Tailoring the Magnetic and Hyperthermic Properties of Biphase Iron Oxide Nanocubes through Post-Annealing
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
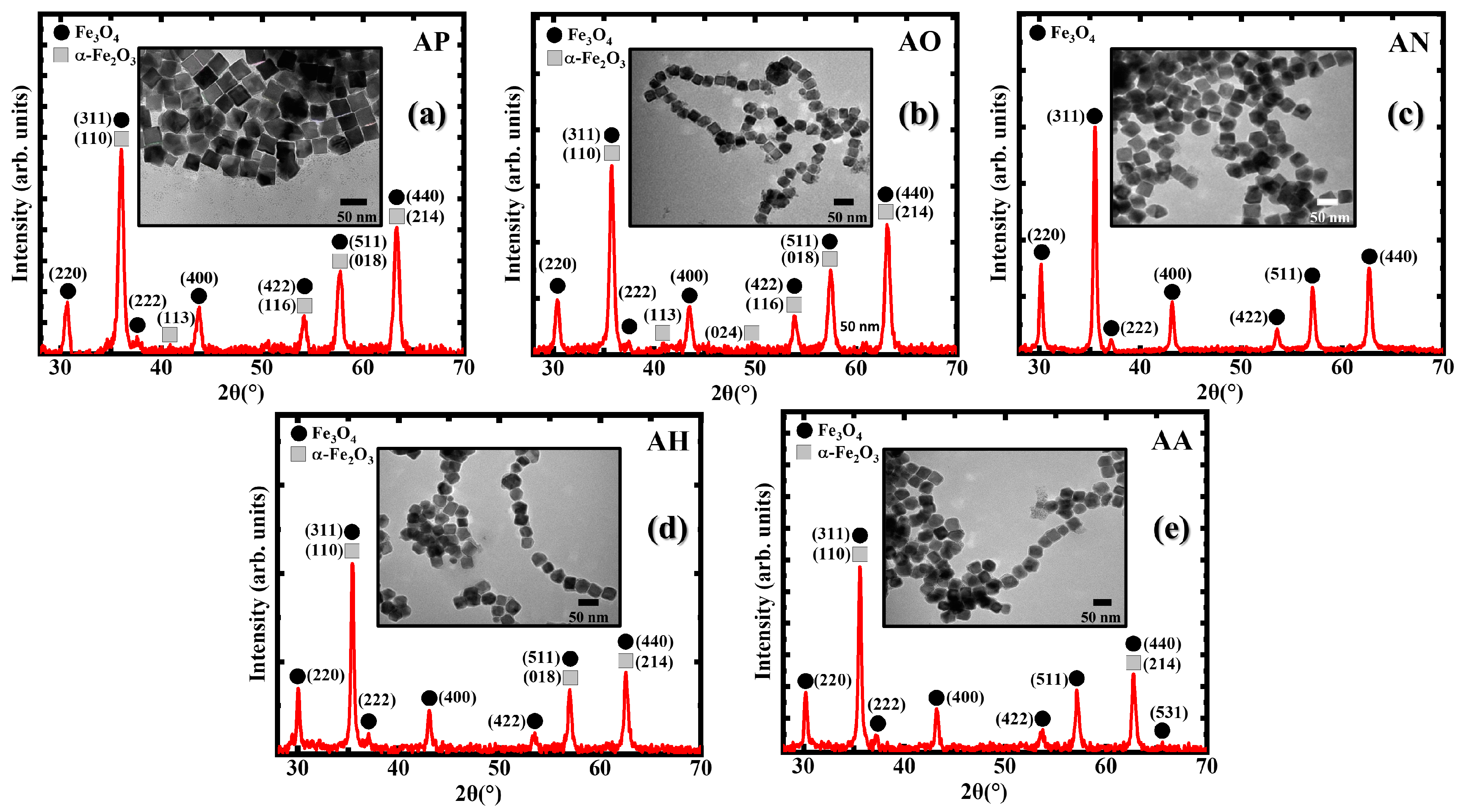
3.1. Structural Characterization
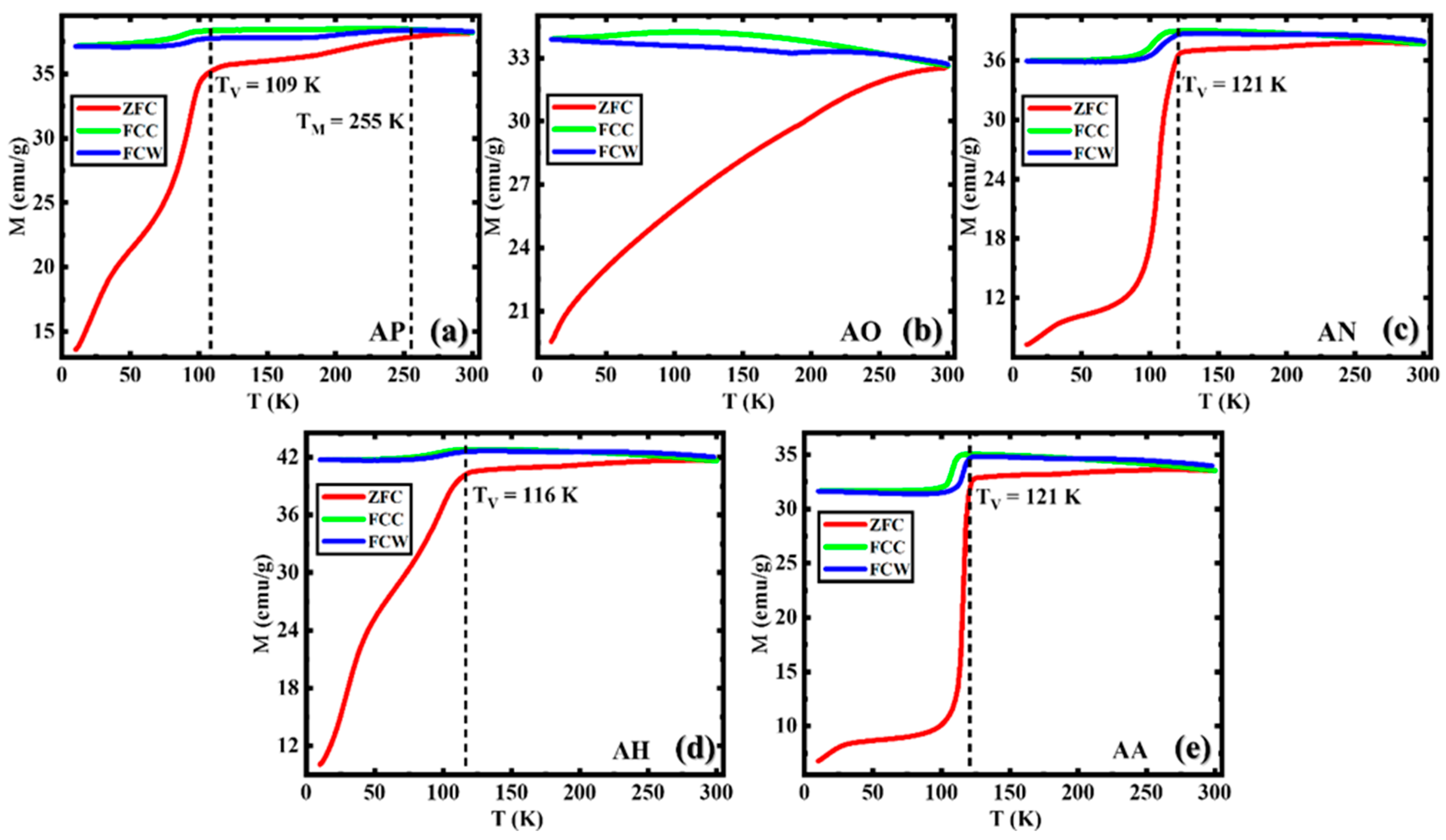
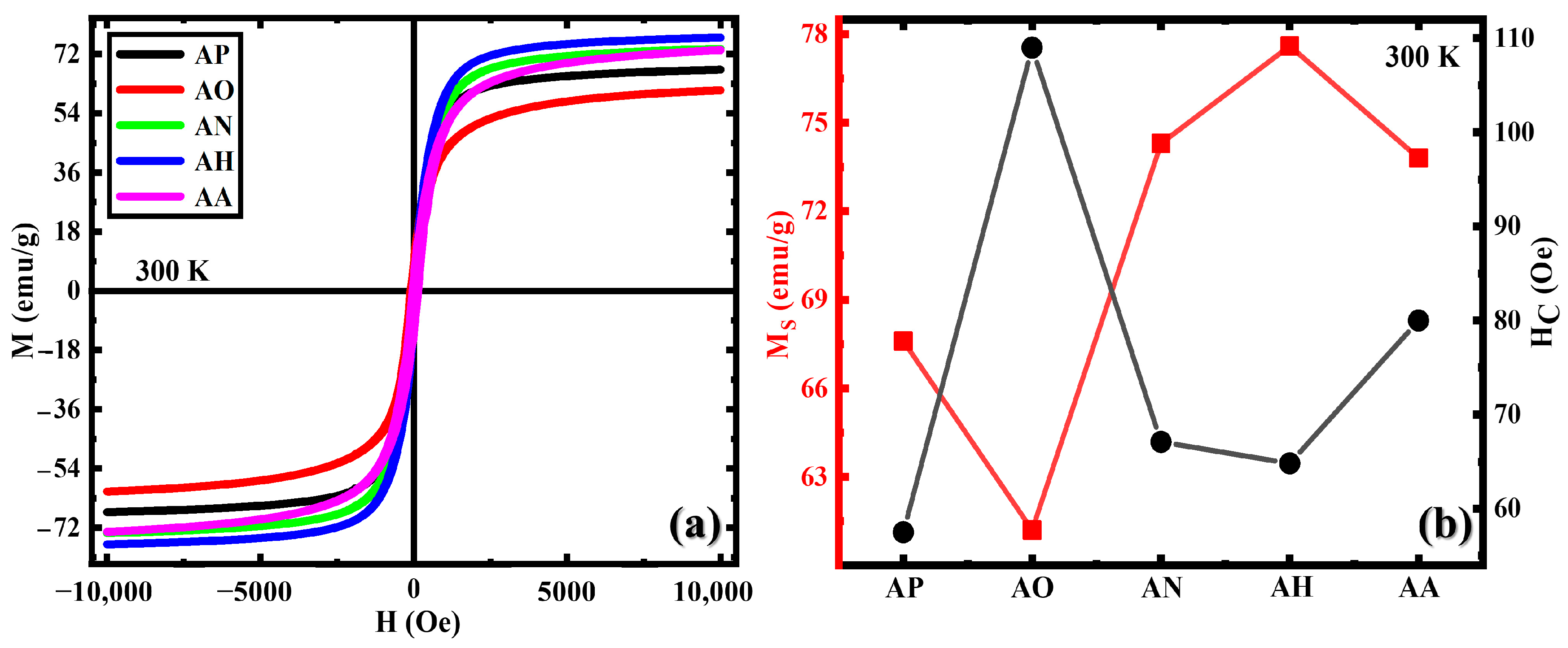
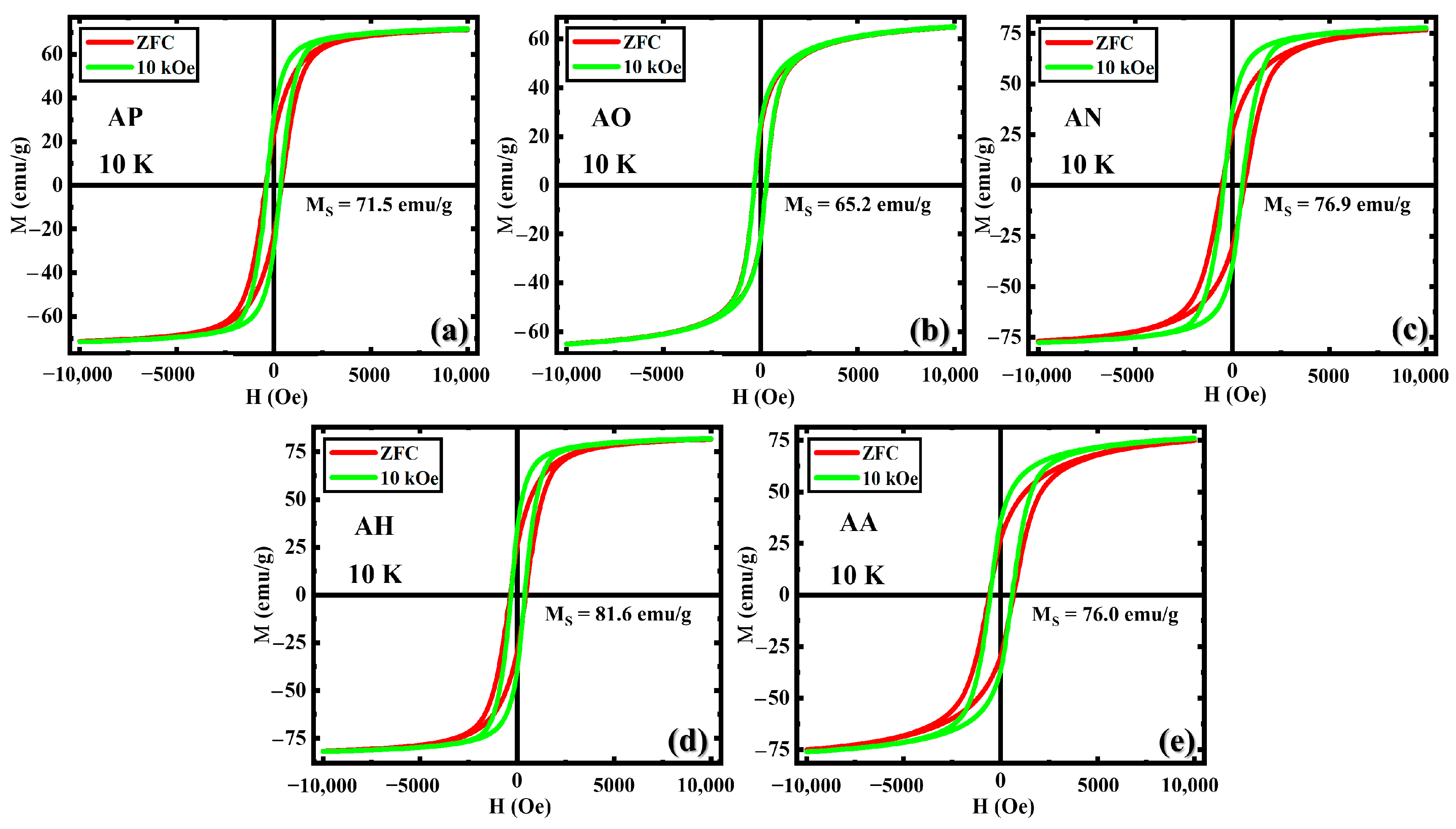
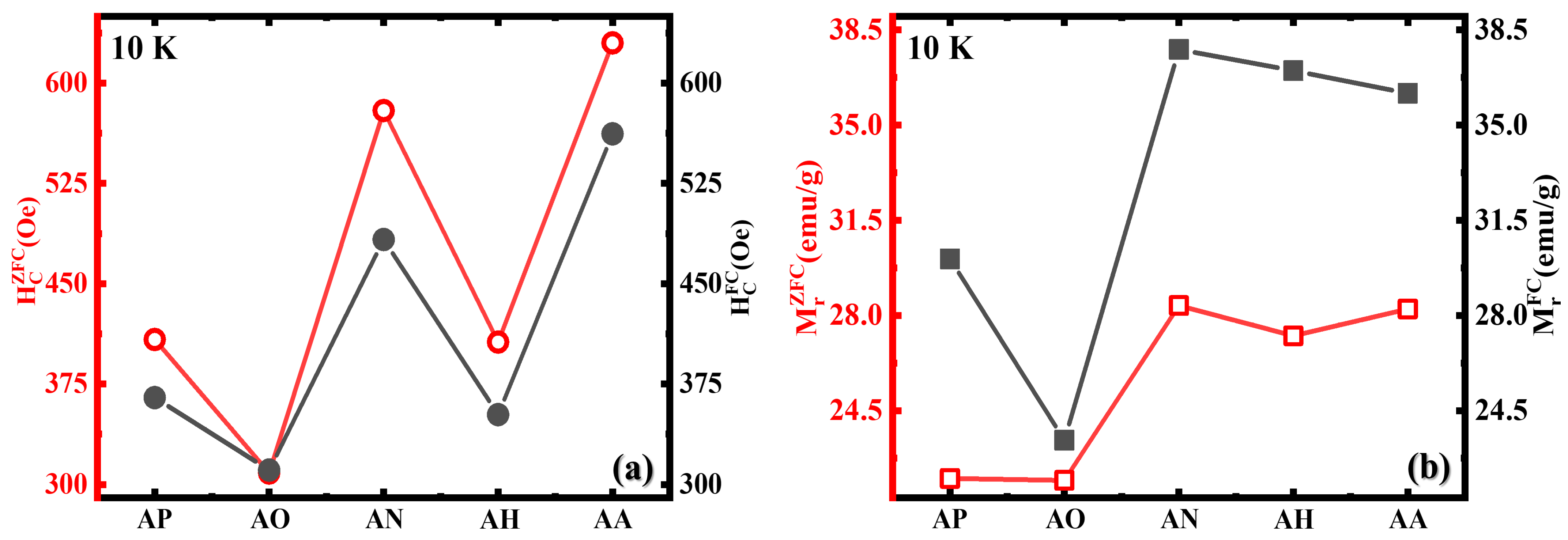
3.2. Magnetic Properties
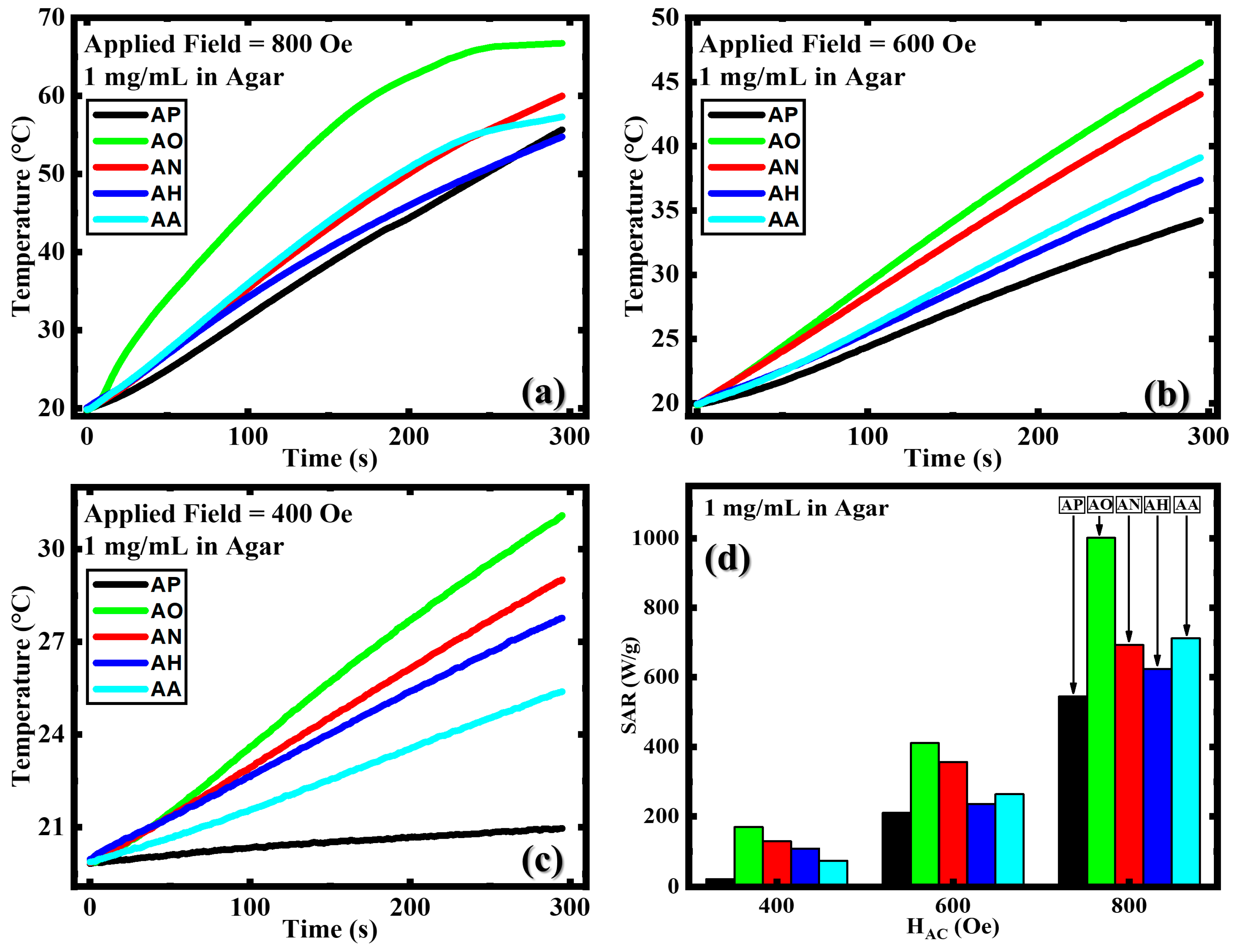
3.3. Magnetic Hyperthermia
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic Nanoparticles in Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, G.C.; Das, R.; Alonso Masa, J.; Phan, M.H.; Srikanth, H. Hybrid Magnetic Nanoparticles as Efficient Nanoheaters in Biomedical Applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef] [PubMed]
- Kons, C.; Krycka, K.L.; Robles, J.; Ntallis, N.; Pereiro, M.; Phan, M.H.; Srikanth, H.; Borchers, J.A.; Arena, D.A. Influence of Hard/Soft Layer Ordering on Magnetization Reversal of Bimagnetic Nanoparticles: Implications for Biomedical/Theranostic Applications. ACS Appl. Nano Mater. 2023, 6, 10986–11000. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Nemati Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.Á.; Sanchez Llamazares, J.L.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Das, R.; Xing, Y.; Robles, J.; Litterst, F.J.; Baggio-Saitovitch, E.; Phan, M.H.; Srikanth, H. Origin and Shell-Driven Optimization of the Heating Power in Core/Shell Bimagnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 1755–1765. [Google Scholar] [CrossRef]
- Chandra, S.; Das, R.; Kalappattil, V.; Eggers, T.; Harnagea, C.; Nechache, R.; Phan, M.H.; Rosei, F.; Srikanth, H. Epitaxial Magnetite Nanorods with Enhanced Room Temperature Magnetic Anisotropy. Nanoscale 2017, 9, 7858–7867. [Google Scholar] [CrossRef]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.Á.; Phan, M.H.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, 1902626. [Google Scholar] [CrossRef]
