Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism
Abstract
:1. Introduction
2. Raw Materials and Experimental Procedure
2.1. Raw Materials
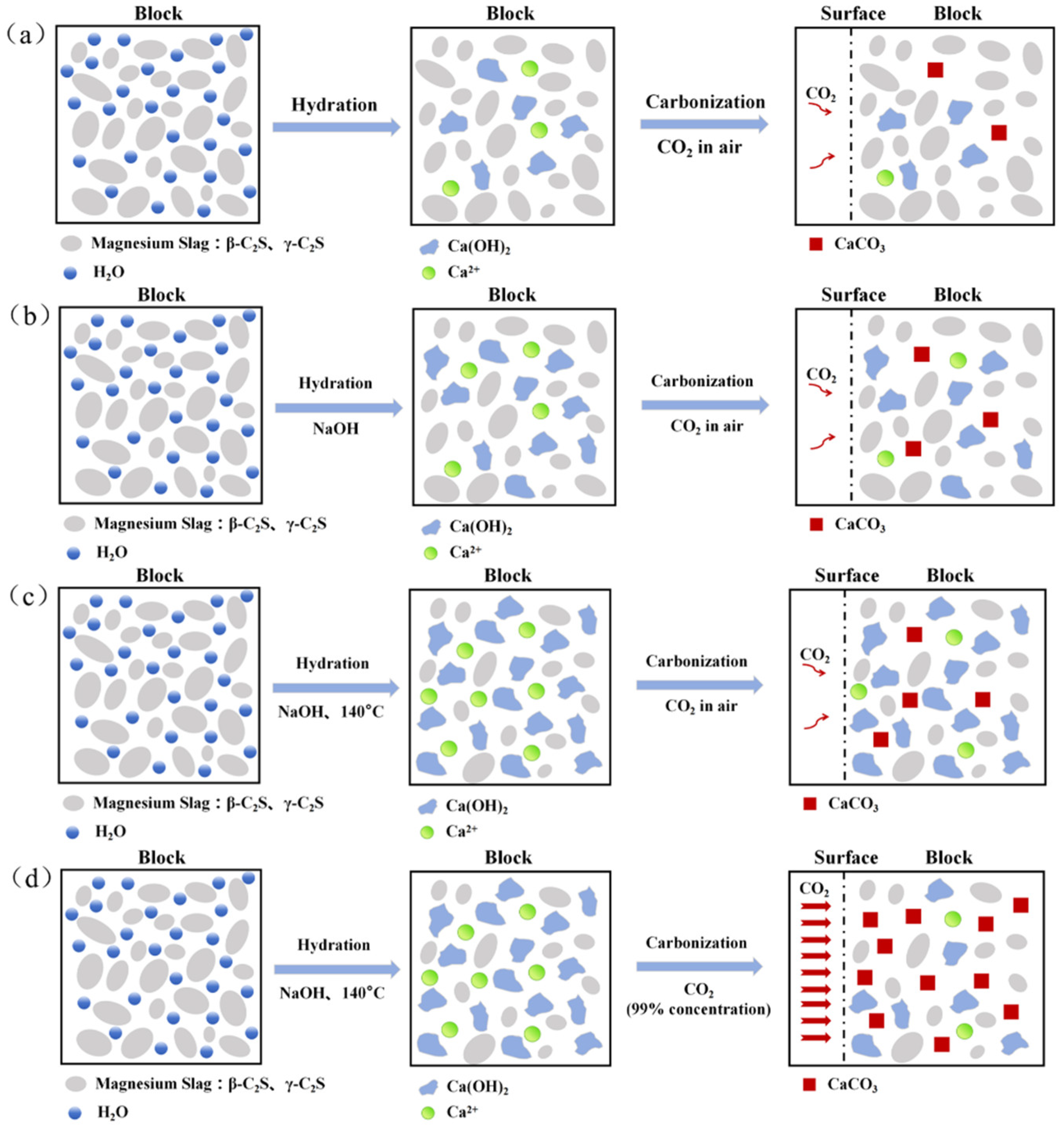
2.2. Specimen Preparation
- (a)
- static treatment at different ambient temperatures: 20 °C or 140 °C for 1 h in air;
- (b)
- carbonized at 140 °C under 99.9% CO2 concentration for 1 h.
