Rapid Bio-Assisted Synthesis and Magnetic Behavior of Zinc Oxide/Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Method
2.3. Characterization Systems
3. Results
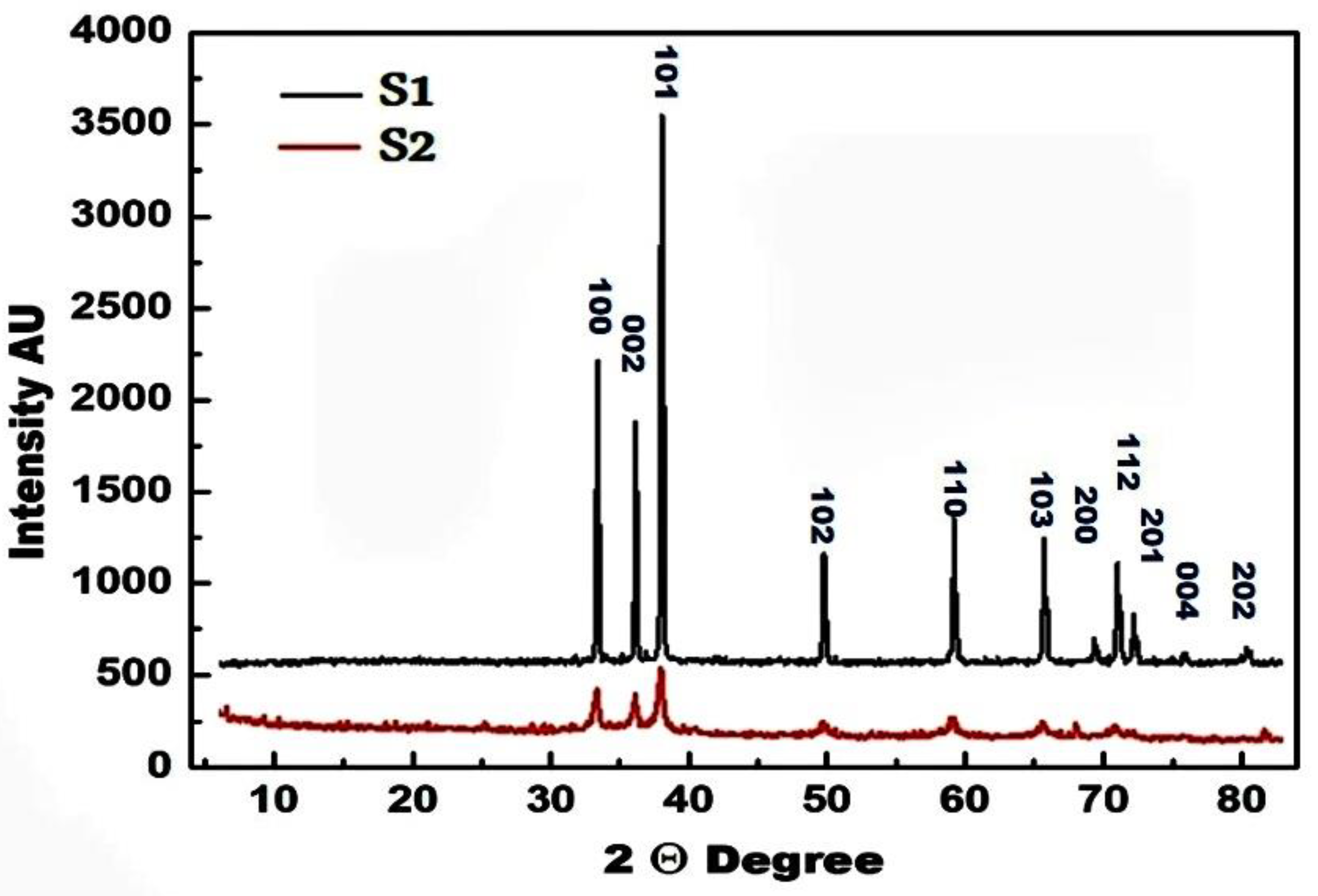
3.1. Structural Analysis
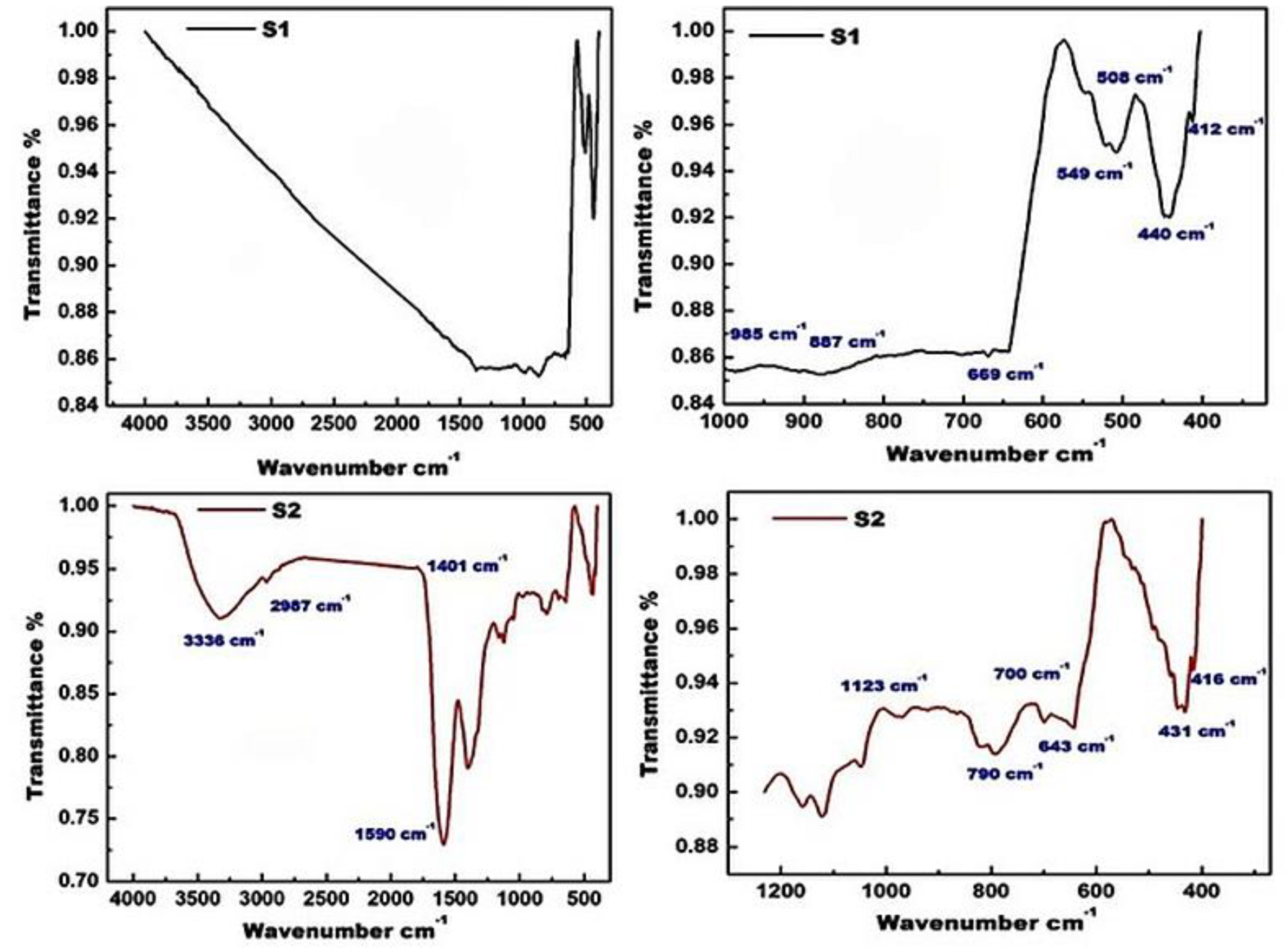
3.2. FTIR Analysis
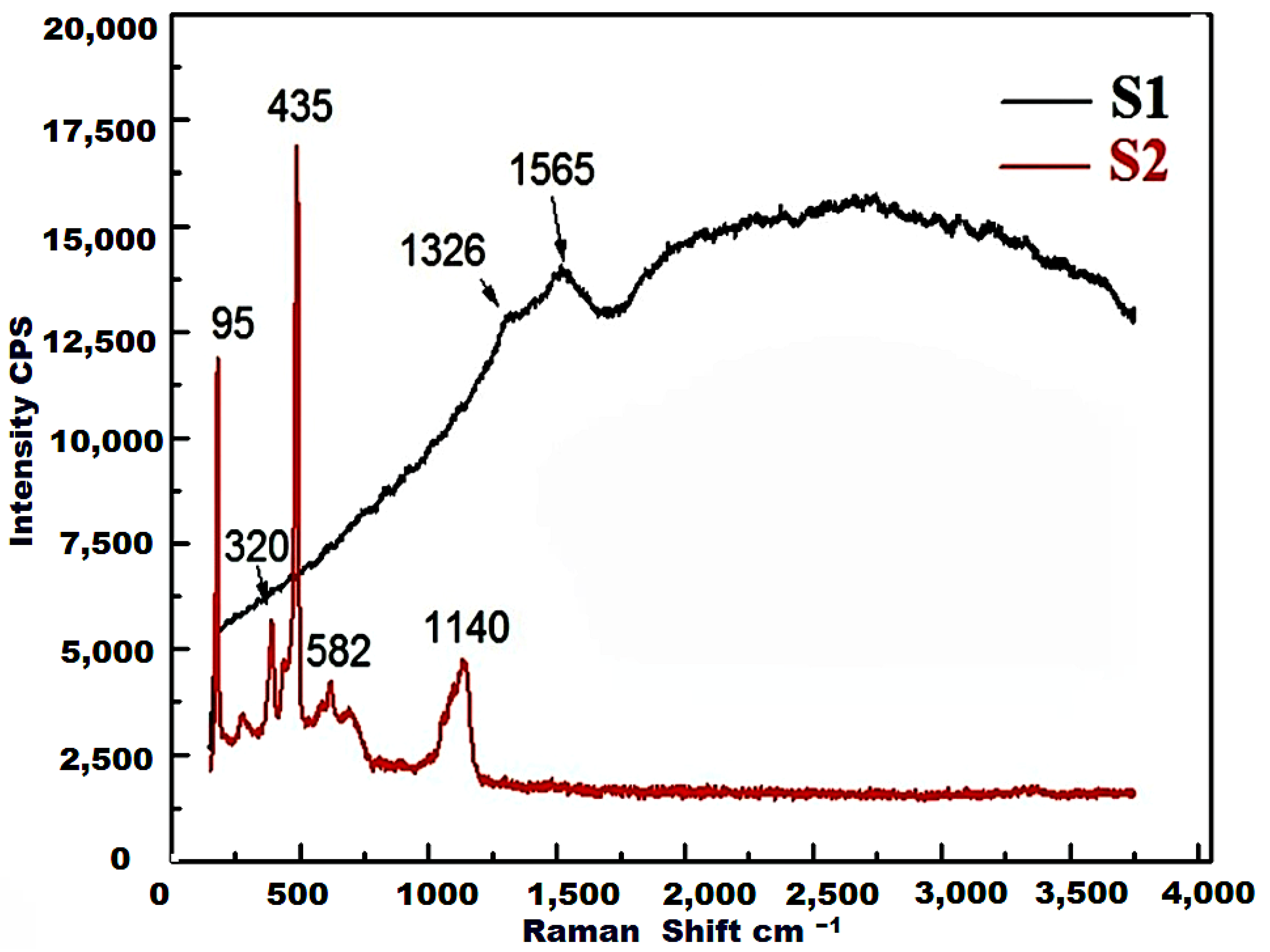
3.3. Raman Analysis
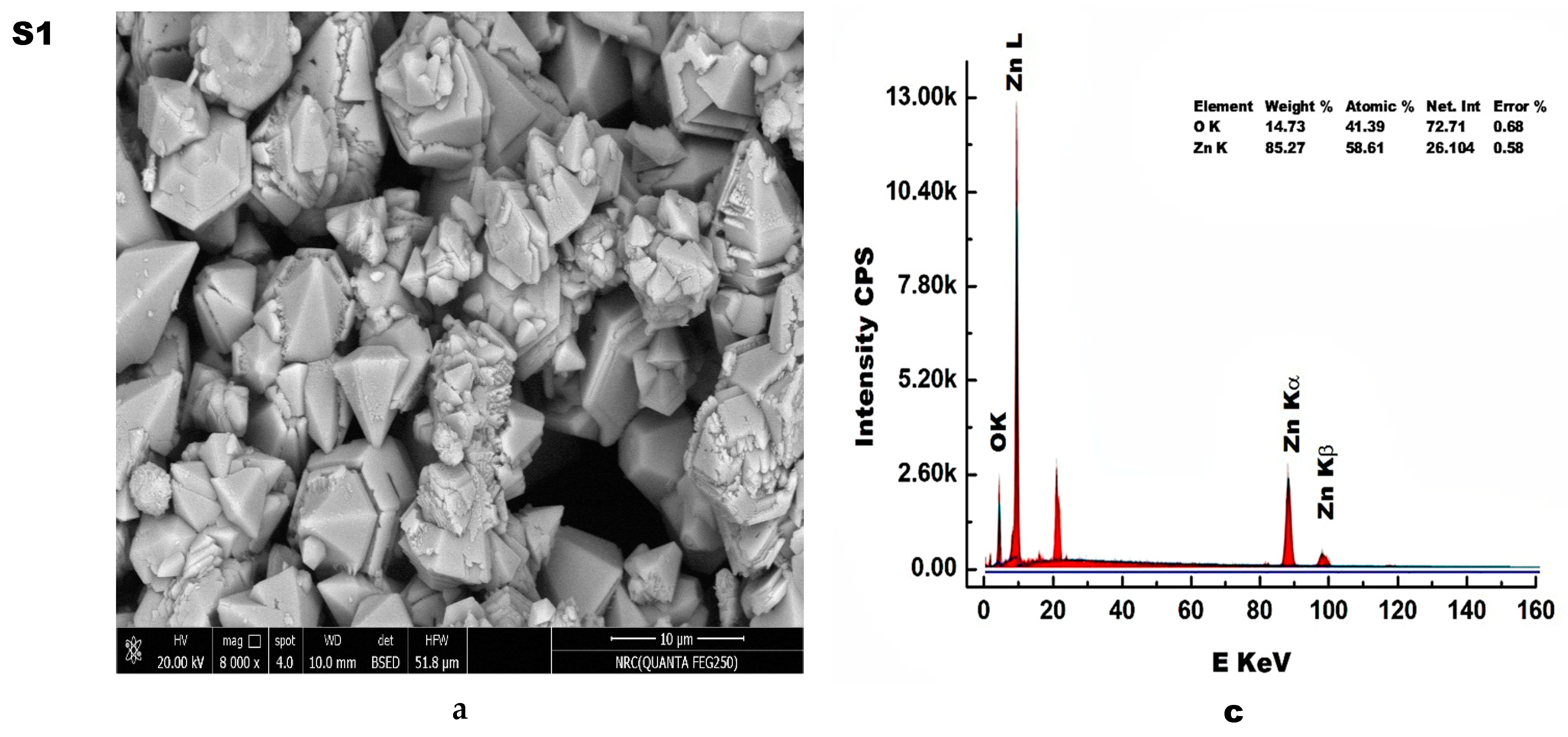
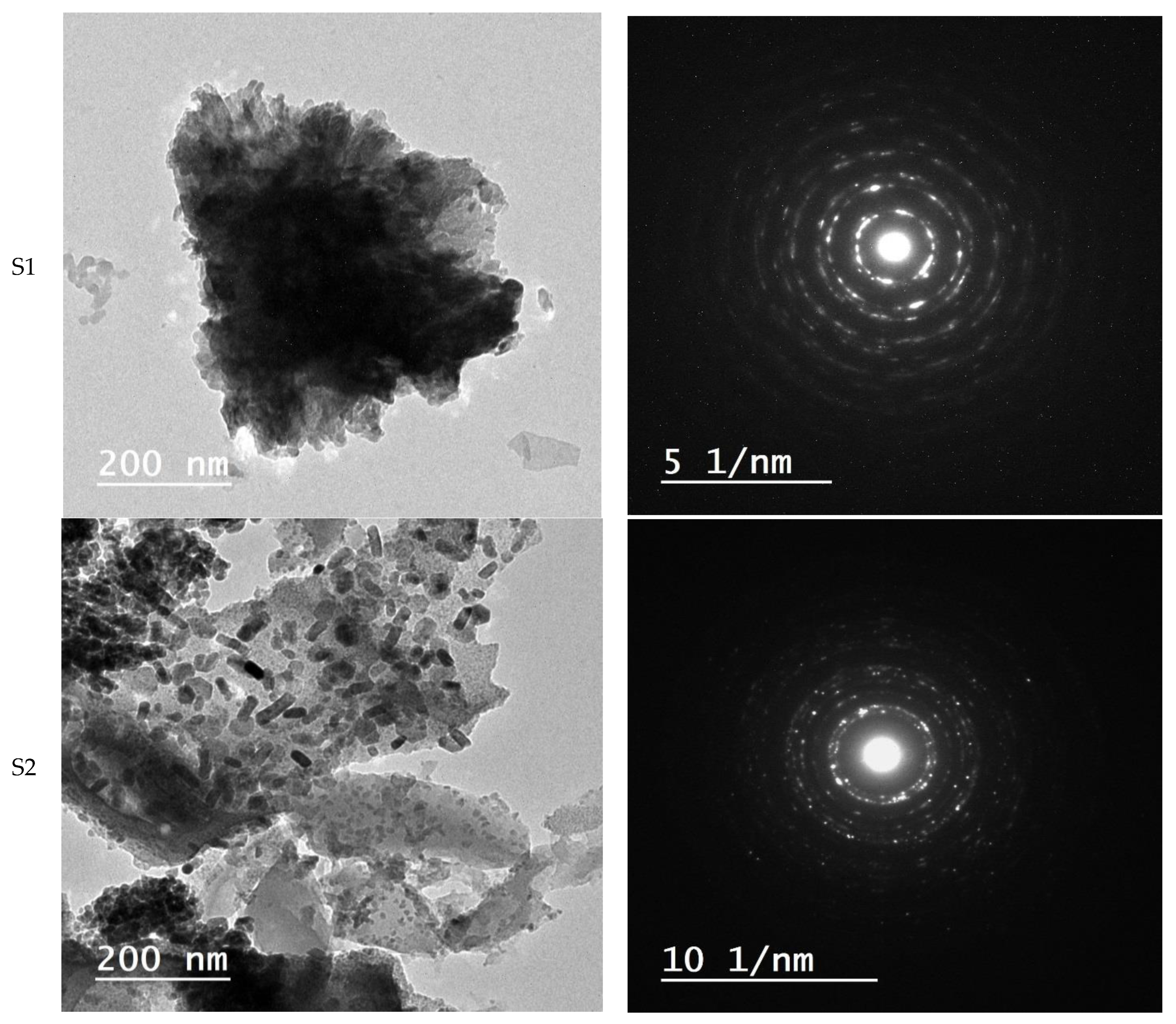
3.4. Morphology Study
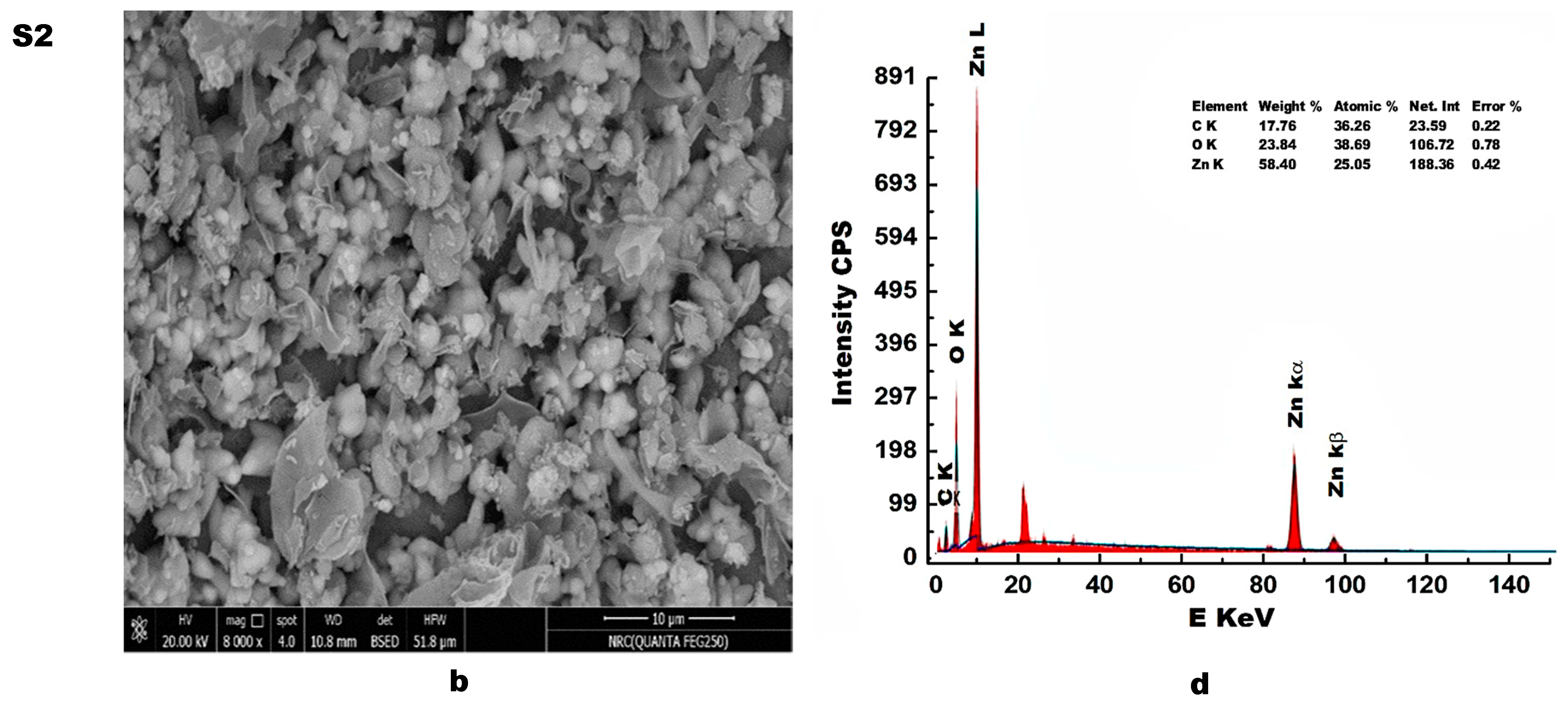
3.5. Elemental Analysis
3.6. Optical Properties
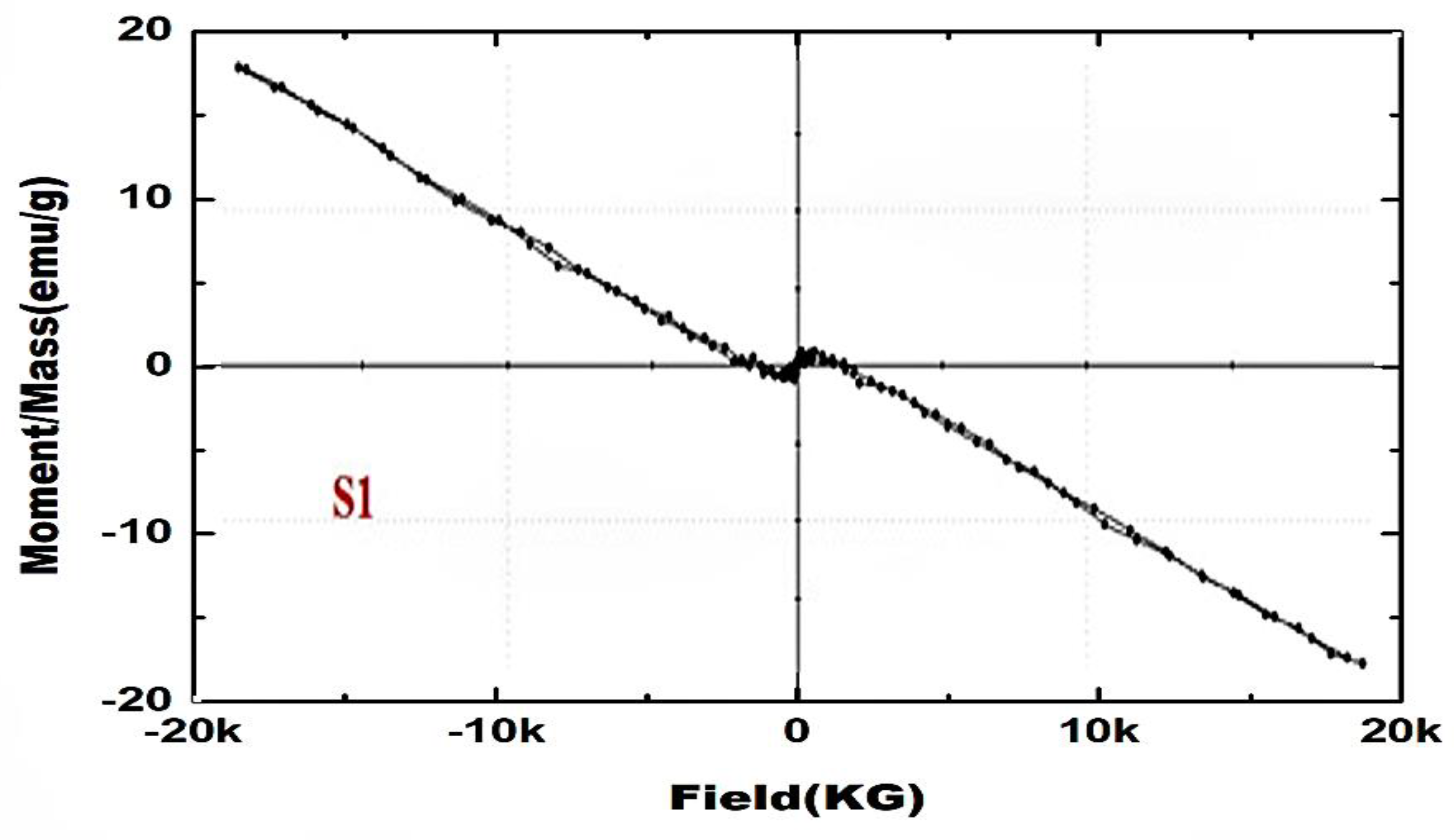
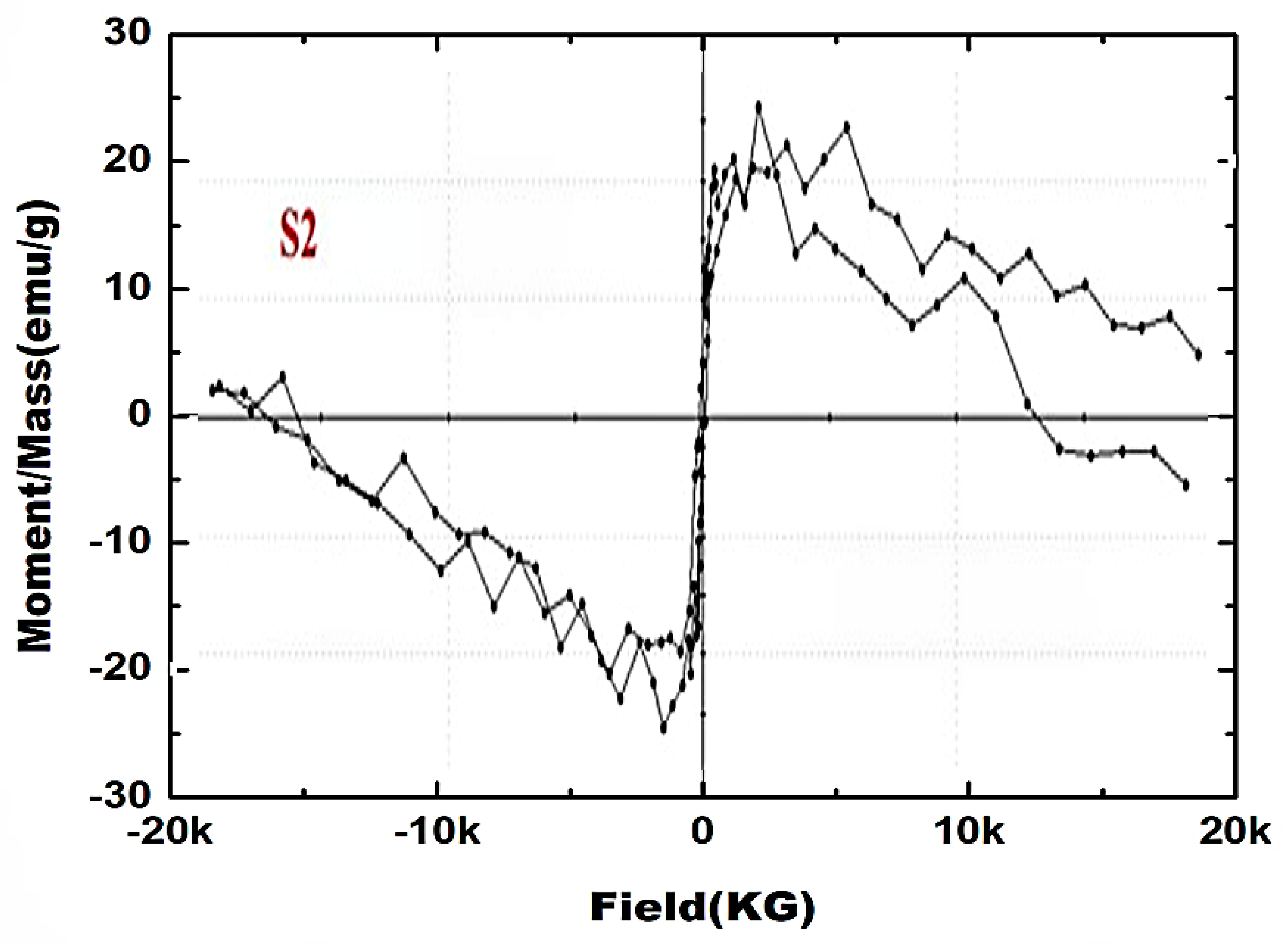
3.7. Magnetic Properties
4. Discussion
5. Conclusions
- The use or exclusion of egg white led to the self-combustion-based fabrication of ZnO or ZnO/C, respectively. The resulting zinc oxide had a wurtzite-type hexagonal structure;
- Using the egg white-assisted self-combustion method led to a decrease in the crystallite size and bond length of the ZnO crystal. The increase in the unit cell volume of ZnO was due to the increase in dislocation density and strain;
- The FTIR spectra confirm the formation of ZnO, based on the presence of typical absorption bands of ZnO in the range of 1000–400 cm−1, and in particular, the existence of fundamental absorption bands at 440 cm−1 and 508 cm−1. The intensity of these bands decreased in the presence of the carbon that resulted from the burning of egg white during the preparation of ZnO using the combustion method. The presence of this carbon resulted in the emergence of absorption bands at 1123, 1401 and 20,968 cm−1, related to C–O, C–H and C–OH, respectively. In the Raman spectra of the S2 sample, the appearance of two broad peaks at 1565 cm−1 and 1326 cm−1, related to the D- and G-bands of the carbonaceous material, confirms the formation of ZnO/C nanocomposite. Thus, the presence of carbon in the composite was detected by FTIR, EDS and Raman analyses;