- Thong, P.Q.; Huong, L.T.T.; Tu, N.D.; Nhung, H.T.M.; Khanh, L.; Manh, D.H.; Nam, P.H.; Phuc, N.X.; Alonso, J.; Qiao, J.; et al. Multifunctional Nanocarriers of Fe3O4@PLA-PEG/Curcumin for MRI, Magnetic Hyperthermia and Drug Delivery. Nanomedicine 2022, 17, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Mohapatra, J.; Silva, E.; He, G.; Gong, L.; Lyu, T.; Madhogaria, R.P.; Zhao, X.; Cheng, Y.; Al-Enizi, A.M.; et al. Metal–Organic Framework as a New Type of Magnetothermally-Triggered On-Demand Release Carrier. Small 2023, 20, 2306940. [Google Scholar] [CrossRef] [PubMed]
- Denmark, D.J.; Hyde, R.H.; Gladney, C.; Phan, M.H.; Bisht, K.S.; Srikanth, H.; Mukherjee, P.; Witanachchi, S. Photopolymerization-Based Synthesis of Iron Oxide Nanoparticle Embedded PNIPAM Nanogels for Biomedical Applications. Drug Deliv. 2017, 24, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, S.B.; Chanda, A.; Das, R.; Kapuruge, N.; Gutierrez, H.R.; Phan, M.H.; Srikanth, H. Emergent Magnetism and Exchange Bias Effect in Iron Oxide Nanocubes with Tunable Phase and Size. J. Phys. Condens. Matter 2022, 34, 495301. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.M.; Srikanth, H. Anisotropy Effects in Magnetic Hyperthermia: A Comparison between Spherical and Cubic Exchange-Coupled FeO/Fe3O4 Nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Keren-Khadmy, N.; Zarivach, R. From Invagination to Navigation: The Story of Magnetosome-Associated Proteins in Magnetotactic Bacteria. Protein Sci. 2016, 25, 338–351. [Google Scholar] [CrossRef]
