Microstructure Evolution and Mechanical Properties of TiB2/Al–Cu–Mn–Cd Composite with the Adoption of Two-Stage Solution and Aging Treatment
Abstract
:1. Introduction
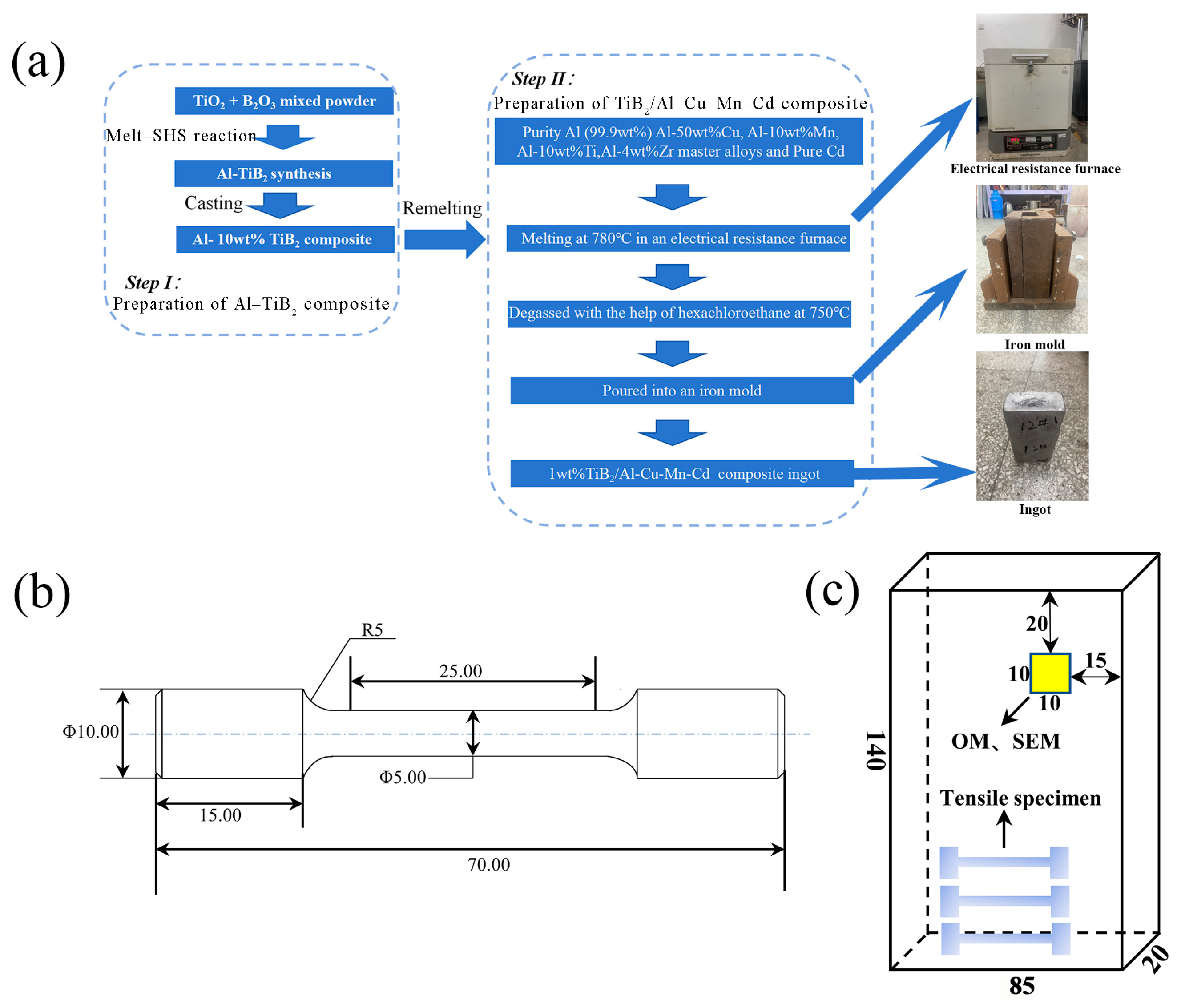
2. Materials and Methods
3. Results
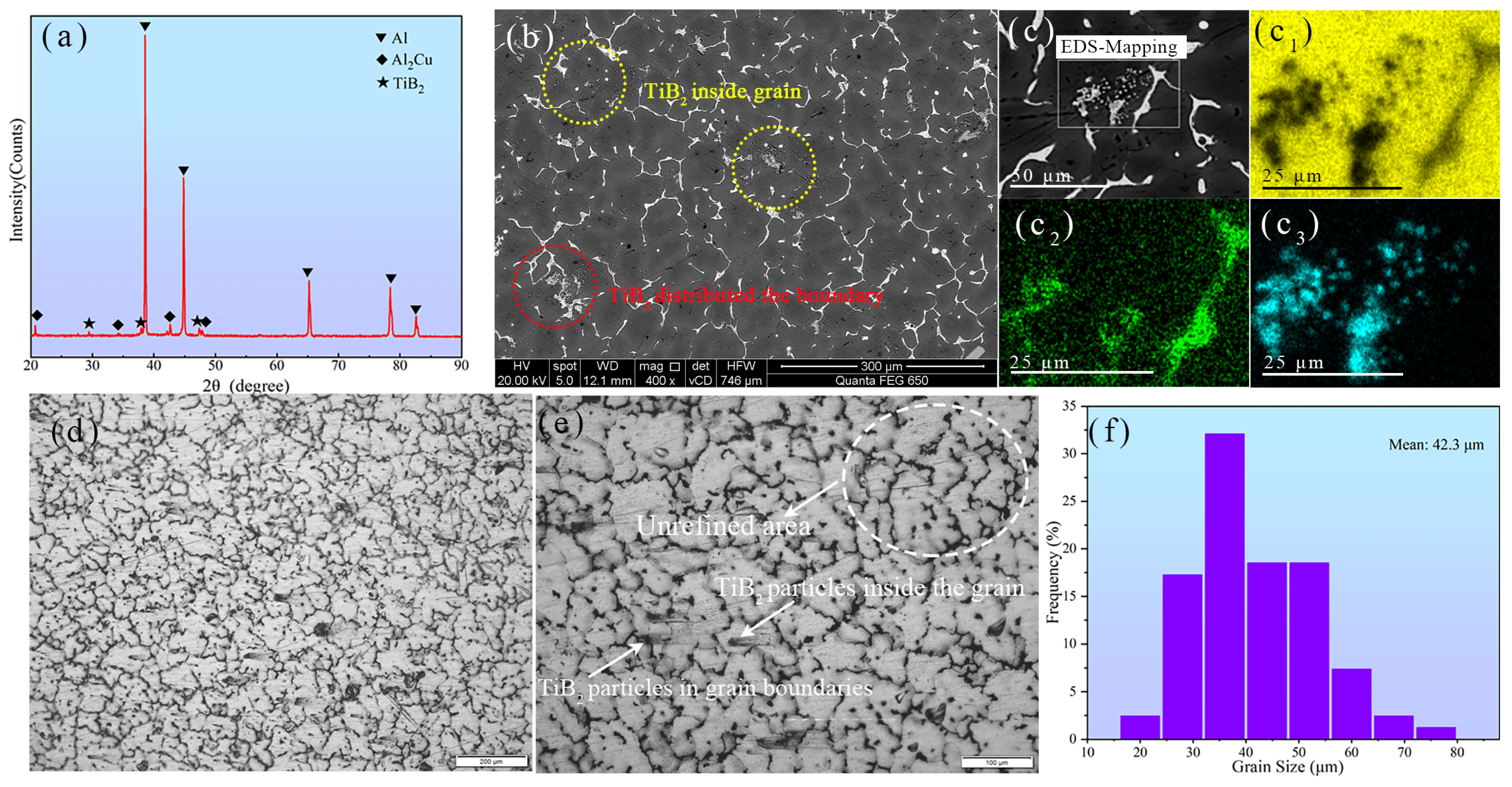
3.1. Microstructure of the As-Cast TiB2/Al–Cu–Mn–Cd Composite