- Using small amount of egg white resulted in the transformation of the shape of ZnO particles from hexagonal cone-type structures to ellipsoidal structures;
- UV–visible spectroscopy showed that the S2 (ZnO/C) sample underwent band gap narrowing compared to the S1 (pure ZnO) sample. The values of optical band gap energy for S1 and S2 are 3.09 and 2.60 eV, respectively;
- The magnetization curve of ZnO/C nanoparticles indicates the alteration of this system to ferromagnetism. The magnetic behavior of ZnO nanoparticles changes from completely diamagnetic to weakly ferromagnetic depending on the incorporation of a carbon atom in the crystal lattice of ZnO.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO–nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohavica, D.; Gladkov, P. ZnO nanoparticles and their applications-new achievements. Olomouc Czech Repub. EU 2010, 10, 12–14. [Google Scholar]
- Sahoo, S.; Maiti, M.; Ganguly, A.; George, J.J.; Bhowmick, A.K. Effect of zinc oxide nanoparticles as cure activator on the properties of natural rubber and nitrile rubber. J. Appl. Polym. Sci. 2007, 105, 2407–2415. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S.; et al. Zinc Oxide Nanostructure-Modified Textile and Its Application to Biosensing, Photocatalysis, and as Antibacterial Material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The synthesis and properties of ZnO–graphene nano hybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Yang, G.Z.; Song, H.W.; Cui, H.; Liu, Y.C.; Wang, C.X. Ultrafast Li-ion battery anode with superlong life and excellent cycling stability from strongly coupled ZnO nanoparticle/conductive nanocarbon skeleton hybrid materials. Nano Energy 2013, 2, 579–585. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Lin, C.Y.; Chen, Y.F.; Lin, J.S. Synthesis of ZnO@ Graphene composites as anode materials for lithium ion batteries. Electrochim. Acta 2013, 111, 359–365. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Ahmad, N.; Ali, N.; Abbas, S. Structure and electrochemical performance of ZnO/CNT composite as anode material for lithium-ion batteries. J. Mater. Sci. 2013, 48, 5429–5436. [Google Scholar] [CrossRef]
- Alshammari, A.S.; Chi, L.; Chen, X.; Bagabas, A.; Kramer, D.; Alromaeh, A.; Jiang, Z. Visible-light photocatalysis on C-doped ZnO derived from polymer-assisted pyrolysis. RSC Adv. 2015, 5, 27690–27698. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kang, Z.H.; Chen, Z.H.; Shafiq, I.; Zapien, J.A.; Bello, I.; Zhang, W.J.; Lee, S.T. Synthesis, Characterization, and Photocatalytic Application of Different ZnO Nanostructures in Array Configurations. Cryst. Growth Des. 2009, 9, 3222–3227. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Omelchenko, M.; Szczytko, J.; Chudoba, T.; Gierlotka, S.; Majhofer, A.; Twardowski, A.; Lojkowski, W. Structural and Magnetic Properties of Co–Mn Codoped ZnO Nanoparticles Obtained by Microwave Solvothermal Synthesis. Crystals 2018, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Abduev, A.K.; Akhmedov, A.K.; Asvarov, A.S.; Rabadanov, K.S.; Emirov, R.M. Formation of a ZnO–C Composite with a Nanocrystalline Structure. Tech. Phys. 2019, 64, 666–673. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Lee, J.S.; Lee, K.-H. Carbon-doped ZnO nanostructures synthesized using vitamin C for visible light photocatalysis. CrystEngComm 2010, 12, 3929–3935. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High photocatalytic activity of ZnO−carbon nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Molaei, P.; Rahimi-Moghadam, F. Optimized synthesis of ZnO nanostructures by egg-white content ratio manipulation for photocatalytic applications. Mater. Res. Express 2019, 6, 1250h7. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.-H. Zinc oxide/activated carbon nanofiber composites for high-performance supercapacitor electrodes. J. Power Sources 2015, 274, 512–520. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Bacsa, R.R.; Benyounes, A.; Axet, R.; Serp, P.; Silva, C.G.; Silva, A.M.; Faria, J.L. Synergistic effect between carbon nanomaterials and ZnO for photocatalytic water decontamination. J. Catal. 2015, 331, 172–180. [Google Scholar] [CrossRef]
- Chung, R.-J.; Wang, A.-N.; Liao, Q.-L.; Chuang, K.-Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, F.; Shen, Q.; Lavernia, E.J.; Zhang, L. Microstructure and Electrical Properties of AZO/Graphene Nanosheets Fabricated by Spark Plasma Sintering. Materials 2016, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardeshi, S.K.; Patil, A.B. Effect of morphology and crystallite size on solar photocatalytic activity of zinc oxide synthesized by solution free mechanochemical method. J. Mol. Catal. A Chem. 2009, 308, 32–40. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Rani, S.; Suri, P.; Shishodia, P.K.; Mehra, R.M. Synthesis of nanocrystalline ZnO powder via sol–gel route for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 1639–1645. [Google Scholar] [CrossRef]