- Kralj, S.; Marchesan, S. Bioinspired Magnetic Nanochains for Medicine. Pharmaceutics 2021, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Cai, T.Y.; Lu, H.S.; Gong, C. De Pressure-Induced Crystal Structure and Spin-State Transitions in Magnetite (Fe3O4). J. Am. Chem. Soc. 2012, 134, 13780–13786. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, D.H. Chapter 1. The crystal chemistry and structure of oxide minerals as exemplified by the Fe-Ti oxides. In Oxide Minerals; De Gruyter: Berlin, Germany, 1976; pp. 1–60. [Google Scholar] [CrossRef]
- Tokoro, H.; Fujii, S.; Oku, T. Iron Nanoparticles Coated with Boron Nitride Nanolayers Synthesized by a Solid Phase Reaction. IEEE Trans. Magn. 2003, 39, 2761–2763. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wang, H.; Yang, Y.; Li, Y. Low-Temperature Activation of Hematite Nanowires for Photoelectrochemical Water Oxidation. ChemSusChem 2014, 7, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Rout, K.; Mohapatra, M.; Layek, S.; Dash, A.; Verma, H.C.; Anand, S. The Influence of Precursors on Phase Evolution of Nano Iron Oxides/Oxyhydroxides: Optical and Magnetic Properties. New J. Chem. 2014, 38, 3492–3506. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R.; et al. Asymmetric Assembling of Iron Oxide Nanocubes for Improving Magnetic Hyperthermia Performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, R.; Durand-Dubief, M.; Chebbi, I.; Mandawala, C.; Lagroix, F.; Valet, J.P.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; et al. Enhanced Antitumor Efficacy of Biocompatible Magnetosomes for the Magnetic Hyperthermia Treatment of Glioblastoma. Theranostics 2017, 7, 4618. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Chebbi, I.; Guyot, F.; Durand-Dubief, M. Use of Bacterial Magnetosomes in the Magnetic Hyperthermia Treatment of Tumours: A Review. Int. J. Hyperth. 2013, 29, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and Its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Walz, F. The Verwey Transition—A Topical Review. J. Phys. Condens. Matter 2002, 14, R285. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J.; Moskowitz, B.M. Changes in Remanence, Coercivity and Domain State at Low Temperature in Magnetite. Earth Planet. Sci. Lett. 2002, 194, 343–358. [Google Scholar] [CrossRef]
