Desulfurization Ability of Blast Furnace Slag Containing High Al2O3 at 1773 K
Abstract
:1. Introduction
2. Experimental
2.1. Desulfurization Experiment
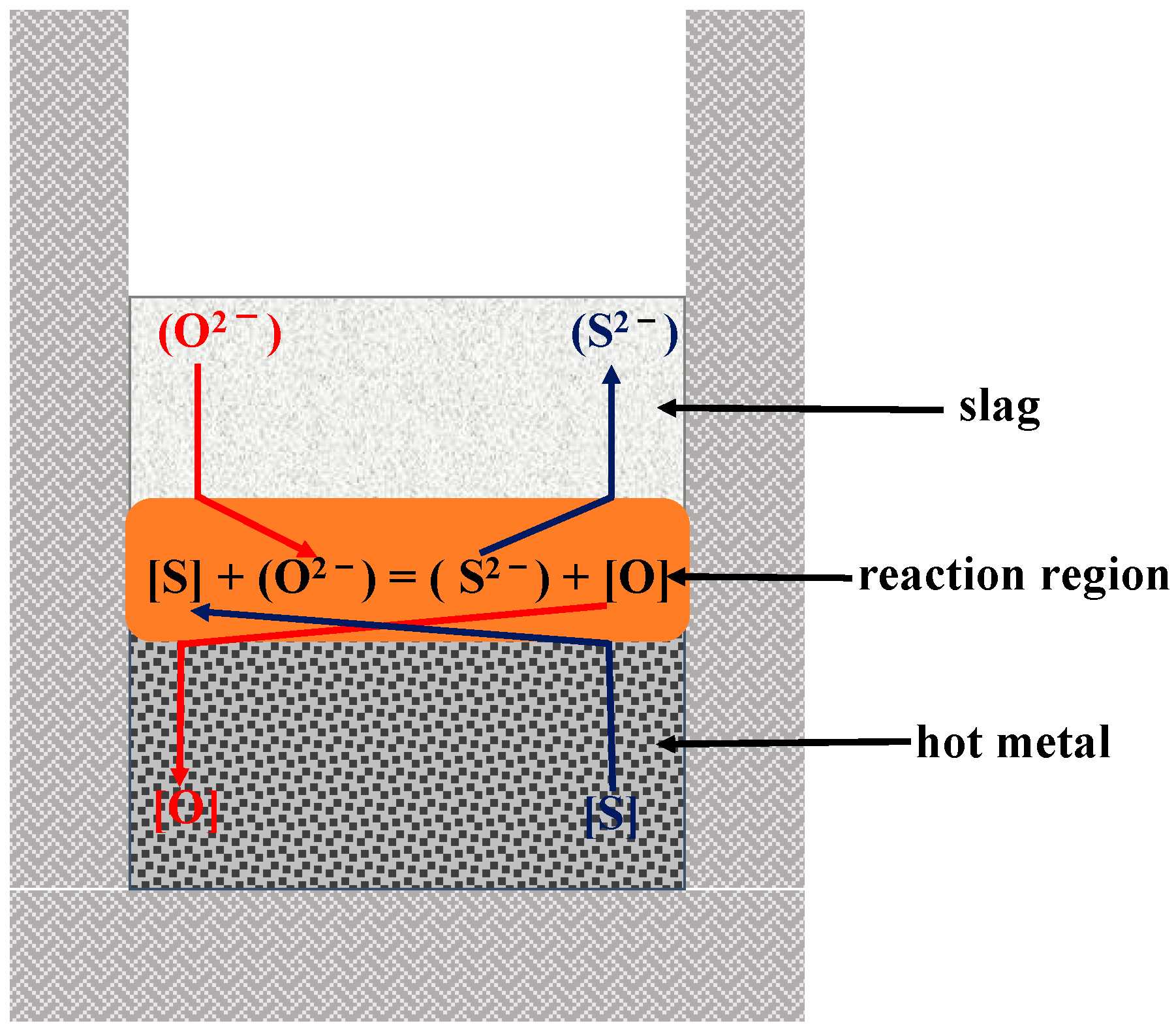
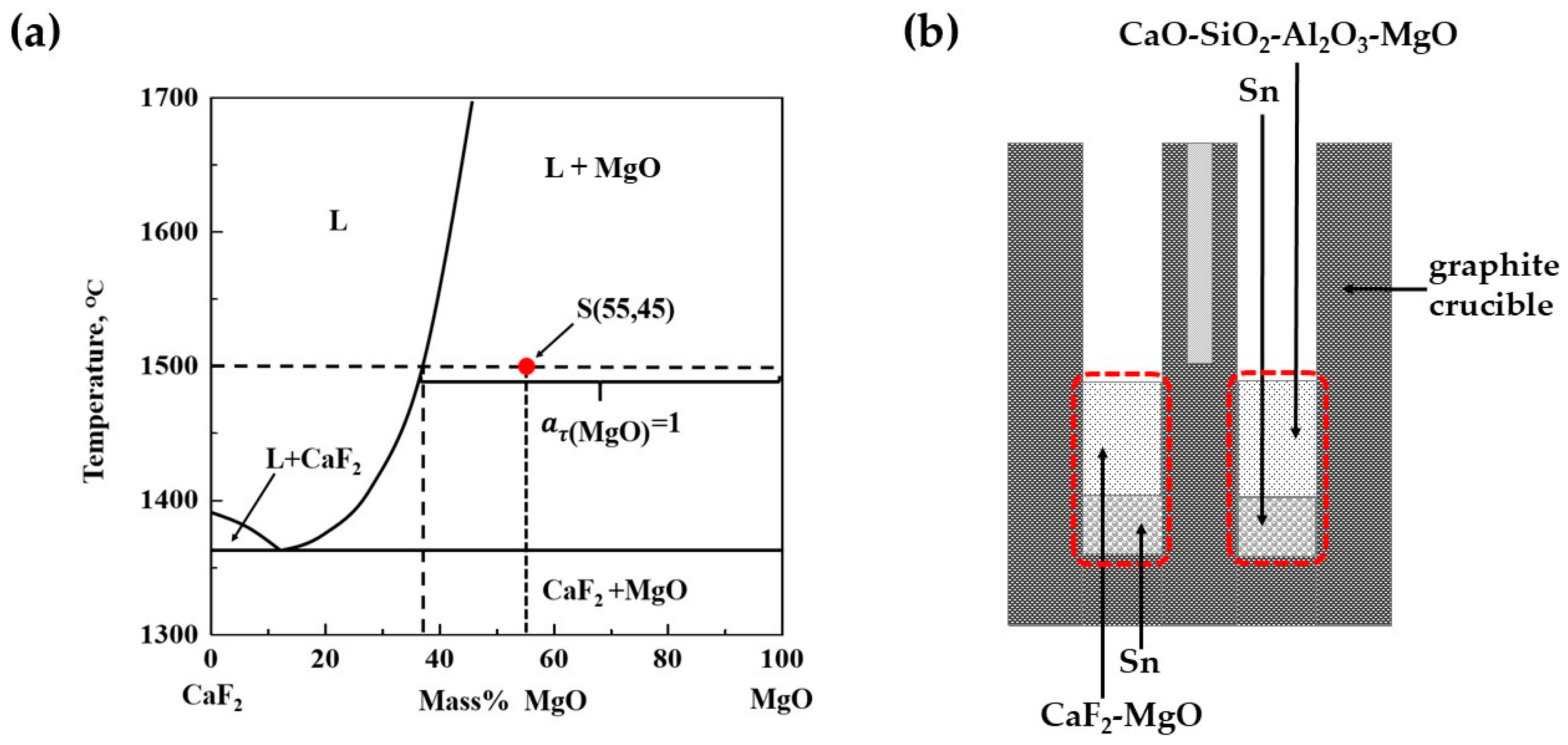
2.1.1. Experimental Principle
ΔGθ = 105784.6 − 28.723T/(J·mol−1)
ΔGθ = 203604.6 − 35.023T/(J·mol−1)
ΔGθ = 124455 − 50.26T/(J·mol−1)
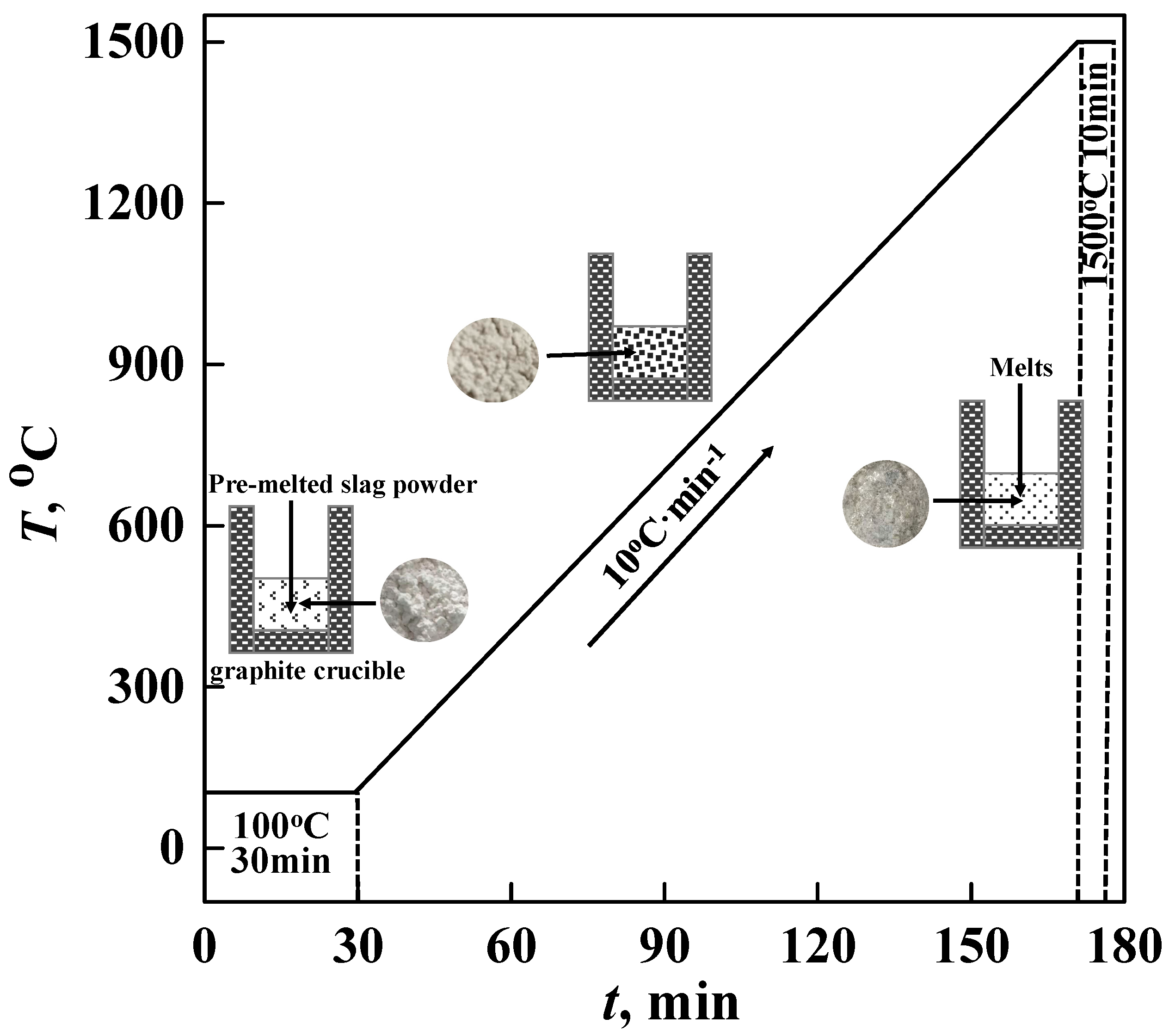
2.1.2. Experimental Procedure
2.2. Activity Experiment
2.2.1. Experimental Procedure
2.2.2. Experimental Principle
3. Results and Discussion
3.1. Effect of B on Desulfurization Ability of Slag
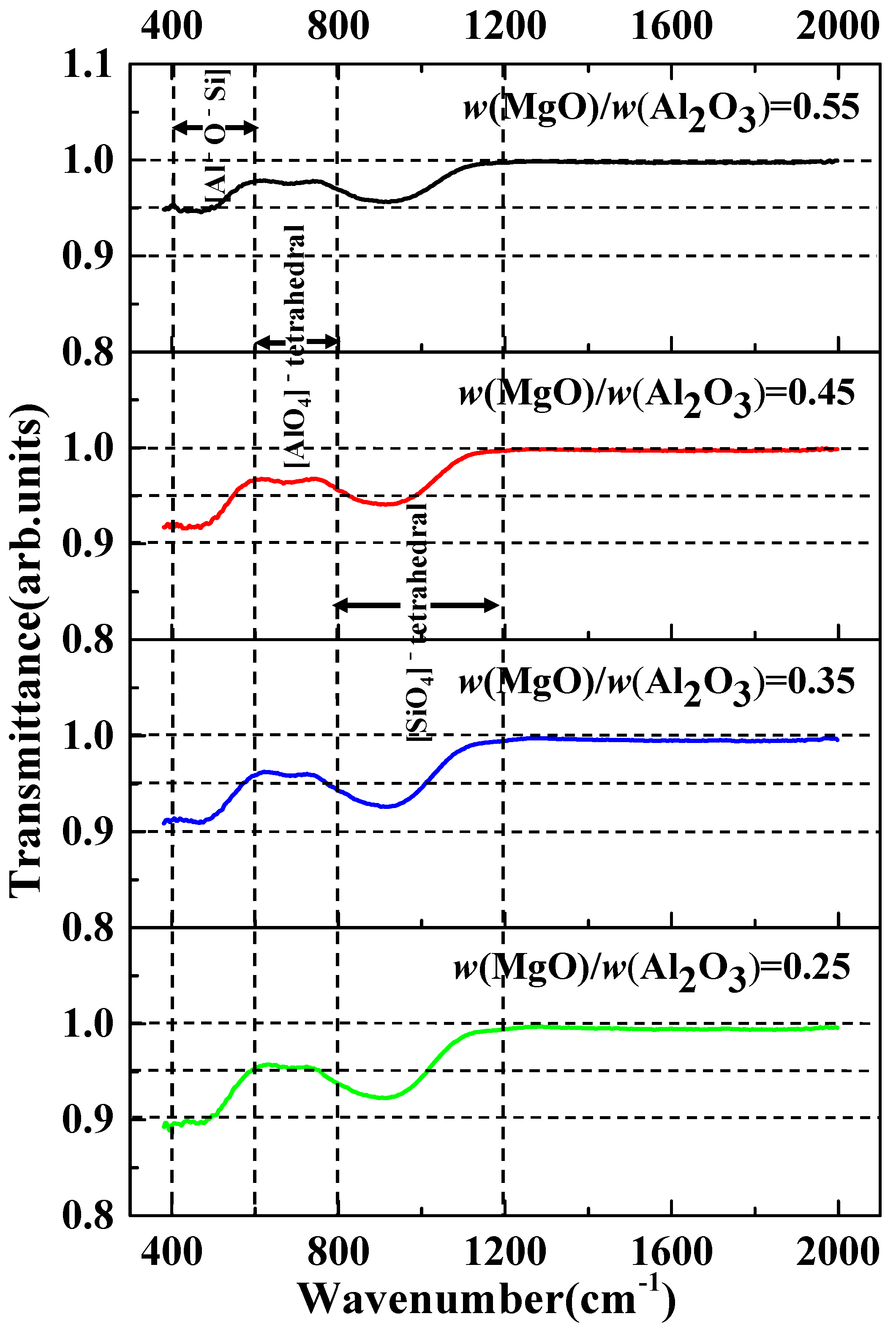
3.2. Effect of w(MgO)/w(Al2O3) on Desulfurization Ability of Slag
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, H.S.; Shen, F.M.; Jiang, X. Fundamental mechanism of effects of MgO on sinter strength. J. Iron Steel Res. Int. 2019, 26, 1171–1177. [Google Scholar] [CrossRef]