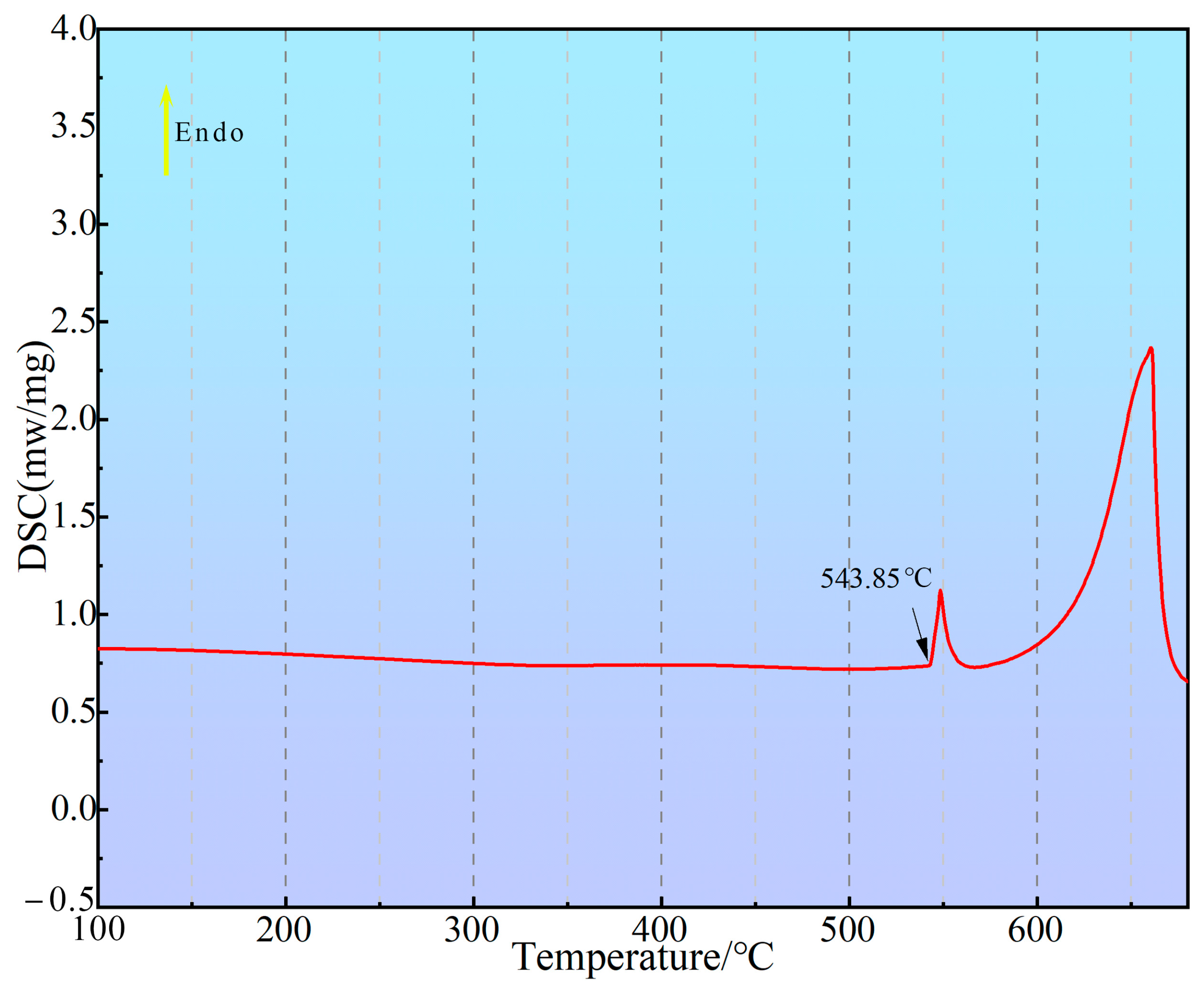
3.2. DSC Analysis
3.3. Optimization of the Solution Process
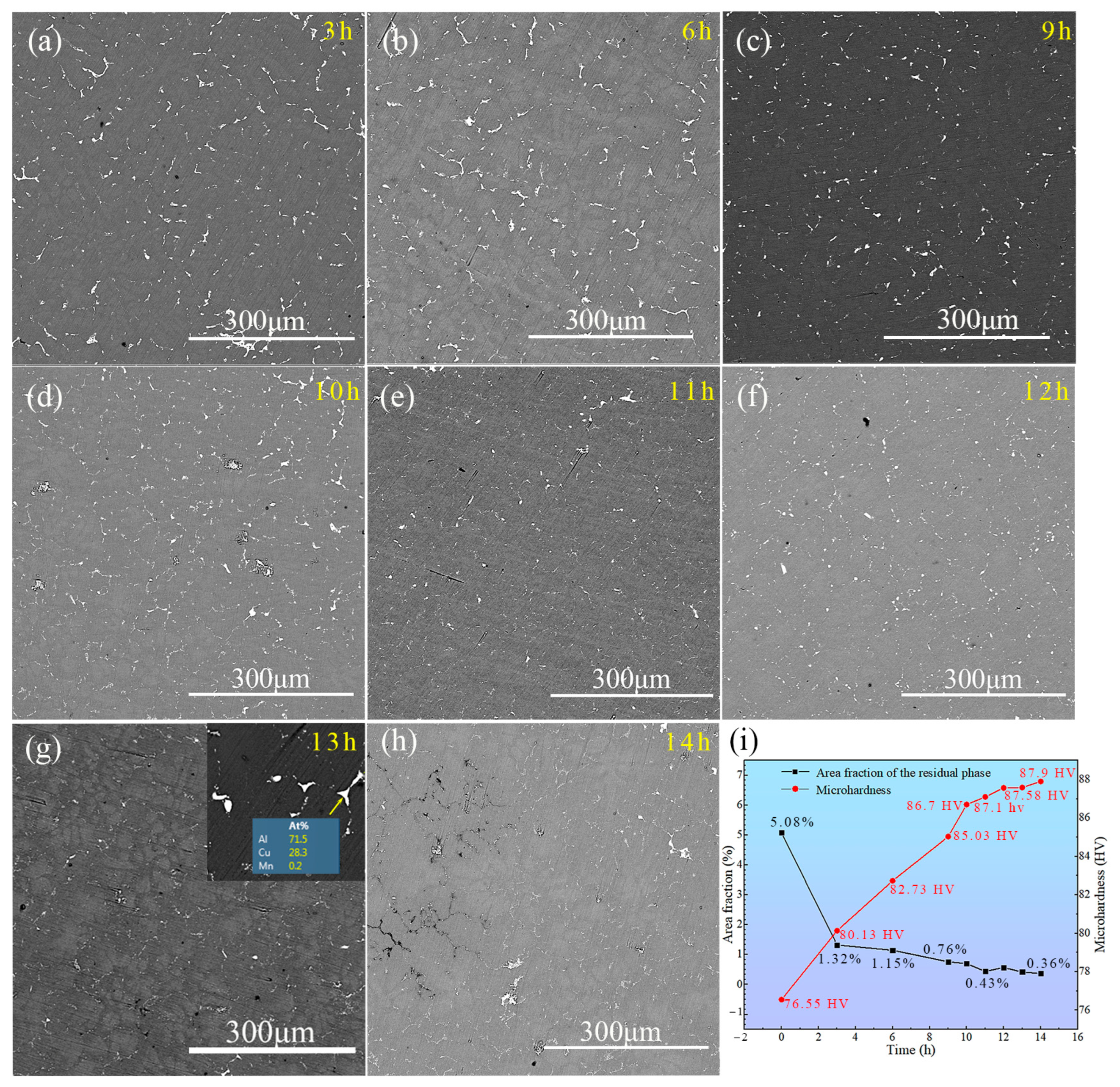
3.3.1. Microstructure Evolution of TiB2/Al–Cu–Mn–Cd Composite in the Single-Stage Solution Treatment
3.3.2. Microstructure Evolution of TiB2/Al–Cu–Mn–Cd Composite in Second-Stage Solution Treatment