- Ahmed, F.; Arshi, N.; Jeong, Y.S.; Anwar, M.S.; Dwivedi, S.; Alsharaeh, E.; Koo, B.H. Novel Biomimatic Synthesis of ZnO Nanorods Using Egg White (Albumen) and Their Antibacterial Studies. J. Nanosci. Nanotechnol. 2016, 16, 5959–5965. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F.; Almufarij, R.S.; Abd-Elkader, O.H.; Deraz, N.M. Magnetic Behavior of Virgin and Lithiated NiFe2O4 Nanoparticles. Crystals 2023, 13, 69. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Zhang, X.; Zeng, W.; Su, Q.; Du, G.; Duan, H. Solid-solution-like ZnO/C composites as excellent anode materials for lithium ion batteries. Electrochim. Acta 2015, 186, 165–173. [Google Scholar] [CrossRef]
- Sedrati, C.; Alleg, S.; Boussafel, H.; Hacine, A.B. Structure and magnetic properties of nickel ferrites synthesized by a facile co-precipitation method: Effect of the Fe/Ni ratio. J. Mater. Sci. Mater. Electron. 2021, 32, 24548–24559. [Google Scholar] [CrossRef]
- Das, J.; Khushalani, D. Nonhydrolytic route for synthesis of ZnO and its use as a recyclable photocatalyst. J. Phys. Chem. C 2010, 114, 2544–2550. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Silva, C.G.; Sampaio, M.J.; Carabineiro, S.A.; Oliveira, J.W.; Baptista, D.L.; Bacsa, R.; Machado, B.F.; Serp, P.; Figueiredo, J.L.; Silva, A.M.; et al. Developing highly active photocatalysts: Gold-loaded ZnO for solar phenol oxidation. J. Catal. 2014, 316, 182–190. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural Characterisation of ZnO Particles Obtained by the Emulsion Precipitation Method. J. Nanomater. 2012, 2012, 656353. [Google Scholar] [CrossRef] [Green Version]
- da Trindade, L.G.; Minervino, G.B.; Trench, A.B.; Carvalho, M.H.; Assis, M.; Li, M.S.; de Oliveira, A.J.; Pereira, E.C.; Mazzo, T.M.; Longo, E. Influence of ionic liquid on the photoelectrochemical properties of ZnO particles. Ceram. Int. 2018, 44, 10393–10401. [Google Scholar] [CrossRef]