- Yan, P.; Nie, P.; Huang, S. Sulphide capacity and mineralogy of BaO and B2O3 modified CaO-Al2O3 top slag. ISIJ Int. 2014, 54, 1570–1577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lv, X.W.; Yan, Z.M. Desulphurisation ability of blast furnace slag containing high Al2O3 and 5mass%TiO2 at 1773K. Ironmak. Steelmak. 2016, 43, 378–384. [Google Scholar] [CrossRef]
- Talapaneni, T.; Yedla, N.; Sarkar, S. Study on desulfurization capacity of high alumina blast furnace slag at 1773 K using slag-metal equilibrium technique. Metall. Res. Technol. 2018, 115, 502. [Google Scholar] [CrossRef]
- Hino, M.; Kitagawa, S. Sulphide capacities of CaO-Al2O3-MgO and CaO-Al2O3-SiO2 Slags. ISIJ Int. 1993, 33, 36–42. [Google Scholar] [CrossRef]
- Ting, T.; Katayama, H.G. Sulphur distribution between liquid iron and CaO-MgO-Al2O3-SiO2 Slags saturated with MgO. Tetsu Hagane. 2009, 72, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lu, Q.; Zhang, S. Study on sulphide capacity of CaO-SiO2-Al2O3-MgO-FetO slags. J. Univ. Sci. Technol. B. 2006, 13, 213–217. [Google Scholar] [CrossRef]