3.4. Optimization of the Aging Process
3.4.1. Aging-Hardening Behavior at Different Temperatures
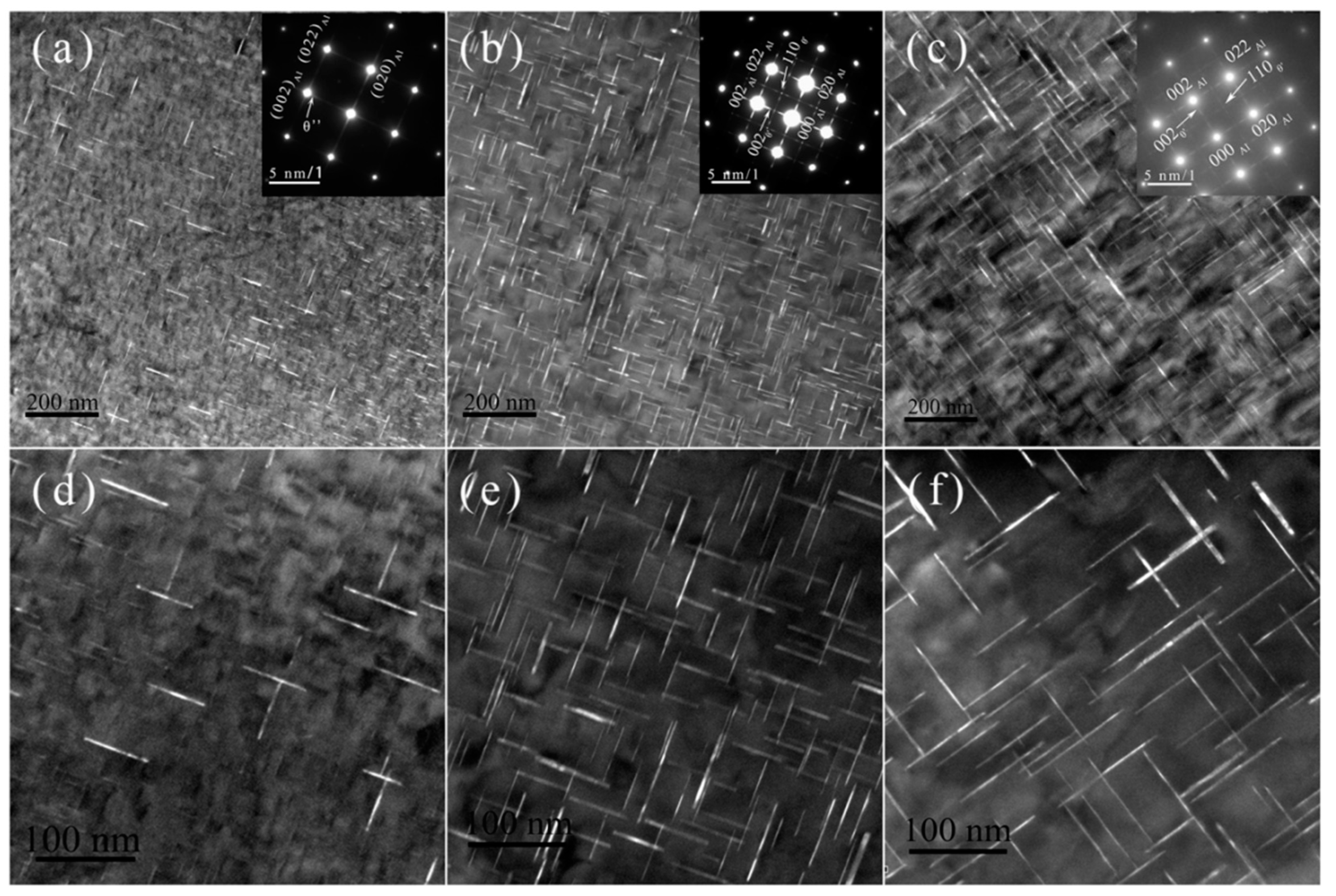
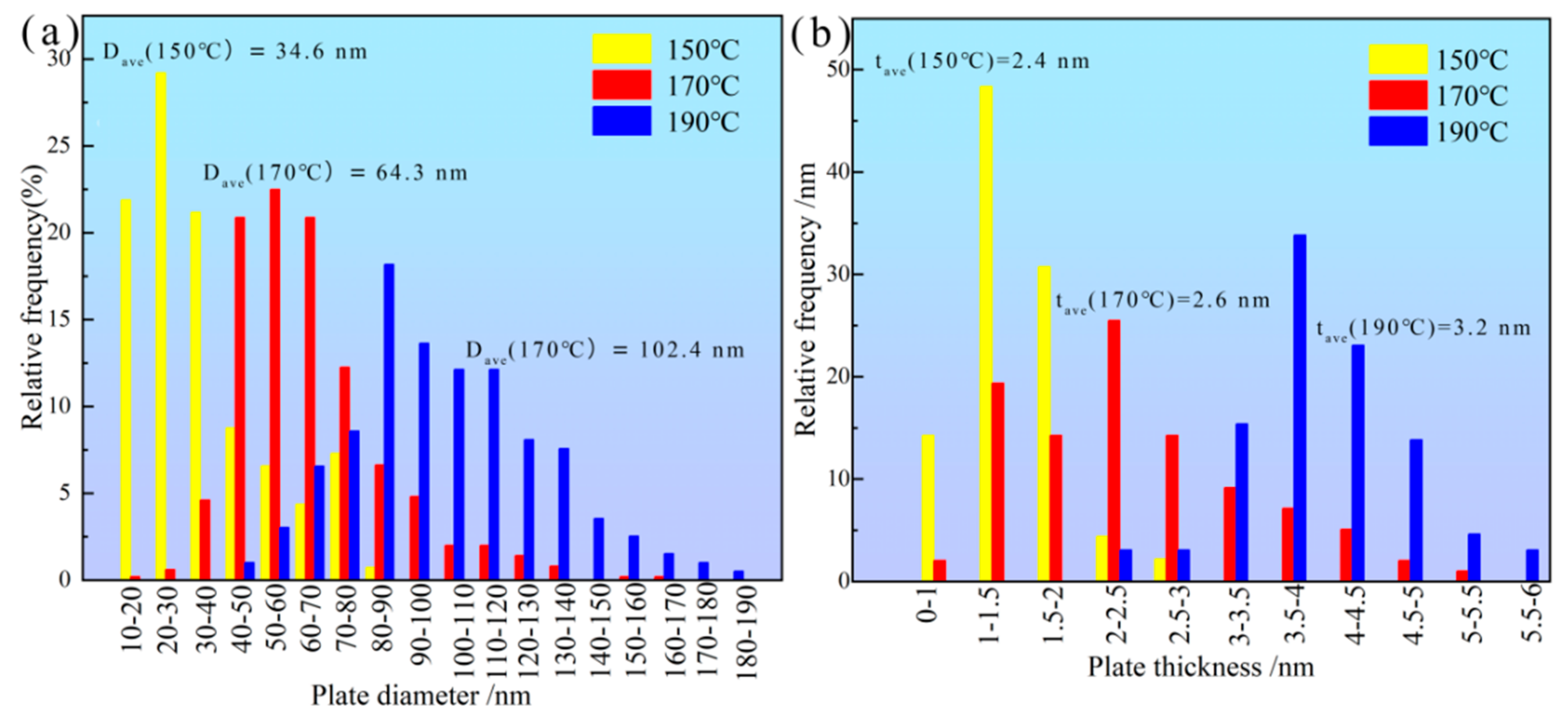
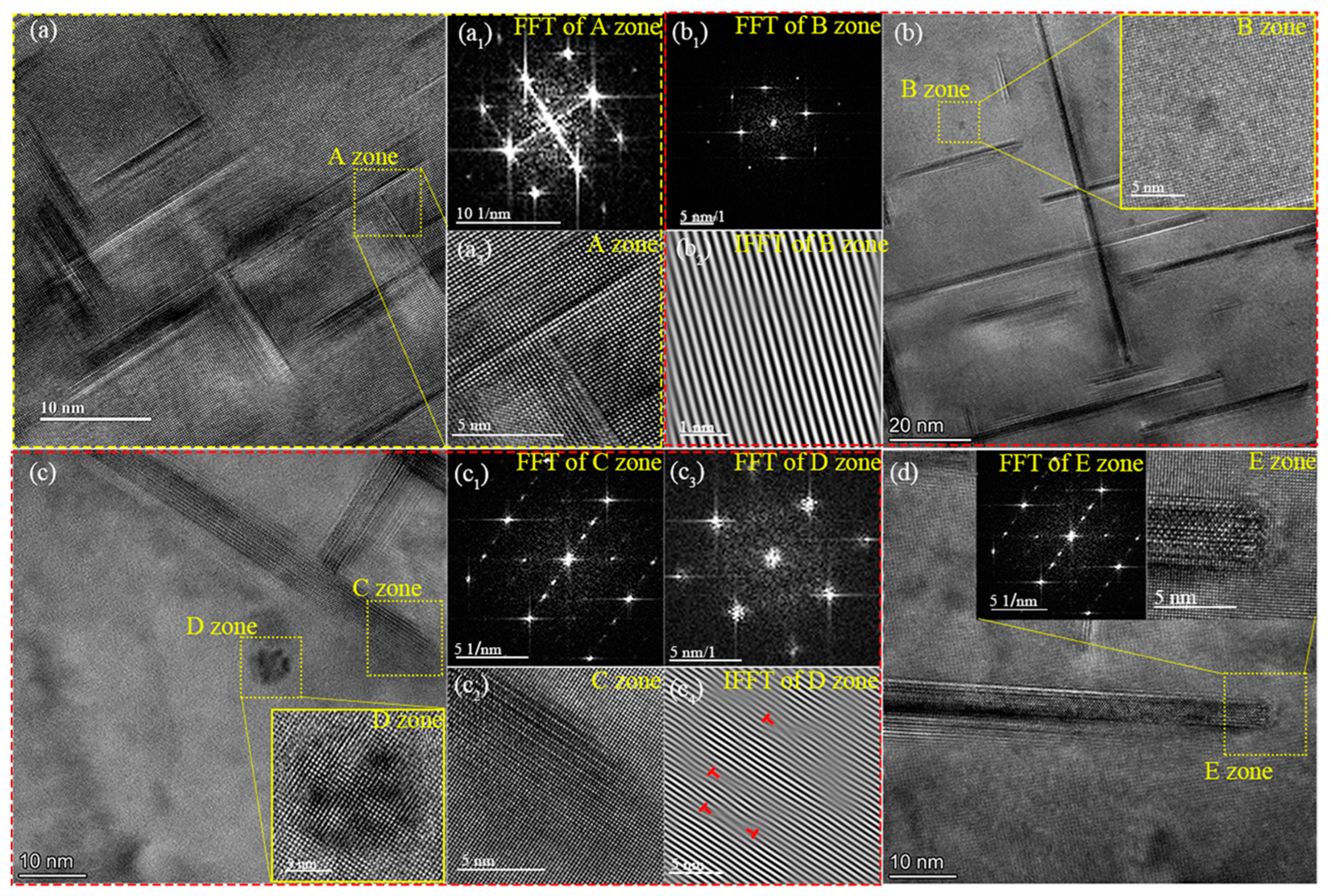
3.4.2. Peak-Aged Microstructure at Different Temperatures
3.5. Mechanical Properties
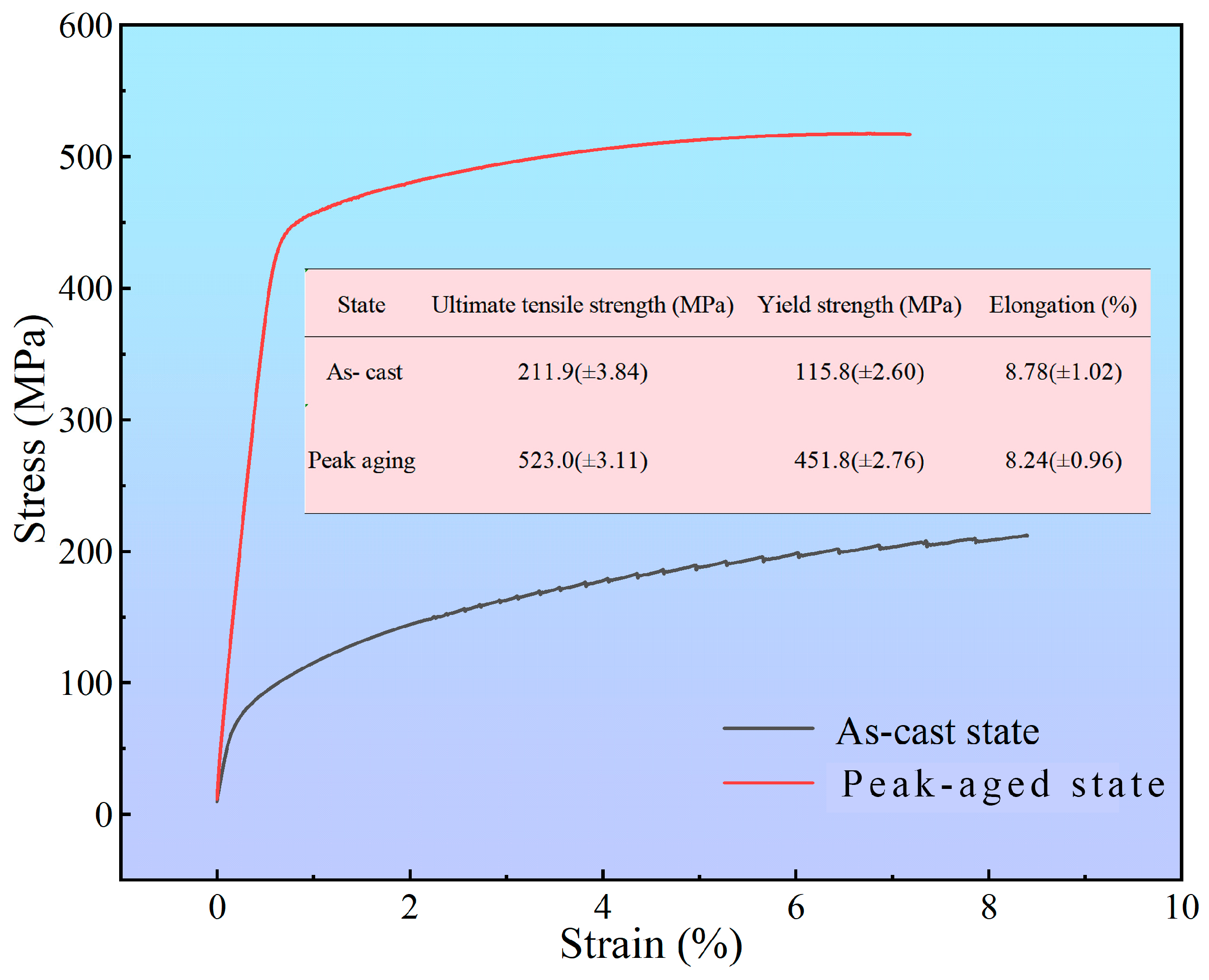