2.3. Experimental Methods
2.3.1. X-ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. TG-DSC
2.3.4. Scanning Electron Microscopy (SEM) Imaging
2.3.5. Compressive Strength
3. Results, Analysis, and Discussion
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. TG-DSC Analysis
3.4. Correlation between Compressive Strength and Reaction Products Formed
3.5. SEM Imaging
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, J.; Ou, D.; Yang, Z.; Gao, X.; Zhong, Y.; Yang, W.; Wu, J.; Yang, Y.; Xia, J.; Liu, Y.; et al. Synergizing economic growth and carbon emission reduction in China: A path to coupling the MFLP and PLUS models for optimizing the territorial spatial functional pattern. Sci. Total Environ. 2024, 929, 171926. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wang, C.; Wang, H.; Zhang, F.; Li, Z. Pathways to achieve carbon emission peak and carbon neutrality by 2060: A case study in the Beijing-Tianjin-Hebei region, China. Renew. Sustain. Energy Rev. 2024, 189, 113955. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.Q. Study on the Challenges and Opportunities for Capture and Storage of CO2 as CDM Activities. Sichuan Environ. 2008, 2, 60–63. (In Chinese) [Google Scholar]
- Ou, Z.D. Research progress in emerging carbon dioxide capture technologies. Resour. Recycl. 2023, 9, 40–43. (In Chinese) [Google Scholar]
- Lu, B.; Fan, Y.; Zhi, X.; Han, Z.; Wu, F.; Li, X.; Luo, C.; Zhang, L. Material design and prospect of dual-functional materials for integrated carbon dioxide capture and conversion. Carbon Capture Sci. Technol. 2024, 12, 100207. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R. A critical review on new and efficient adsorbents for CO2 capture. Chem. Eng. J. 2024, 485, 149495. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Li, Z.; Xing, Y.; Ma, M.; Su, W.; Cui, Y.; Tian, J.; Fei, F. Towards the co-benefits of carbon capture, utilization and sequestration: A life cycle assessment study for steel slag disposal. J. Clean. Prod. 2024, 443, 141166. [Google Scholar] [CrossRef]
- He, D.; Chen, M.; Liu, H.; Wang, J. Preparation of activated electrolytic manganese residue-slag-cement ternary blended cementitious material: Hydration characteristics and carbon reduction potential. Constr. Build. Mater. 2024, 425, 135990. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Du, H.; Ma, B.; Tang, C.; Ni, W.; Yan, X.; Zhang, H. Performance optimization and carbon reduction effect of solid waste-based cementitious materials from iron and steel metallurgical slags and ammonia-soda residue. Chem. Eng. J. Adv. 2024, 17, 100584. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Du, Z.; Hou, H.; Zheng, Y. Research on Utilizable Calcium from Calcium Carbide Slag with Different Extractors and Its Effect on CO2 Mineralization. Materials 2024, 17, 1068. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.A.; Fayaz, H.; Saleel, C.A. Developments in mineral carbonation for Carbon sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, H.H.; Ni, W.; Fu, P.F.; Wang, F. Ratio Optimization and Hydration Characteristics of Kr Desulfurization Slag Alkali-Activated Granulated Blast-Furnace Slag. Bull. Chin. Ceram. Soc. 2023, 42, 170–179. (In Chinese) [Google Scholar]
- Miyan, N.; Omur, T.; Kabay, N.; Birol, B. The potential usage of waste ferrochrome slag in alkali-activated mixes. J. Build. Eng. 2023, 75, 107026. [Google Scholar] [CrossRef]
- LI, P.; Tang, J.; Bai, Y.; Chen, X.; Chen, J. Experimental study on the pH for activating ground granulated blast-furnace slag activity at different temperatures. Sādhanā 2019, 44, 213. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Ma, Y.; Ji, T. Effect of hydration assemblage on the autogenous shrinkage of alkali-activated slag mortars. Mag. Concr. Res. 2023, 75, 447–463. [Google Scholar] [CrossRef]
- Kumar Sinha, A.; Talukdar, S. Mechanical and bond behaviour of high volume Ultrafine-slag blended fly ash based alkali activated concrete. Constr. Build. Mater. 2023, 383, 131368. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wu, A.; Shi, D.; Zhang, M.; Ruan, Z.; Wang, S. Study on the performance of alkali-activated phosphorus slag cemented paste backfill material: Effect of activator type and amount. Constr. Build. Mater. 2024, 425, 136036. [Google Scholar] [CrossRef]