- Zheng, H.; Schenk, J.; Spreitzer, D.; Wolfinger, T.; Daghagheleh, O. Review on the Oxidation Behaviors and Kinetics of Magnetite in Particle Scale. Steel Res. Int. 2021, 92, 2000687. [Google Scholar] [CrossRef]
- Anupama, A.V.; Keune, W.; Sahoo, B. Thermally Induced Phase Transformation in Multi-Phase Iron Oxide Nanoparticles on Vacuum Annealing. J. Magn. Magn. Mater. 2017, 439, 156–166. [Google Scholar] [CrossRef]
- Attanayake, S.B.; Chanda, A.; Das, R.; Phan, M.H.; Srikanth, H. Effects of Annealing Temperature on the Magnetic Properties of Highly Crystalline Biphase Iron Oxide Nanorods. AIP Adv. 2023, 13, 025333. [Google Scholar] [CrossRef]
- Attanayake, S.B.; Chanda, A.; Hulse, T.; Das, R.; Phan, M.-H.; Srikanth, H. Competing Magnetic Interactions and Field-Induced Metamagnetic Transition in Highly Crystalline Phase-Tunable Iron Oxide Nanorods. Nanomaterials 2023, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Zhou, X.D.; Yelon, W.B.; James, W.J.; Cai, Q.; Gopalakrishnan, K.V.; Malik, S.K.; Sun, X.C.; Nikles, D.E. Magnetic and Structural Studies of the Verwey Transition in Fe3−δO4 Nanoparticles. J. Appl. Phys. 2004, 95, 7540. [Google Scholar] [CrossRef]
- Schmitz-Antoniak, C.; Schmitz, D.; Warland, A.; Darbandi, M.; Haldar, S.; Bhandary, S.; Sanyal, B.; Eriksson, O.; Wende, H. Suppression of the Verwey Transition by Charge Trapping. Ann. Phys. 2018, 530, 1700363. [Google Scholar] [CrossRef]
- Attanayake, S.B.; Chanda, A.; Das, R.; Phan, M.H.; Srikanth, H. Emergent Magnetic Properties of Biphase Iron Oxide Nanorods. AIP Adv. 2022, 12, 035136. [Google Scholar] [CrossRef]