3.5.1. Tensile Property at Room Temperature
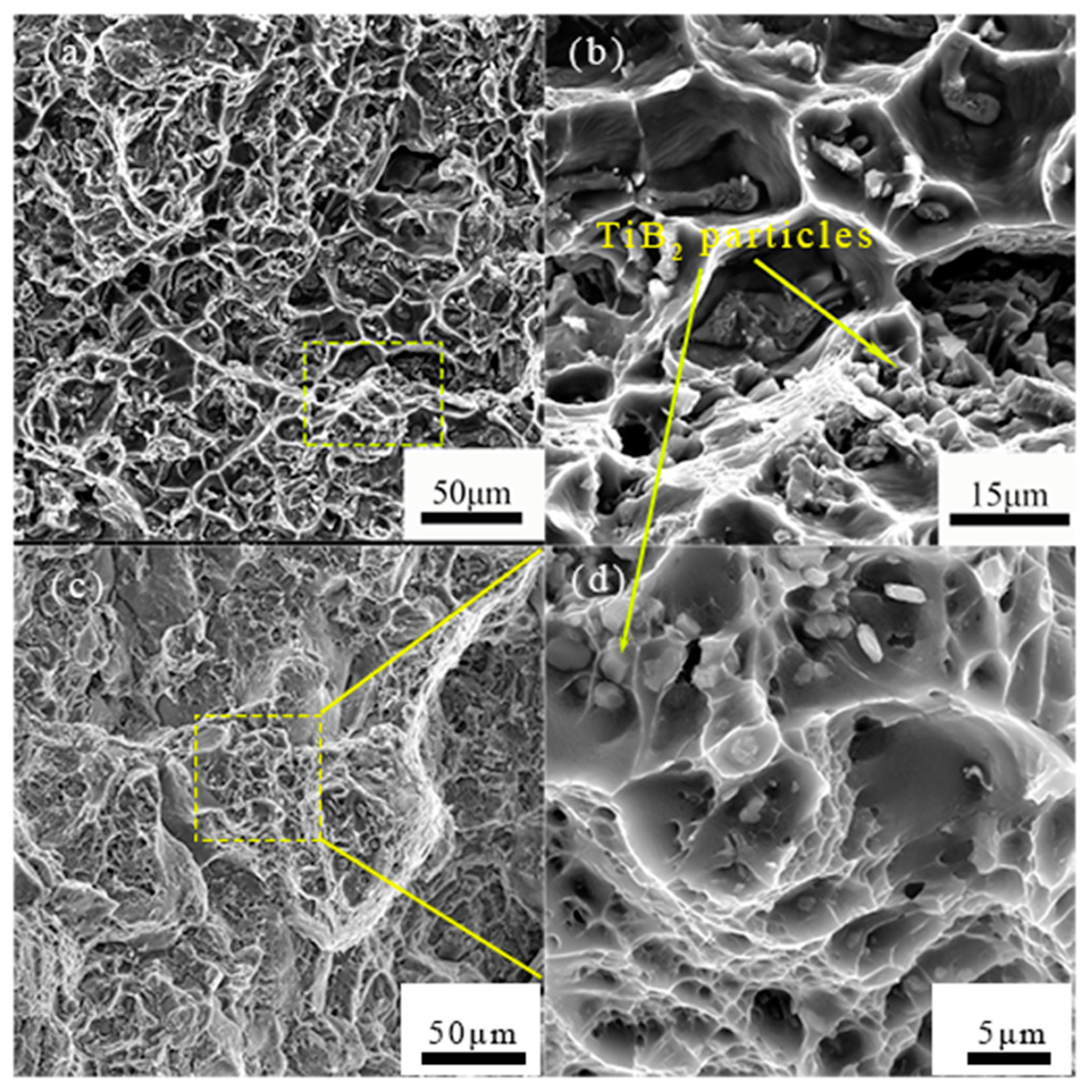
3.5.2. Fractography
4. Discussion
4.1. Nucleation Behavior at Different Aging Temperatures
4.2. Kinetics Behavior of Precipitation during Aging at 170 °C
4.3. The Connection between Hardness and Precipitates Evolution during Aging Process
5. Conclusions
- An optimized two-stage solution treatment for TiB2/Al–Cu–Mn–Cd composite was proposed. The second and eutectic Al2Cu phases in the as-cast state completely dissolved into the Al matrix with the adoption of the optimized solution treatment, i.e., solutionized at 523 °C for 11 h followed by the high-temperature short-time solution at 535 °C for 1 h.