- Ansari, S.P.; Mohammad, F. Studies on Nanocomposites of Polyaniline and Zinc Oxide Nanoparticles with Supporting Matrix of Polycarbonate. ISRN Mater. Sci. 2012, 2012, 129869. [Google Scholar] [CrossRef] [Green Version]
- PK Shishodia, M.T.; K Shishodia, M.T. Ferromagnetism in sol-gel derived ZnO: Mn nanocrystalline thin films. Adv. Mater. Lett. 2016, 7, 116–122. [Google Scholar] [CrossRef]
- Sahoo, S.; Sharma, G.L.; Katiyar, R.S. Raman spectroscopy to probe residual stress in ZnO nanowire. J. Raman Spectrosc. 2011, 43, 72–75. [Google Scholar] [CrossRef]
- Pal, B.; Giri, P.K. Defect mediated magnetic interaction and high T c ferromagnetism in Co doped ZnO nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 9167–9174. [Google Scholar] [CrossRef]
- Wang, J.B.; Huang, G.J.; Zhong, X.L.; Sun, L.Z.; Zhou, Y.C.; Liu, E.H. Raman scattering and high temperature ferromagnetism of Mn-doped ZnO nanoparticles. Appl. Phys. Lett. 2006, 88, 252502. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibanez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Thangavel, R.; Moirangthem, R.S.; Lee, W.S.; Chang, Y.C.; Wei, P.K.; Kumare, J. Cesium doped and undoped ZnO nanocrystalline thin films: A comparative study of structural andmicro-Raman investigation of optical phonons. J. Raman Spectrosc. 2010, 41, 1594–1600. [Google Scholar] [CrossRef]
- Silambarasan, M.; Saravanan, S.; Soga, T. Raman and photoluminescence studies of Ag and Fe-doped ZnO nanoparticles. Int. J. Chemtech Res. 2015, 7, 1644–1650. [Google Scholar]
- Zhang, R.; Fan, L.; Fang, Y.; Yang, S. Electrochemical route to the preparation of highly dispersed composites of ZnO/carbon nanotubes with significantly enhanced electrochemiluminescence from ZnO. J. Mater. Chem. 2008, 18, 4964–4970. [Google Scholar] [CrossRef]