- Xiang, J.; He, Y.; Cui, X.; Liu, L. Enhancement of setting times and hardening of alkali-activated slag binder using CO2-modified slag. Cem. Concr. Compos. 2022, 134, 104797. [Google Scholar] [CrossRef]
- Coffetti, D.; Candamano, S.; Crea, F.; Coppola, L. On the role of alkali content on one-part alkali activated slag pastes produced with tri-blend solid activators. Constr. Build. Mater. 2023, 409, 133868. [Google Scholar] [CrossRef]
- Cota, T.G.; Brigolini, G.J.; Lima, R.M.F.; Reis, É.L. Alkaline activation for production of slag-based binders from the manufacture of manganese ferroalloys. J. Build. Eng. 2023, 79, 107842. [Google Scholar] [CrossRef]
- Lu, G.; Han, J.; Ma, Z. Experimental design of alkali activated cementitious properties of magnesium slag. Exp. Technol. Manag. 2022, 39, 31–36+47. (In Chinese) [Google Scholar]
- Hamdy, A.S.; Butt, D.P. Corrosion mitigation of rare-earth metals containing magnesium EV31A-T6 alloy via chrome-free conversion coating treatment. Electrochim. Acta 2013, 108, 852–859. [Google Scholar] [CrossRef]
- Wang, H.; Luo, X.C.; Zhang, D.T.; Qiu, C.; Chen, D.L. High-strength extruded magnesium alloys: A critical review. J. Mater. Sci. Technol. 2024, 199, 27–52. [Google Scholar] [CrossRef]
- Bai, J.; Yang, Y.; Wen, C.; Chen, J.; Zhou, G.; Jiang, B.; Peng, X.; Pan, F. Applications of magnesium alloys for aerospace: A review. J. Magnes. Alloys 2023, 11, 3609–3619. [Google Scholar] [CrossRef]
- He, M.; Yang, L.; He, Q.; Zhang, M.; Xian, G.; Liu, W. Comparative study on the corrosion resistance of Al, AlTiSi and AlTiSiN coated Mg-Gd-Y magnesium alloy. Mater. Lett. 2024, 359, 135945. [Google Scholar] [CrossRef]
- Gao, M.; Dai, J.; Jing, H.; Ye, W.; Sesay, T. Investigation of the performance of cement-stabilized magnesium slag as a road base material. Constr. Build. Mater. 2023, 403, 133065. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, W.; Zhang, S.; Hou, G.; Lu, B. Preparation of aragonite whisker-rich materials by wet carbonation of magnesium slag: A sustainable approach for CO2 sequestration and reinforced cement. Constr. Build. Mater. 2024, 418, 135429. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, L.; He, W.; Han, K.; Lan, H.; Gao, Y.; Sun, W.; Han, Z.; Xia, L.; Yang, P. Strength characteristics and carbonation depth evolution of modified magnesium slag based solid waste storage backfill materials. J. Environ. Chem. Eng. 2024, 12, 111975. [Google Scholar] [CrossRef]
- Sun, H.; Pan, G.; Lu, X.; Iqbal, S.; Meng, H. The in-situ grown nano-γ-Al2O3@FA composite synthesized by carbonization method improves the properties of gypsum slag cement. Constr. Build. Mater. 2024, 426, 136172. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Y.; Zhou, Y.; Wei, W.; Wang, Y. Municipal solid waste incineration bottom ash recycling assessment: Carbon emission analysis of bottom ash applied to pavement materials. Constr. Build. Mater. 2024, 421, 135774. [Google Scholar] [CrossRef]
- Sun, R.; Wu, Z.J.; Wang, D.M.; Ding, Y.; Fang, K.Z. Properties and Hydration Mechanism of Ultrafine Magnesium Slag Powder-CementComposites Cementitious Materials. Mater. Rep. 2023, 37, 98–108. (In Chinese) [Google Scholar]
- Jin, K.; Zhou, X.; Wang, D.; Bi, W.; Lu, Y.; Wang, J. Performance of cementitious materials prepared with magnesium slag and concrete slurry waste. J. Build. Eng. 2024, 89, 109379. [Google Scholar] [CrossRef]
- Ye, J.; Liu, S.; Fang, J.; Zhang, H.; Zhu, J.; Guan, X. Effect of temperature on wet carbonation products of magnesium slag. Constr. Build. Mater. 2024, 425, 135949. [Google Scholar] [CrossRef]
- Lei, M.; Deng, S.; Huang, K.; Liu, Z.; Wang, F.; Hu, S. Preparation and characterization of a CO2 activated aerated concrete with magnesium slag as carbonatable binder. Constr. Build. Mater. 2022, 353, 129112. [Google Scholar] [CrossRef]
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. People’s Republic of China Industry Standard: Beijing, China, 2009.