- Xiang, D.W.; Shen, F.M.; Jiang, X. Effect of unburned pulverized coal on the melting characteristics and fluidity of blast furnace slag. Crystals 2021, 11, 579. [Google Scholar] [CrossRef]
- Shufeng, Y.E.; Xie, Y.; Guo, Z. Desulfurization ability of metallurgical dusts from blast furnace ironmaking process. J. Chem. Eng. Jpn. 2001, 34, 1103–1107. [Google Scholar] [CrossRef]
- Ling, J.; Pang, Z.; Jiang, Y. Blast furnace ironmaking process with super-high TiO2 in the slag: Sulfide capacity. Metall. Mater. Trans. B. 2021, 52, 2786–2795. [Google Scholar] [CrossRef]
- Shankar, A. Sulphur partition between hot metal and high alumina blast furnace slag. Ironmak. Steelmak. 2006, 33, 413–418. [Google Scholar] [CrossRef]
- Ma, X.D.; Chen, M.; Xu, H.F. Sulphide capacity of CaO-SiO2-Al2O3-MgO system relevant to low MgO blast furnace slags. ISIJ Int. 2016, 56, 2126–2131. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.D.; Chen, M.; Zhu, J. Properties of low-MgO ironmaking blast furnace slags. ISIJ Int. 2018, 58, 1402–1405. [Google Scholar] [CrossRef]