- The peak hardness for TiB2/Al–Cu–Mn–Cd reached 164.6 HV with the optimized aging at 170 °C for 12 h. Compared to the mechanical properties in the as-cast state, the average ultimate tensile strength and yield strength increased from 211.9 MPa to 523.0 MPa and 115.8 MPa to 451.8 MPa, respectively. However, the value of elongation slightly decreased from 8.78% to 8.24%.
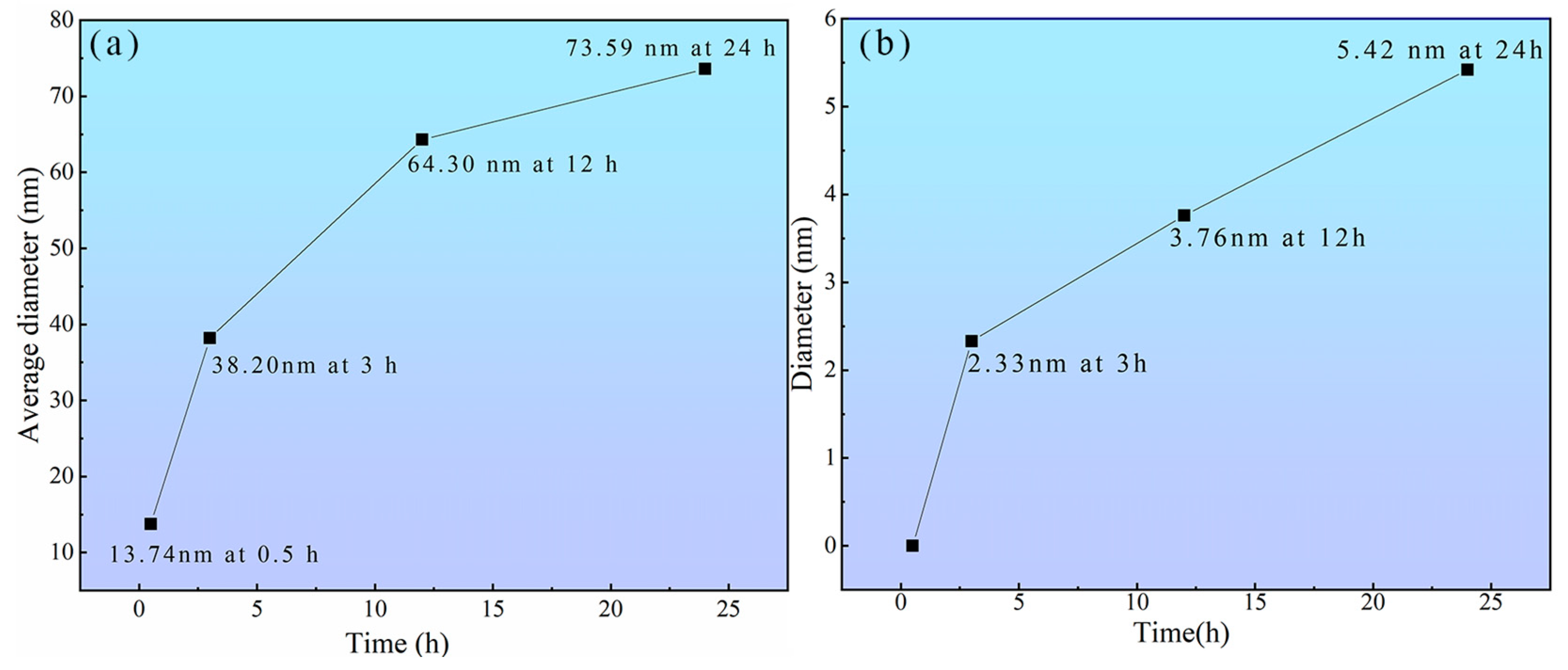
- The strength contribution of the peak-aged TiB2/Al–Cu–Mn–Cd composite mainly ascribed to the Cd-rich, θ″ and θ′ precipitates. In the peak-aged state, the number density and average diameter of the plate-like precipitates reached 4.266 × 1021 m3 and 64.30 nm, which provided the most powerful precipitate strengthening. The smaller sizes and sparser number densities of the precipitates were the reasons for the low hardnesses of the under-aged and over-aged composites, respectively.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, K.; Kang, H.; Wu, Y.; Liu, M.; Guo, E.; Chen, Z.; Wang, T. Manipulating the particle distribution of in situ TiB2p/Al composites via acoustic vibration and cooling rate. Mater. Lett. 2019, 262, 127063. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zou, B.L.; Tao, S.Y.; Zhang, M.X.; Cao, X.Q. Fabrication and properties of Al2O3-TiB2-TiC/Al metal matrix composite coatings by atmospheric plasma spraying of SHS powders. J. Alloys Compd. 2016, 672, 251–259. [Google Scholar] [CrossRef]
- Ma, G.N.; Wang, D.; Liu, Z.Y.; Xiao, B.L.; Ma, Z.Y. An investigation on particle weakening in T6-treated SiC/Al-Zn-Mg-Cu composites. Mater. Charact. 2019, 158, 109966. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Fan, J.Z.; Liu, Y.Q.; Nie, J.H.; Yang, Z.Y.; Kang, Y.L. Strengthening and Weakening Effects of Particles on Strength and Ductility of SiC Particle Reinforced Al-Cu-Mg Alloys Matrix Composites. Materials 2021, 14, 1219. [Google Scholar] [CrossRef]
- Zhong, X.M.; Li, Q.Y.; Gong, Y.Z.; Duan, X.Z.; Shao, Y.; Yang, H.Y.; Qiu, F.; Jiang, Q.C. Effects of various proportions TiB2p-TiCp reinforced Al-Cu-Mg composites in high-temperature mechanical properties and sliding wear behaviors. J. Mater. Res. Technol. 2024, 30, 4169–4180. [Google Scholar] [CrossRef]
- Gao, T.; Liu, L.Y.; Song, J.P.; Liu, G.L.; Liu, X.F. Synthesis and characterization of an in-situ Al2O3/Al-Cu composite with a heterogeneous structure. J. Alloys Compd. 2021, 868, 159283. [Google Scholar] [CrossRef]
- Diao, E.; Fan, J.Z.; Yang, Z.Y.; Lv, Z.C.; Gao, H.; Nie, J.H. Hot Deformation Behavior and Mechanisms of SiC Particle Reinforced Al-Zn-Mg-Cu Alloy Matrix Composites. Materials 2023, 16, 7430. [Google Scholar] [CrossRef]
- Jin, P.; Zhou, J.; Zhou, J.; Liu, Y.; Sun, Q. Contribution made by double-sized TiC particles addition to the ductility-strength synergy in wire and arc additively manufactured Al-Cu alloys. Compos. Part B Eng. 2024, 268, 111078. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.Y.; Chen, Z.; Wang, M.L.; Zhu, H.; Wang, H.W. Understanding alloying behaviors of Sc, Ni and Zn additions on Al/TiB2 interfaces based on interfacial characteristics and solute properties. Surf. Interface 2021, 26, 101427. [Google Scholar] [CrossRef]
- Schaffer, P.L.; Miller, D.N.; Dahle, A.K. Crystallography of engulfed and pushed TiB2 particles in aluminum. Scr. Mater. 2007, 57, 1129–1132. [Google Scholar] [CrossRef]
- Li, L.; Han, Z.; Gao, M.; Li, S.; Wang, H.; Kang, H.; Guo, E.; Chen, Z.; Wang, T. Microstructures, mechanical properties, and aging behavior of hybrid-sized TiB2 particulate-reinforced 2219 aluminum matrix composites. Mater. Sci. Eng. A 2022, 829, 142180. [Google Scholar] [CrossRef]
- Tian, W.S.; Zhao, Q.L.; Zhang, Q.Q.; Qiu, F.; Jiang, Q.C. Simultaneously increasing the high-temperature tensile strength and ductility of nano-sized TiCp reinforced Al-Cu matrix composites. Mater. Sci. Eng. A 2018, 717, 105–112. [Google Scholar] [CrossRef]
- Sun, J.W.; Wu, G.H.; Zhang, L.; Zhang, X.L.; Liu, L.L.; Zhang, J.S. Microstructure characteristics of an ultra-high strength extruded Al-4.7Cu-1Li-0.5Mg-0.1Zr-1Zn alloy during heat treatment. J. Alloys Compd. 2020, 813, 152216. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Samuel, F.H.; Kahtani, S.A. Influence of Mg and solution heat treatment on the occurrence of incipient melting in Al-Si-Cu-Mg cast alloys. Mater. Sci. Eng. A 2012, 543, 22–34. [Google Scholar] [CrossRef]
- Han, Y.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Optimizing the tensile properties of Al-Si-Cu-Mg 319-type alloys: Role of solution heat treatment. Mater. Des. 2014, 58, 426–438. [Google Scholar] [CrossRef]