- Min, Y.; Zhang, K.; Chen, L.; Chen, Y.; Zhang, Y. Ionic liquid assisting synthesis of ZnO/graphene heterostructure photocatalysts with tunable photoresponse properties. Diam. Relat. Mater. 2012, 26, 32–38. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, L.C.; Leong, K.H.; Ibrahim, S.; Saravanan, P. Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J. Mater. Chem. A 2014, 2, 5315–5322. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.M.; Deraz, N.M.; Saleh, H.A. Fabrication and characterization of chemically deposited copper–manganese sulfide thin films. Appl. Phys. A 2020, 126, 700. [Google Scholar] [CrossRef]
- Jindal, K.; Tomar, M.; Katiyar, R.S.; Gupta, V. Transition from diamagnetic to ferromagnetic state in laser ablated nitrogen doped ZnO thin films. AIP Adv. 2015, 5, 027117. [Google Scholar] [CrossRef]
- Il’ves, V.G.; Sokovnin, S.Y. Structural and Magnetic Properties of Nanopowders and Coatings of Carbon-Doped Zinc Oxide Prepared by Pulsed Electron Beam Evaporation. J. Nanotechnol. 2017, 2017, 4628193. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Yi, J.B.; Shen, L.; Wu, R.Q.; Yang, J.H.; Lin, J.Y.; Feng, Y.P.; Ding, J.; Van, L.H.; Yin, J.H. Room-Temperature Ferromagnetism in Carbon-Doped ZnO. Phys. Rev. Lett. 2007, 99, 127201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Cheng, H.; Zong, R.; Zhu, Y. Photocorrosion Suppression of ZnO Nanoparticles via Hybridization with Graphite-like Carbon and Enhanced Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 2368–2374. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tu, Y.-J.; Wang, Y.-S.; Kan, R.-S.; Huang, C.-M. Characterization and photoreactivity of N-, S-, and C-doped ZnO under UV and visible light illumination. J. Photochem. Photobiol. A Chem. 2008, 199, 170–178. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Yu, J.; Xiang, Q. Improved visible-light photocatalytic activity of porous carbon self-doped ZnO nanosheet-assembled flowers. CrystEngComm 2011, 13, 2533–2541. [Google Scholar] [CrossRef]