- Condo, A.; Allertz, C.; Du, S.C. Experimental determination of sulphide capacities of blast furnace slags with higher MgO contents. Ironmak. Steelmak. 2017, 46, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Wang, X.; Wang, W. Study on desulfurization ability of CaO-Al2O3-SiO2 and CaO-CaF2 slags at 1600 °C. Steel Res. Int. 2015, 86, 1455–1460. [Google Scholar]
- Wang, Y.; Wang, X.; Zhou, H. Sulphide capacity calculation and desulphurization analysis of ultralow oxygen spring steel. J. Univ. Sci. Technol. B. 2008, 30, 986–992. [Google Scholar]
- Nzotta, M.M.; Nilsson, R. Sulphide capacities in MgO-SiO2 and CaO-MgO-SiO2 slags. Ironmak. Steelmak. 1997, 24, 300–305. [Google Scholar]
- Wang, L.J.; Wang, Y.X.; Wang, Q. Raman structure investigations of CaO-MgO-Al2O3-SiO2-CrOx and its correlation with sulfide capacity. Metall. Mater. Trans. B 2016, 47, 10–15. [Google Scholar] [CrossRef]
- Lee, S.; Dong, J.M. Viscous behavior of FeO-bearing slag melts considering structure of slag. J. Iron Steel Res. Int. 2018, 89, 1800055. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, Y.; Lee, S. Cationic effect of charge compensation on the sulfide capacity of aluminosilicate slags. J. Am. Ceram. Soc. 2018, 101, 2856–2867. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, T.J.; Dong, J.M. Structure–property relationship amphoteric oxide systems via phase stability and ionic structural analysis. J. Am. Ceram. Soc. 2021, 104, 140–156. [Google Scholar] [CrossRef]
- Yang, X.M.; Jiao, J.S.; Ding, R.C. A thermodynamic model for calculating sulphur distribution ratio between CaO-SiO2-Al2O3-MgO ironmaking slags and carbon saturated hot metal based on the ion and molecule coexistence theory. ISIJ Int. 2009, 49, 1828–1837. [Google Scholar] [CrossRef] [Green Version]