- Gaviría, J.P.; Bohé, A.; Pasquevich, A.; Pasquevich, D.M. Hematite to Magnetite Reduction Monitored by Mössbauer Spectroscopy and X-Ray Diffraction. Phys. B Condens. Matter 2007, 389, 198–201. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhu, Y.J.; Ruan, M.L. Microwave-Assisted Synthesis and Magnetic Property of Magnetite and Hematite Nanoparticles. J. Nanopart. Res. 2007, 9, 419–426. [Google Scholar] [CrossRef]
- Artman, J.O.; Murphy, J.C.; Foner, S. Magnetic Anisotropy in Antiferromagnetic Corundum-Type Sesquioxides. Phys. Rev. 1965, 138, A912. [Google Scholar] [CrossRef]
- Mitra, S.; Das, S.; Basu, S.; Sahu, P.; Mandal, K. Shape- and Field-Dependent Morin Transitions in Structured α-Fe2O3. J. Magn. Magn. Mater. 2009, 321, 2925–2931. [Google Scholar] [CrossRef]
- Mørup, S.; Madsen, D.E.; Frandsen, C.; Bahl, C.R.H.; Hansen, M.F. Experimental and Theoretical Studies of Nanoparticles of Antiferromagnetic Materials. J. Phys. Condens. Matter 2007, 19, 213202. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J.; Berquó, T.S. Morin Transition in Hematite: Size Dependence and Thermal Hysteresis. Geochem. Geophys. Geosystems 2008, 9, 10–11. [Google Scholar] [CrossRef]
- Shimomura, N.; Pati, S.P.; Sato, Y.; Nozaki, T.; Shibata, T.; Mibu, K.; Sahashi, M. Morin Transition Temperature in (0001)-Oriented α-Fe2O3 Thin Film and Effect of Ir Doping. J. Appl. Phys. 2015, 117, 17C736. [Google Scholar] [CrossRef]
- Wang, J.; Aguilar, V.; Li, L.; Li, F.G.; Wang, W.Z.; Zhao, G. meng Strong Shape-Dependence of Morin Transition in α-Fe2O3 Single-Crystalline Nanostructures. Nano Res. 2015, 8, 1906–1916. [Google Scholar] [CrossRef]
- Sun, Q.J.; Lu, X.G.; Liang, G.Y. Controlled Template-Free Hydrothermal Synthesis of Hematite Nanoplatelets. Mater. Lett. 2010, 64, 2006–2008. [Google Scholar] [CrossRef]
- Jacob, J.; Abdul Khadar, M. VSM and Mössbauer Study of Nanostructured Hematite. J. Magn. Magn. Mater. 2010, 322, 614–621. [Google Scholar] [CrossRef]