| Parameters | S1 | Error % | S2 | Error % |
|---|---|---|---|---|
| d, nm | 71 | 0.408 | 19 | 0.526 |
| a, nm | 0.32481 | 0.250 | 0.32578 | 0.549 |
| c, nm | 0.52040 | 0.306 | 0.52153 | −0.090 |
| c/a | 1.6022 | 0.137 | 1.6001 | 0.006 |
| V, nm3 | 0.04755 | 0.316 | 0.04935 | 0.411 |
| μ | 0.3797 | 0.184 | 0.3902 | 0.295 |
| L, nm | 0.1977 | 0.253 | 0.1966 | −0.304 |
| δ, Lines/nm2 | 0.198 × 10−3 | 0.421 | 2.77 × 10−3 | 0.457 |
| ε | 0.0016 | 0.142 | 0.0061 | 0.335 |
| Samples | Ms × 10−3 (emu/g) | Mr × 10−3 (emu/g) | Mr/Ms | Hc (Oe) | μm × 10−4 | Ka (erg/cm3) |
|---|---|---|---|---|---|---|
| S2 | 21.12 | 12.06 | 0.5710 | 107.25 | 3.077 | 2.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elkader, O.H.; Deraz, N.M.; Aleya, L. Rapid Bio-Assisted Synthesis and Magnetic Behavior of Zinc Oxide/Carbon Nanoparticles. Crystals 2023, 13, 1081. https://doi.org/10.3390/cryst13071081
Abd-Elkader OH, Deraz NM, Aleya L. Rapid Bio-Assisted Synthesis and Magnetic Behavior of Zinc Oxide/Carbon Nanoparticles. Crystals. 2023; 13(7):1081. https://doi.org/10.3390/cryst13071081
Chicago/Turabian StyleAbd-Elkader, Omar H., Nasrallah M. Deraz, and Lotfi Aleya. 2023. "Rapid Bio-Assisted Synthesis and Magnetic Behavior of Zinc Oxide/Carbon Nanoparticles" Crystals 13, no. 7: 1081. https://doi.org/10.3390/cryst13071081
APA StyleAbd-Elkader, O. H., Deraz, N. M., & Aleya, L. (2023). Rapid Bio-Assisted Synthesis and Magnetic Behavior of Zinc Oxide/Carbon Nanoparticles. Crystals, 13(7), 1081. https://doi.org/10.3390/cryst13071081