- Hong, T.R.; Li, X.F.; Wang, H.W.; Chen, D. Influence of solution temperature on microstructure and properties of in-situ TiB2/2009 composites. Mater. Sci. Eng. A 2015, 634, 1–4. [Google Scholar] [CrossRef]
- Du, R.; Gao, Q.; Wu, S.S.; Lü, S.L.; Zhou, X. Influence of TiB2 particles on aging behavior of in-situ TiB2/Al-4.5Cu composites. Mater. Sci. Eng. A 2018, 721, 244–250. [Google Scholar] [CrossRef]
- Li, J.; Lü, S.; Wu, S.; Zhao, D.; Li, F.; Guo, W. Effects of nanoparticles on the solution treatment and mechanical properties of nano-SiCp/Al-Cu composites. J. Mater. Process. Technol. 2021, 296, 117195. [Google Scholar] [CrossRef]
- Yang, X.F.; Xu, C.; Lu, G.X.; Guan, S.K. Towards strength-ductility synergy through an optimized two-stage solution treatment in Al-7Si-3Cu-0.5Mg alloys. Mater. Sci. Eng. A 2022, 849, 143504. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, C.; Li, X.F.; Ma, N.H.; Wang, H.W. Microstructural evolution and mechanical properties of cast high-Li-content TiB2/Al-Li-Cu composite during heat treatment. J. Alloys Compd. 2018, 739, 270–279. [Google Scholar] [CrossRef]
- Möller, H.; Govender, G. The Heat Treatment of Rheo-High Pressure Die Cast Al-Cu-Mg-Ag Alloy 2139. Solid State Phenom. 2012, 173, 192–193. [Google Scholar] [CrossRef]
- Stemper, L.; Tunes, M.A.; Dumitraschkewitz, P.; Mendez-Martin, F.; Tosone, R.; Marchand, D.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Giant hardening response in AlMgZnCu alloys. Acta Mater. 2021, 206, 116617. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, H.; Bian, Z.; Geng, J.; Chen, Z.; Wang, L.; Wang, M.; Wang, H. Insight into the recrystallization behavior and precipitation reaction of in-situ nano TiB2/Al-Cu-Mg composite during heat treatment. Mater. Charact. 2021, 181, 111458. [Google Scholar] [CrossRef]
- Ma, P.P.; Liu, C.H.; Zhan, L.H.; Huang, M.H.; Ma, Z.Y. Formation of a new intermediate phase and its evolution toward θ’ during aging of pre-deformed Al-Cu alloys. J. Mater. Sci. Technol. 2019, 35, 885–890. [Google Scholar] [CrossRef]
- Shahani, A.; Xiao, X.; Skinner, K.; Peters, M.; Voorhees, P. Voorhees, Ostwald ripening of faceted Si particles in an Al-Si-Cu melt. Mater. Sci. Eng. A 2016, 673, 307–320. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Lin, Y.; Wu, Q.; Jiang, Y.-Q. Effects of aging on precipitation behavior and mechanical propertiesof a tensile deformed Al-Cu alloy. J. Alloys Compd. 2020, 843, 155975. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. The heat treatment of Al–Si–Cu–Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef]
- Alvarez-Antolin, J.F.; Segurado-Frutos, E.; Neira-Castaño, H.; Asensio-Lozano, J. Heat Treatment Optimization in Al-Cu-Mg-Si Alloys with or without Prior Deformation. Metals 2018, 8, 739. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.M.; Chai, L.H.; Wang, H.J.; Chen, Z.Y.; Xiang, Z.L.; Jin, T.N. Microstructure and mechanical properties of an in-situ TiB2/Al-Zn-Mg-Cu-Zr composite fabricated by Melt-SHS process. Mater. Sci. Eng. A 2018, 720, 60–68. [Google Scholar] [CrossRef]
- Zhang, M.S.; Wang, J.S.; Wang, B.; Xue, C.P.; Liu, X.G. Quantifying the effects of Sc and Ag on the microstructure and mechanical properties of Al-Cu alloys. Mater. Sci. Eng. A 2022, 831, 142355. [Google Scholar] [CrossRef]
- Delmas, F.; Casanove, M.J.; Lours, P. Quantitative TEM study of the precipitation microstructure in aluminium alloy Al(MgSiCu) 6056 T6. Mater. Sci. Eng. A 2004, 373, 80–89. [Google Scholar] [CrossRef]
- Kelly, P.M.; Jostsons, A.; Blake, R.G. The determination of foil thickness by scanning transmission electron microscopy. Phys. Status Solidi (a) 1975, 31, 771–780. [Google Scholar] [CrossRef]
- Chen, J.L.; Liao, H.C.; Xu, H.T. Uneven Precipitation Behavior during the Solutionizing Course of Al-Cu-Mn Alloys and Their Contribution to High Temperature Strength. Adv. Mater. Sci. Eng. 2018, 12, 6741502. [Google Scholar] [CrossRef]
- Chisholm, M.F.; Shin, D.; Duscher, G.; Oxley, M.P.; Allard, L.F.; Poplawsky, J.D. Atomic structures of interfacial solute gateways to θ’ precipitates in Al-Cu alloys. Acta Mater. 2021, 212, 116891. [Google Scholar] [CrossRef]
- Gao, L.; Li, K.; Ni, S.; Du, Y.; Song, M. The growth mechanisms of θ’ precipitate phase in an Al-Cu alloy during aging treatment. J. Mater. Sci. Technol. 2021, 61, 25–32. [Google Scholar] [CrossRef]
- Chen, Y.; Weyland, M.; Hutchinson, C.R. The effect of interrupted aging on the yield strength and uniform elongation of precipitation-hardened Al alloys. Acta Mater. 2013, 61, 5877–5894. [Google Scholar] [CrossRef]