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; LI, L.; Wang, J.; Shao, N.; Wang, D. Microstructure and phase evolution of alkali-activated steel slag during early age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Feng, J.; Sun, J. A comparison of the 10-year properties of converter steel slag activated by high temperature and an alkaline activator. Constr. Build. Mater. 2020, 234, 116948. [Google Scholar] [CrossRef]
- Li, W.; Cao, M.; Liu, F.; Wang, D.; Chang, J. Pretreatment of alkali activation and carbonation of steel slag for using as binding material. Cem. Concr. Compos. 2024, 149, 105521. [Google Scholar] [CrossRef]
- Šauman, Z. Carbonization of porous concrete and its main binding components. Cem. Concr. Res. 1971, 1, 645–662. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Ye, J.; Ren, X.; Liu, L.; Shen, W. Influence of alkaline carbonates on the hydration characteristics of β-C2S. Constr. Build. Mater. 2021, 296, 123661. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Wang, Y.; Chen, J.; Qi, C. The carbon uptake and mechanical property of cemented paste backfill carbonation curing for low concentration of CO2. Sci. Total Environ. 2022, 852, 158516. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, W.; Ye, J.; Li, J.; Lu, Z.; Ren, X. Mechanism of interaction between hydration–carbonation of C2S. Constr. Build. Mater. 2024, 412, 134891. [Google Scholar] [CrossRef]
- Shi, T.; Yang, Z.P.; Zheng, L.W. FTIR spectra for early age hydration of cement-basedcomposites incorporatted with CNTs. Acta Mater. Compos. Sin. 2017, 34, 653–660. (In Chinese) [Google Scholar]
- Chen, H.M.; Qin, Z.G.; Chen, J.; Zhang, Y.D.; Wu, P. Experimental study on drying shrinkage and microscopic characteristics of borax-sodium silicate alkali-activated slag mortar. Exp. Technol. Manag. 2024, 41, 25–31. (In Chinese) [Google Scholar]
- Fang, Y.; Chang, J. Rapid hardening β-C2S mineral and microstructure changes activated by accelerated carbonation curing. J. Therm. Anal. Calorim. 2017, 129, 681–689. [Google Scholar] [CrossRef]
- Khan, R.I.; Ashraf, W.; Olek, J. Amino acids as performance-controlling additives in carbonation-activated cementitious materials. Cem. Concr. Res. 2021, 147, 106501. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Banthia, N.; Zhang, Y.; Zhang, Z. Interpreting the early-age reaction process of alkali-activated slag by using_combined embedded ultrasonic measurement, thermal analysis, XRD, FTIR_and SEM. Compos. Part B 2020, 186, 107840. [Google Scholar] [CrossRef]
- Hansen, M.R.; Jakobsen, H.J.; Skibsted, J. 29Si chemical shift anisotropies in calcium silicates from high-field 29Si MAS NMR spectroscopy. Inorg. Chem. 2003, 42, 2368–2377. [Google Scholar] [CrossRef]
- Gupta, A.; Armatis, P.D.; Sabharwall, P.; Fronk, B.M.; Utgikar, V. Energy and exergy analysis of Ca(OH)2/CaO dehydration-hydration chemical heat pump system: Effect of reaction temperature. J. Energy Storage 2021, 39, 102633. [Google Scholar] [CrossRef]
- Xu, R.; Kong, F.; Yang, R.; Wang, H.; Hong, T. Influences of silicate modulus and alkali content on macroscopic properties and microstructure of alkali-activated blast furnace slag-copper slag. Constr. Build. Mater. 2024, 442, 137622. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; He, W.; Zhu, M.; Xia, L.; Yang, P.; Liu, D.; He, J. Carbonation curing of magnesium-coal slag solid waste backfill material: Study on properties of flow, mechanics and carbon sequestration. Case Stud. Constr. Mater. 2024, 20, e03204. [Google Scholar] [CrossRef]