- Dunlop, D.J.; Ozdemir, O. Rock Magnetism: Fundamentals and Frontiers; Cambridge University Press: Cambridge, UK, 1997; Volume 135, p. 573. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.J. Size-Dependent Superparamagnetic Properties of MgFe2O4 Spinel Ferrite Nanocrystallites. Appl. Phys. Lett. 1998, 73, 3156–3158. [Google Scholar] [CrossRef]
- Coore, R.R.; Love, S.; Helps, C.R.; Anil, M.H. Frequency of Brain Tissue Embolism Associated with Captive Bolt Gun Stunning of Sheep. Foodborne Pathog. Dis. 2004, 1, 291–294. [Google Scholar] [CrossRef]
- Lak, A.; Disch, S.; Bender, P. Embracing Defects and Disorder in Magnetic Nanoparticles. Adv. Sci. 2021, 8, 2002682. [Google Scholar] [CrossRef] [PubMed]
- Muzzi, B.; Lottini, E.; Yaacoub, N.; Peddis, D.; Bertoni, G.; De Julián Fernández, C.; Sangregorio, C.; López-Ortega, A. Hardening of Cobalt Ferrite Nanoparticles by Local Crystal Strain Release: Implications for Rare Earth Free Magnets. ACS Appl. Nano Mater. 2022, 5, 14871–14881. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Munjal, S.; Khare, N. Strain/Defect Induced Enhanced Coercivity in Single Domain CoFe2O4 Nanoparticles. J. Magn. Magn. Mater. 2015, 386, 69–73. [Google Scholar] [CrossRef]
- Kneller, E.F.; Luborsky, F.E. Particle Size Dependence of Coercivity and Remanence of Single-Domain Particles. J. Appl. Phys. 2004, 34, 656. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Safari, B.; Nshingabigwi, E. Temperature-Dependent Magnetic Properties of Magnetite Nanoparticles Synthesized via Coprecipitation Method. J. Alloys Compd. 2020, 846, 156344. [Google Scholar] [CrossRef]
- Waldow, S.M.; Dougherty, T.J. Interaction of Hyperthermia and Photoradiation Therapy. Radiat. Res. 1984, 97, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Crezee, J.; Franken, N.A.P.; Oei, A.L. Hyperthermia-Based Anti-Cancer Treatments. Cancers 2021, 13, 1240. [Google Scholar] [CrossRef]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current State of the Art of Regional Hyperthermia Treatment Planning: A Review. Radiat. Oncol. 