRef]
- Wen, Y.-T.; Dai, N.-T.; Hsu, S.-H. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Schreck, M.; Kleger, N.; Matter, F.; Kwon, J.; Tervoort, E.; Masania, K.; Studart, A.R.; Niederberger, M. 3D Printed Scaffolds for Monolithic Aerogel Photocatalysts with Complex Geometries. Small 2021, 17, 2104089. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, X.; Wang, Y.; Zhang, D.; Chen, C.; Yang, Z. In-situ growth pH-adjusted iodine defects engineering BiOI film on 3D-printed polymer substrate for efficient organic pollutant and microorganism purification. Sep. Purif. Technol. 2023, 318, 123974. [Google Scholar] [CrossRef]
- Latif, A.; Memon, A.M.; Gadhi, T.A.; Bhurt, I.A.; Channa, N.; Mahar, R.B.; Ali, I.; Chiadò, A.; Bonelli, B. Bi2O3 immobilized 3D structured clay filters for solar photocatalytic treatment of wastewater from batch to scaleup reactors. Mater. Chem. Phys. 2022, 276, 125297. [Google Scholar] [CrossRef]
- Li, N.; Tong, K.; Yang, L.; Du, X. Review of 3D printing in photocatalytic substrates and catalysts. Mater. Today Energy 2022, 29, 101100. [Google Scholar] [CrossRef]
- Qiu, H.; Zhang, R.; Yu, Y.; Shen, R.; Gao, H. BiOI-on-SiO2 microspheres: A floating photocatalyst for degradation of diesel oil and dye wastewater. Sci. Total. Environ. 2020, 706, 136043. [Google Scholar] [CrossRef]
- Kiss, J.; Kukovecz, Z.; Kónya, Z. Beyond Nanoparticles: The Role of Sub-nanosized Metal Species in Heterogeneous Catalysis. Catal. Lett. 2019, 149, 1441–1454. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Li, J.; Gao, G.; Zhi, J. Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B Environ. 2022, 308, 121216. [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. State Administration for Market Regulation, National Standardization Administration: Beijing, China, 2022.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, Y.; Deng, Z.; Wang, J.; Wei, X.; Wang, P.; Zhang, D. Shining a Light on Sewage Treatment: Building a High-Activity and Long-Lasting Photocatalytic Reactor with the Elegance of a “Kongming Lantern”. Catalysts 2024, 14, 645. https://doi.org/10.3390/catal14090645
Xu X, Wang Y, Deng Z, Wang J, Wei X, Wang P, Zhang D. Shining a Light on Sewage Treatment: Building a High-Activity and Long-Lasting Photocatalytic Reactor with the Elegance of a “Kongming Lantern”. Catalysts. 2024; 14(9):645. https://doi.org/10.3390/catal14090645
Chicago/Turabian StyleXu, Xiaohan, Yi Wang, Zhuo Deng, Jin Wang, Xile Wei, Peng Wang, and Dun Zhang. 2024. "Shining a Light on Sewage Treatment: Building a High-Activity and Long-Lasting Photocatalytic Reactor with the Elegance of a “Kongming Lantern”" Catalysts 14, no. 9: 645. https://doi.org/10.3390/catal14090645