- Jun, Y.; Han, S.H.; Kim, J.H. Performance of CO2-cured alkali-activated blast-furnace slag incorporating magnesium oxide. Constr. Build. Mater. 2024, 429, 136462. [Google Scholar] [CrossRef]
- Zhong, X.; Li, L.; Jiang, Y.; Ling, T. Elucidating the dominant and interaction effects of temperature, CO2 pressure and carbonation time in carbonating steel slag blocks. Constr. Build. Mater. 2021, 302, 124158. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Tang, P.; Guan, X.; Shi, C. Enhancing the hardening properties and microstructure of magnesium slag blocks by carbonation-hydration sequential curing. J. Build. Eng. 2023, 76, 107414. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, S.M.; Jun, Y.; Han, T.H.; Kim, J.H. Carbon-captured sodium hydroxide solution for sustainable alkali-activated slag. Sustain. Mater. Technol. 2024, 40, e00915. [Google Scholar] [CrossRef]
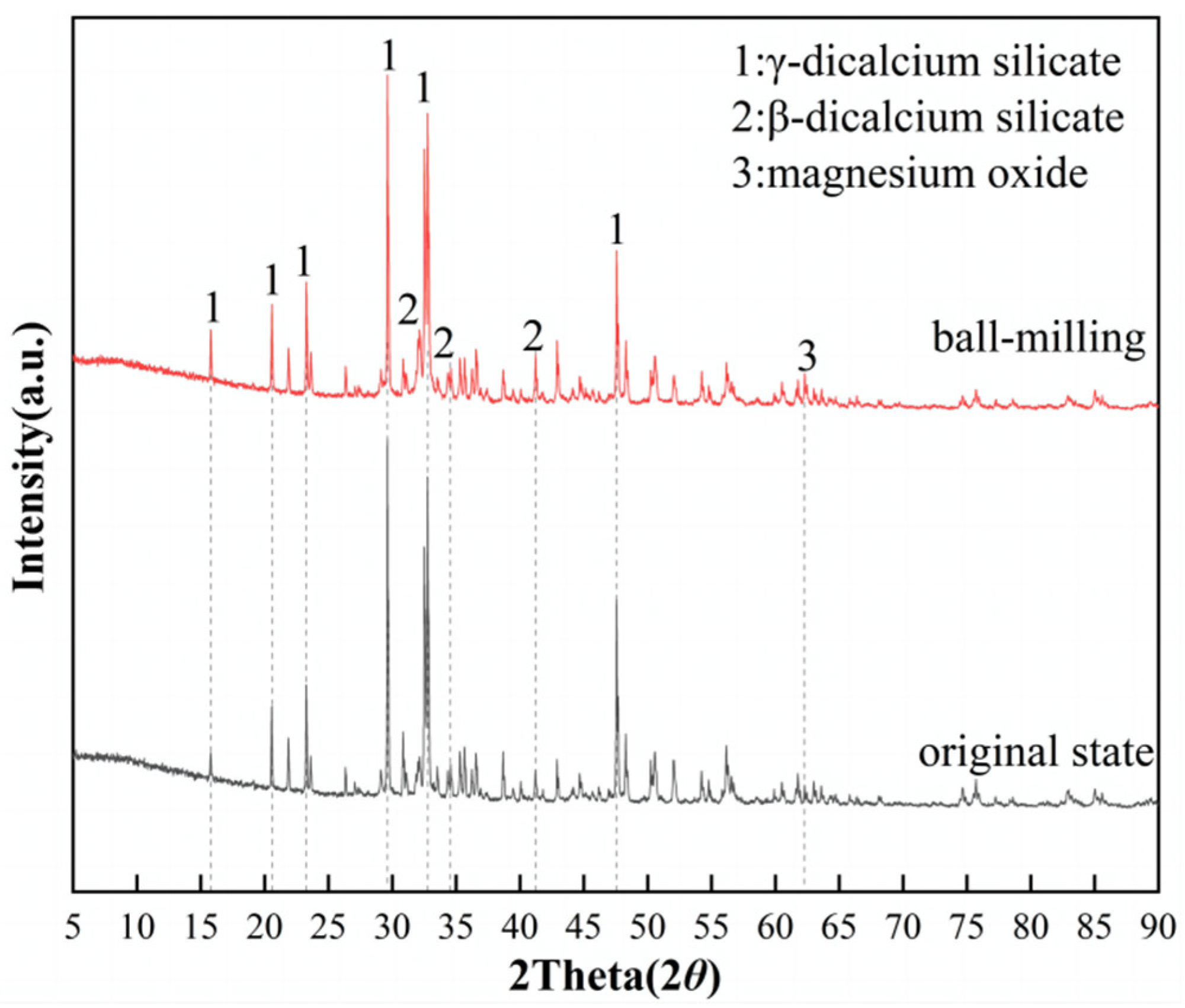
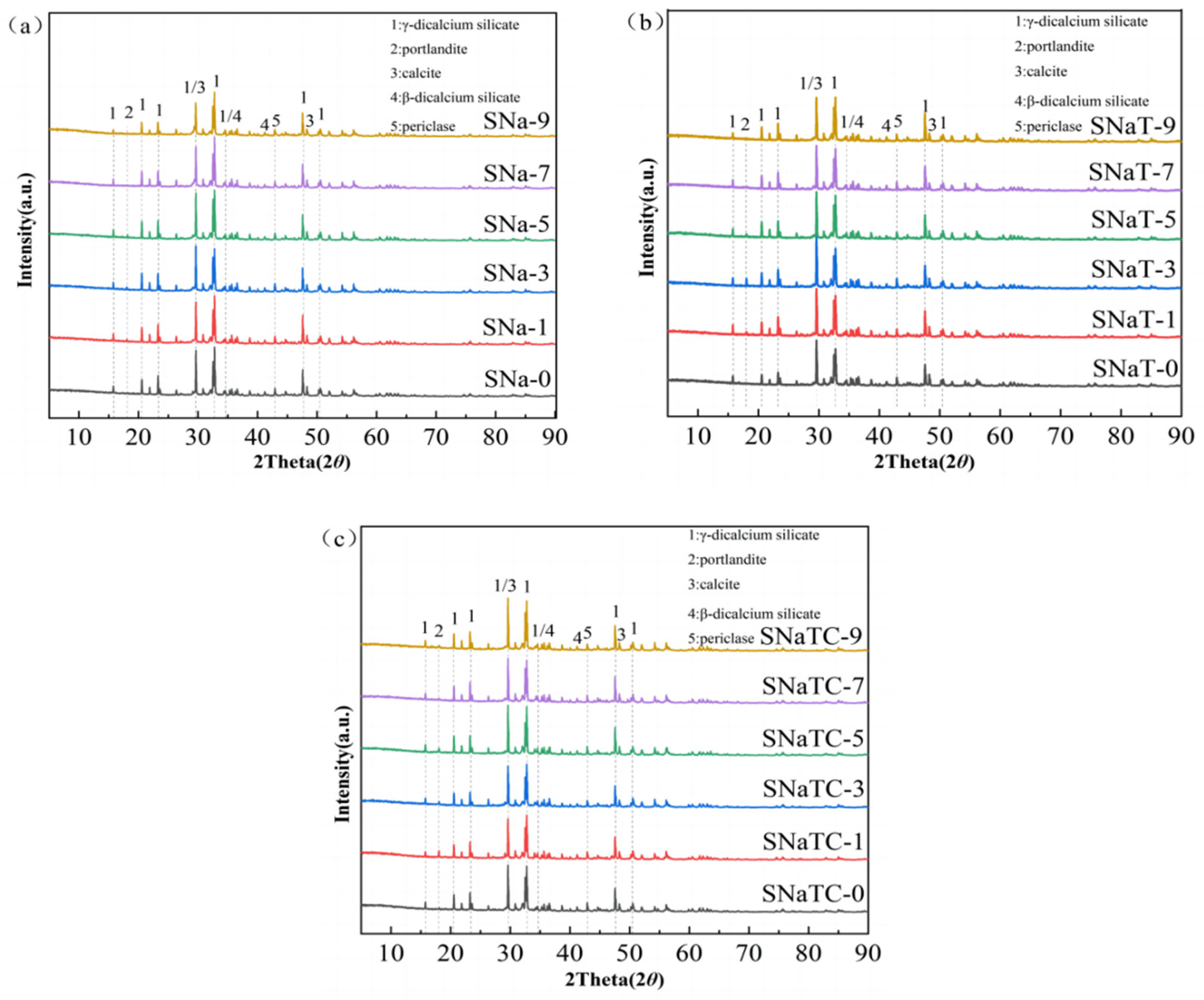
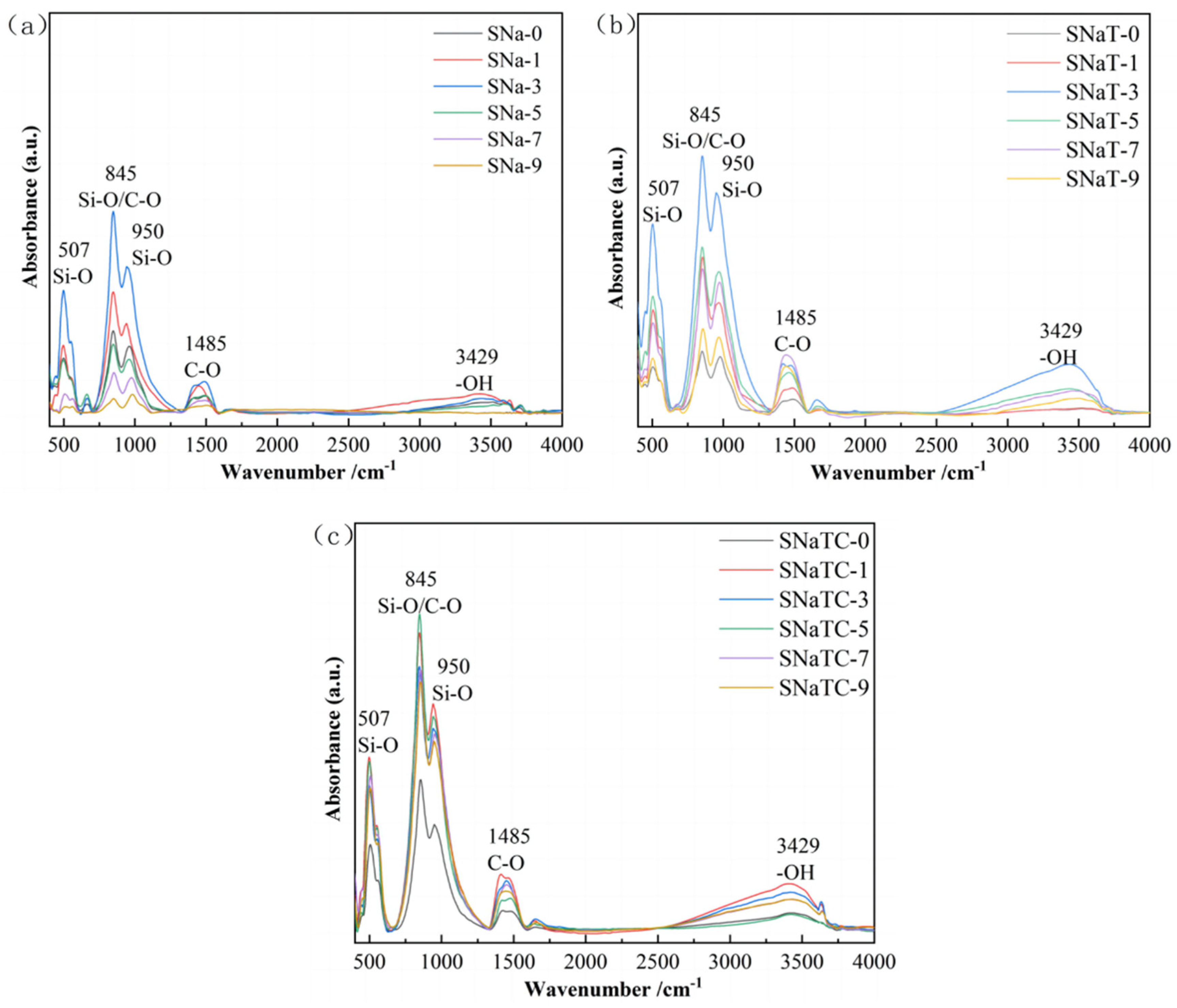

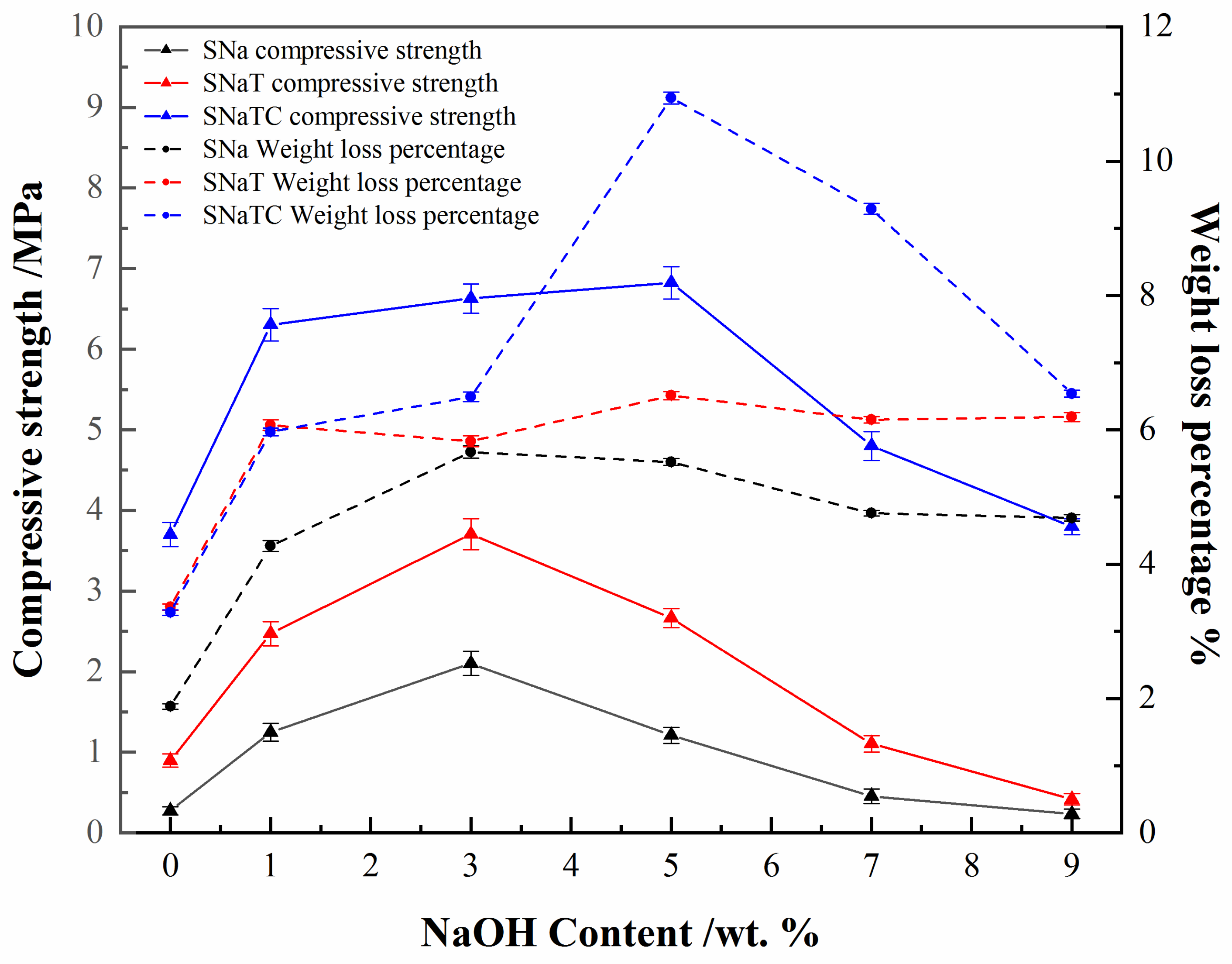

| Composition | Na2O | MgO | Al2O3 | SiO2 | P2O5 | CaO | TiO2 |
|---|---|---|---|---|---|---|---|