- Yuang, X.; Zhang, J.L.; Mao, R. Effect of w(MgO)/w(Al2O3) ratio on desulfurization capacity of BF slag. J. Northeast. Univ. 2015, 36, 1609–1613. [Google Scholar]
- Allibert, M.; Gaye, H. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995; pp. 158, 188. [Google Scholar]
- Shen, F.M.; Hu, X.G.; Zheng, H.Y. Proper MgO/Al2O3 ratio in blast-furnace slag: Analysis of proper MgO/Al2O3 ratio based on observed data. Metals 2020, 10, 784. [Google Scholar] [CrossRef]
- Bian, T.; Gao, Y.H. Influence of B2O3 and basicity on viscosity and structure of medium titanium bearing blast furnace slag. J. Chem. 2016, 12, 1–8. [Google Scholar]
- Jiang, C.; Li, K.; Zhang, J. Effect of MgO/Al2O3 ratio on the structure and properties of blast furnace slags: A molecular dynamics simulation. J. Non-Cryst. Solids. 2018, 502, 76–82. [Google Scholar] [CrossRef]
- Ren, Z.X.; Jian, L.Z.; Wang, X.H. Effect of BaO and Na2O on the viscosity and structure of blast furnace slag. Ironmak. Steelmak. 2018, 47, 168–172. [Google Scholar]