- Tian, W.-S.; Zhao, Q.-L.; Zhang, Q.-Q.; Qiu, F.; Jiang, Q.-C. Enhanced strength and ductility at room and elevated temperatures of Al-Cu alloy matrix composites reinforced with bimodal-sized TiCp compared with monomodal-sized TiCp. Mater. Sci. Eng. A 2018, 724, 368–375. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Z.W.; Song, Y.G.; Wang, B.; Zhu, Y.L.; Qiu, F.; Zhao, Q.L.; Jiang, Q.C. Synergistic effects of the TiC nanoparticles and cold rolling on the microstructure and mechanical properties of Al–Cu strips fabricated by twin-roll casting. Mater. Sci. Eng. A 2021, 812, 141110. [Google Scholar] [CrossRef]
- Whelan, M.J. On the Kinetics of Precipitate Dissolution. Met. Sci. J. 1968, 2, 192–193. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Luo, X.; Zhou, H.; Cai, Q.; Li, M.; Ma, Y. Microstructural evoluation and mechanical properties of 7075 aluminum alloy prepared by controlled diffusion solidification. China Foundry 2019, 16, 238–247. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Wang, Z.; Chen, K.; Tang, W.; Pan, S.; Yang, X.; Qin, J.; Li, S.; Wang, Y. Effect of Cd micro-addition on microstructure and mechanical properties in ternary Al-Si-Cu alloy. J. Alloys Compd. 2021, 851, 156739. [Google Scholar] [CrossRef]
- Ma, P.; Zhan, L.; Liu, C.; Wang, Q.; Li, H.; Liu, D.; Hu, Z. Pre-strain-dependent natural ageing and its effect on subsequent artificial ageing of an Al-Cu-Li alloy. J. Alloys Compd. 2019, 790, 8–19. [Google Scholar] [CrossRef]
- Bourgeois, L.; Dwyer, C.; Weyland, M.; Nie, J.-F.; Muddle, B.C. Structure and energetics of the coherent interface between the θ‘precipitate phase and aluminium in Al-Cu. Acta Mater. 2011, 59, 7043–7050. [Google Scholar] [CrossRef]
- Shen, Z.; Ding, Q.; Liu, C.; Wang, J.; Tian, H.; Li, J.; Zhang, Z. Atomic-scale mechanism of the θ″→θ′ phase transformation in Al-Cu alloys. J. Mater. Sci. Technol. 2017, 10, 95–100. [Google Scholar] [CrossRef]
- Qian, F.; Li, J.; Zhao, D.; Jin, S.; Mørtsell, E.A.; Li, Y. Comparative study of Cd addition effects on precipitation in different aluminium alloys. Mater. Sci. Technol. 2023, 39, 2469–2477. [Google Scholar] [CrossRef]
- Heugue, P.; Larouche, D.; Breton, F.; Martinez, R.; Chen, X.G. Evaluation of the Growth Kinetics of θ’ and h-Al2Cu Precipitates in a Binary Al-3.5 wt per Cu Alloy. Metall. Mater. Trans. A 2019, 50, 3048–3060. [Google Scholar] [CrossRef]
- Ostwald, W. Studies on the formation and transformation of solid bodies. Z. Phys. Chem. 1897, 22, 289–330. [Google Scholar] [CrossRef]





| Composition | Cu | Mn | B | Cd | Zr | Ti | TiB2 | Al |
|---|---|---|---|---|---|---|---|---|
| Designed composition | 5.30 | 0.40 | 0.05 | 0.20 | 0.2 | 0.15 | 1.0 | Balance |
| Actual composition | 5.26 | 0.38 | 0.04 | 0.18 | 0.19 | 0.15 | 1.2 | Balance |
| Solution Treatment | Temperature/°C | Time/h | Quenching Transfer Time/s |
|---|---|---|---|
| First step | 518, 523, 528, 532 | 12 h | ≤10 s |
| Second step | OST | 9, 10, 11, 12, 13, 14 | ≤10 s |
| Composite | Heat Treatment | σs (MPa) | σb (MPa) | El (%) |
|---|---|---|---|---|
| TiB2/Al–Cu–Mn (this study) | 523 °C, 11 h + 535 °C, 1 h 170 °C 12 h | 523.0 | 451.8 | 8.24 |
| TiC/Al–Cu–Mn [37] | 538 °C, 12 h 165 °C, 10 h | 542.0 | 367.0 | 11.0 |
| TiB2/Al–Cu–Li [20] | 500 °C, 32 h + 560 °C 24 h 175 °C 8 h | 302.0 | 206.0 | 3.7 |
| TiB2/Al–Cu–Mn [11] | 535 °C, 2 h 175 °C 15 h | 446.3 | 344.8 | 8.1 |
| TiC/Al–Cu [39] | 535 °C, 1 h 165 °C, 10 h | 401.0 | 245.0 | 13.1 |
| SiC/Al–Cu–Mn [18] | 540 °C, 10 h 180 °C, 10 h | 500.1 | 358.0 | 8.5 |
| Aging Time | Precipitates Type | A/nm2 | Dt/nm | NV/m−3 |
|---|---|---|---|---|
| 0.5 h | θ″ | 52,982.83 | 13.74 | 7.011 × 1022 |
| 3 h | θ′, θ″ | 39,302.58 | 38.20 | 2.482 × 1022 |
| 12 h | θ′, θ″ | 86,010.66 | 64.30 | 4.266 × 1021 |
| 24 h | θ′ | 87,325.47 | 73.59 | 3.872 × 1021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xiang, Z.; Shen, G.; Huang, J.; Sun, W.; Yang, Z.; Han, Y.; Li, L.; Li, M.; Chen, Z. Microstructure Evolution and Mechanical Properties of TiB2/Al–Cu–Mn–Cd Composite with the Adoption of Two-Stage Solution and Aging Treatment. Crystals 2024, 14, 836. https://doi.org/10.3390/cryst14100836
Li J, Xiang Z, Shen G, Huang J, Sun W, Yang Z, Han Y, Li L, Li M, Chen Z. Microstructure Evolution and Mechanical Properties of TiB2/Al–Cu–Mn–Cd Composite with the Adoption of Two-Stage Solution and Aging Treatment. Crystals. 2024; 14(10):836. https://doi.org/10.3390/cryst14100836
Chicago/Turabian StyleLi, Jihao, Zhilei Xiang, Gaoliang Shen, Jingcun Huang, Wenchao Sun, Zian Yang, Yang Han, Leizhe Li, Meng Li, and Ziyong Chen. 2024. "Microstructure Evolution and Mechanical Properties of TiB2/Al–Cu–Mn–Cd Composite with the Adoption of Two-Stage Solution and Aging Treatment" Crystals 14, no. 10: 836. https://doi.org/10.3390/cryst14100836