| wt% | 0.14 | 5.20 | 0.87 | 36.50 | 0.05 | 53.17 | 0.04 |
| Nomenclature | SNa | SNaT | SNaTC | |
|---|---|---|---|---|
| Conditions | ||||
| NaOH content within slag, wt% | 0, 1, 3, 5, 7, 9 | 0, 1, 3, 5, 7, 9 | 0, 1, 3, 5, 7, 9 | |
| Temperature, °C | 20 | 140 | 140 | |
| CO2 concentration, % | 0 | 0 | 99.9 | |
| NaOH Content (wt%) | 0 | 1 | 3 | 5 | 7 | 9 | |
|---|---|---|---|---|---|---|---|
| Conditions | |||||||
| SNa | 0.70 | 1.03 | 1.20 | 0.93 | 1.33 | 1.21 | |
| SNaT | 0.98 | 1.60 | 1.32 | 1.17 | 1.73 | 1.72 | |
| SNaTC | 1.20 | 1.21 | 2.00 | 4.74 | 3.29 | 1.55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Zhai, R.; Zhu, M.; He, J. Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism. Crystals 2024, 14, 847. https://doi.org/10.3390/cryst14100847
Zhu M, Zhai R, Zhu M, He J. Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism. Crystals. 2024; 14(10):847. https://doi.org/10.3390/cryst14100847
Chicago/Turabian StyleZhu, Miaomiao, Ruoxin Zhai, Mingming Zhu, and Jiabei He. 2024. "Evaluating Alkali Activation in Magnesium Slag Carbonization and Its Mechanism" Crystals 14, no. 10: 847. https://doi.org/10.3390/cryst14100847