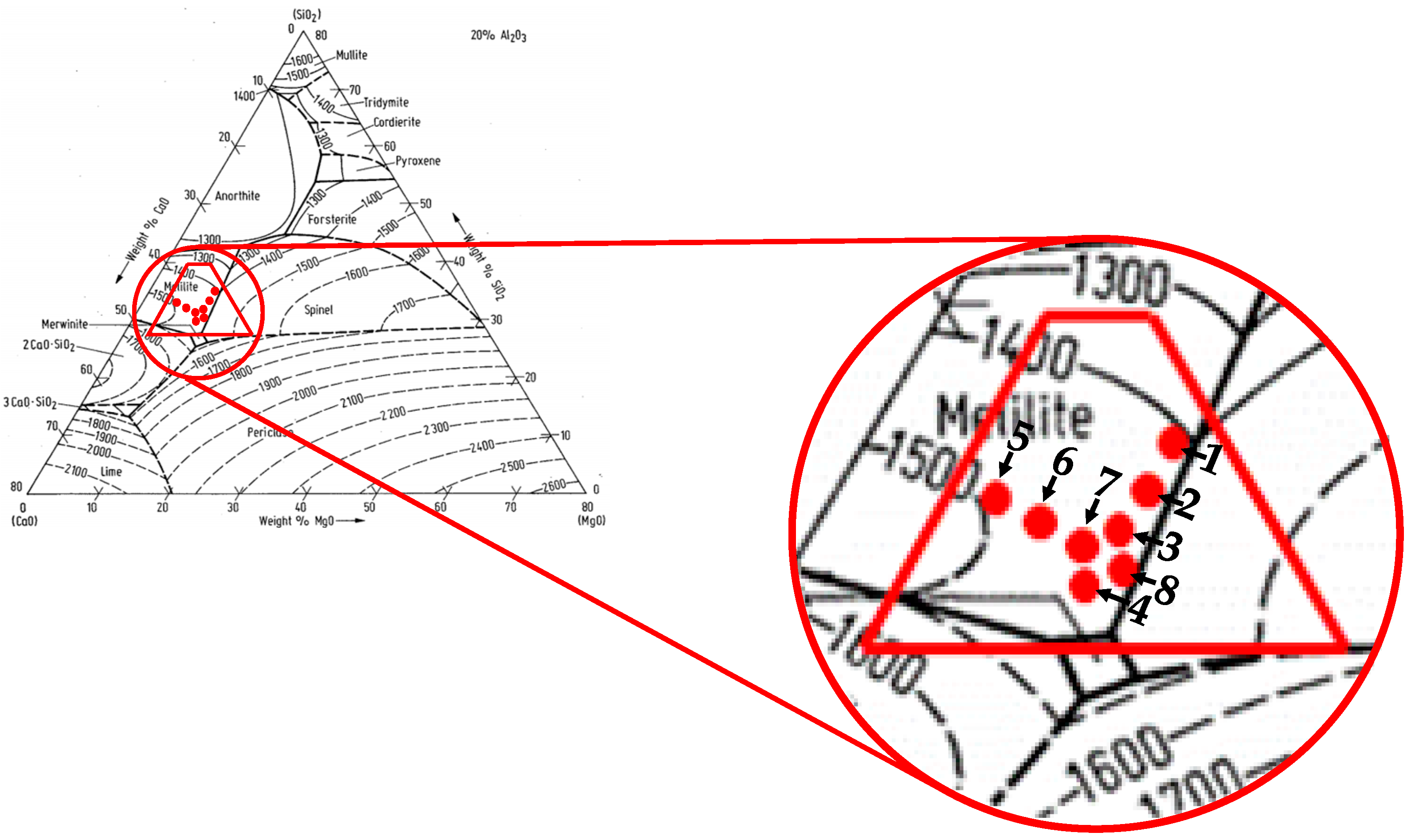


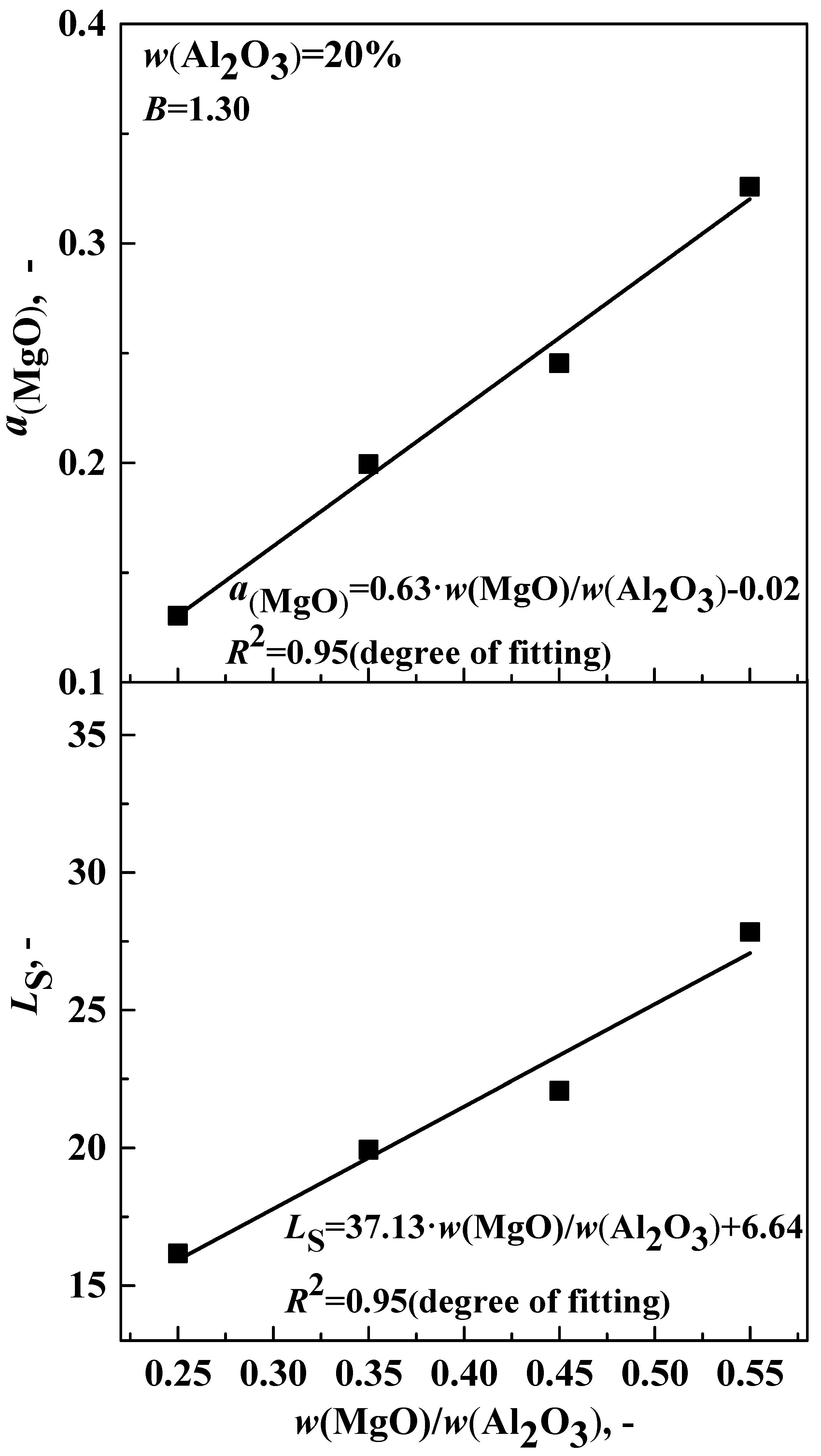
| No. | Slag Compositions | B, - | w(MgO)/w(Al2O3), - | |||
|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Al2O3 | |||
| 1 | 35.85 | 34.14 | 10.0 | 20 | 1.05 | 0.50 |
| 2 | 37.51 | 32.49 | 10.0 | 20 | 1.15 | 0.50 |
| 3 | 38.89 | 31.11 | 10.0 | 20 | 1.25 | 0.50 |
| 4 | 40.21 | 29.78 | 10.0 | 20 | 1.35 | 0.50 |
| 5 | 42.39 | 32.60 | 5.0 | 20 | 1.30 | 0.25 |
| 6 | 41.26 | 31.73 | 7.0 | 20 | 1.30 | 0.35 |
| 7 | 40.13 | 30.86 | 9.0 | 20 | 1.30 | 0.45 |
| 8 | 39.00 | 30.00 | 11.0 | 20 | 1.30 | 0.55 |
| C | Si | P | S | Mn | Fe |
|---|---|---|---|---|---|
| 4.63 | 0.260 | 0.104 | 0.105 | 0.229 | 94.672 |
| No. | w(Al2O3), - | B, - | w(MgO)/w(Al2O3), - | w(S), - | w[S], - | LS, - | - |
|---|---|---|---|---|---|---|---|
| 1 | 20 | 1.05 | 0.50 | 0.302 | 0.02 | 15.10 | 0.1917 |
| 2 | 20 | 1.15 | 0.50 | 0.332 | 0.016 | 20.75 | 0.2032 |
| 3 | 20 | 1.25 | 0.50 | 0.347 | 0.014 | 24.79 | 0.2531 |