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.; Pankhurst, Q.A. Magnetic Hyperthermia. In Nanoscience: Volume 1: Nanostructures through Chemistry; Royal Society of Chemistry: London, UK, 2012; pp. 60–88. [Google Scholar] [CrossRef]
- Arora, D.; Skliar, M.; Roemer, R.B. Model-Predictive Control of Hyperthermia Treatments. IEEE Trans. Biomed. Eng. 2002, 49, 629–639. [Google Scholar] [CrossRef]
- Moroz, P.; Jones, S.K.; Gray, B.N.; Morozy, P.; Jonesz, S.K.; Grayz, B.N. Magnetically Mediated Hyperthermia: Current Status and Future Directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef]
- Bellizzi, G.; Bucci, O.M.; Chirico, G. Numerical Assessment of a Criterion for the Optimal Choice of the Operative Conditions in Magnetic Nanoparticle Hyperthermia on a Realistic Model of the Human Head. Int. J. Hyperth. 2016, 32, 688–703. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Ranoo, S.; Philip, J. Effect of Orientational Ordering of Magnetic Nanoemulsions Immobilized in Agar Gel on Magnetic Hyperthermia. J. Magn. Magn. Mater. 2018, 451, 254–268. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of Available Methods for Quantifying the Heat Power Generation of Nanoparticles for Magnetic Hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef]
- Hilger, I.; Frühauf, K.; Andrä, W.; Hiergeist, R.; Hergt, R.; Kaiser, W.A. Heating Potential of Iron Oxides for Therapeutic Purposes in Interventional Radiology. Acad. Radiol. 2002, 9, 198–202. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Arshad, S.; Riaz, S.; Naseem, S. Synthesis of Iron Oxide Nanoparticles by Sol-Gel Technique and Their Characterization. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.; Zhang, Y.; Gu, N. Size Dependence of Specific Power Absorption of Fe3O4 Particles in AC Magnetic Field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-Dependent Magnetic and Inductive Heating Properties of Fe3O4 Nanoparticles: Scaling Laws across the Superparamagnetic Size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Fan, D.D.; Ding, J. Optimization of Surface Coating on Fe3O4 Nanoparticles for High Performance Magnetic Hyperthermia Agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Klekotka, U.; Satuła, D.; Basa, A.; Kalska-Szostko, B. Importance of Surfactant Quantity and Quality on Growth Regime of Iron Oxide Nanoparticles. Materials 2020, 13, 1747. [Google Scholar] [CrossRef]
- Panda, R.N.; Gajbhiye, N.S.; Balaji, G. Magnetic Properties of Interacting Single Domain Fe3O4 Particles. J. Alloys Compd. 2001, 326, 50–53. [Google Scholar] [CrossRef]
| Sample | HC (Oe) | MS (emu/g) | SAR in Agar @ 800 Oe (W/g) | Lattice Parameter (a) of Fe3O4 (Å) |
|---|---|---|---|---|
| 30 nm Nanocubes [6] | 33.7 | 67.5 | ~540 | Not Applicable |
| AP | 57.5 | 67.6 | 544 | 8.27 |
| AO | 109 | 61.2 | 1001 | 8.32 |
| AN | 67.1 | 74.3 | 692.8 | 8.37 |
| AH | 64.8 | 77.6 | 624.4 | 8.40 |
| AA | 80 | 73.8 | 712.3 | 8.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attanayake, S.B.; Chanda, A.; Das, R.; Phan, M.-H.; Srikanth, H. Tailoring the Magnetic and Hyperthermic Properties of Biphase Iron Oxide Nanocubes through Post-Annealing. Crystals 2024, 14, 519. https://doi.org/10.3390/cryst14060519
Attanayake SB, Chanda A, Das R, Phan M-H, Srikanth H. Tailoring the Magnetic and Hyperthermic Properties of Biphase Iron Oxide Nanocubes through Post-Annealing. Crystals. 2024; 14(6):519. https://doi.org/10.3390/cryst14060519
Chicago/Turabian StyleAttanayake, Supun B., Amit Chanda, Raja Das, Manh-Huong Phan, and Hariharan Srikanth. 2024. "Tailoring the Magnetic and Hyperthermic Properties of Biphase Iron Oxide Nanocubes through Post-Annealing" Crystals 14, no. 6: 519. https://doi.org/10.3390/cryst14060519