| 4 | 20 | 1.35 | 0.50 | 0.348 | 0.011 | 31.64 | 0.2914 |
| 5 | 20 | 1.30 | 0.25 | 0.291 | 0.018 | 16.17 | 0.1304 |
| 6 | 20 | 1.30 | 0.35 | 0.319 | 0.016 | 19.94 | 0.1994 |
| 7 | 20 | 1.30 | 0.45 | 0.309 | 0.014 | 22.07 | 0.2454 |
| 8 | 20 | 1.30 | 0.55 | 0.334 | 0.012 | 27.83 | 0.3259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Shen, F.; Zheng, H.; Wang, S.; Jiang, X.; Gao, Q. Desulfurization Ability of Blast Furnace Slag Containing High Al2O3 at 1773 K. Crystals 2021, 11, 910. https://doi.org/10.3390/cryst11080910
Guo Y, Shen F, Zheng H, Wang S, Jiang X, Gao Q. Desulfurization Ability of Blast Furnace Slag Containing High Al2O3 at 1773 K. Crystals. 2021; 11(8):910. https://doi.org/10.3390/cryst11080910
Chicago/Turabian StyleGuo, Yongchun, Fengman Shen, Haiyan Zheng, Shuo Wang, Xin Jiang, and Qiangjian Gao. 2021. "Desulfurization Ability of Blast Furnace Slag Containing High Al2O3 at 1773 K" Crystals 11, no. 8: 910. https://doi.org/10.3390/cryst11080910






