Evolution of the Microstructure, Phase Composition and Thermomechanical Properties of the CuZnIn Alloys, Achieved by Thermally Controlled Phase Transitions
Abstract
:1. Introduction
2. Materials and Methods
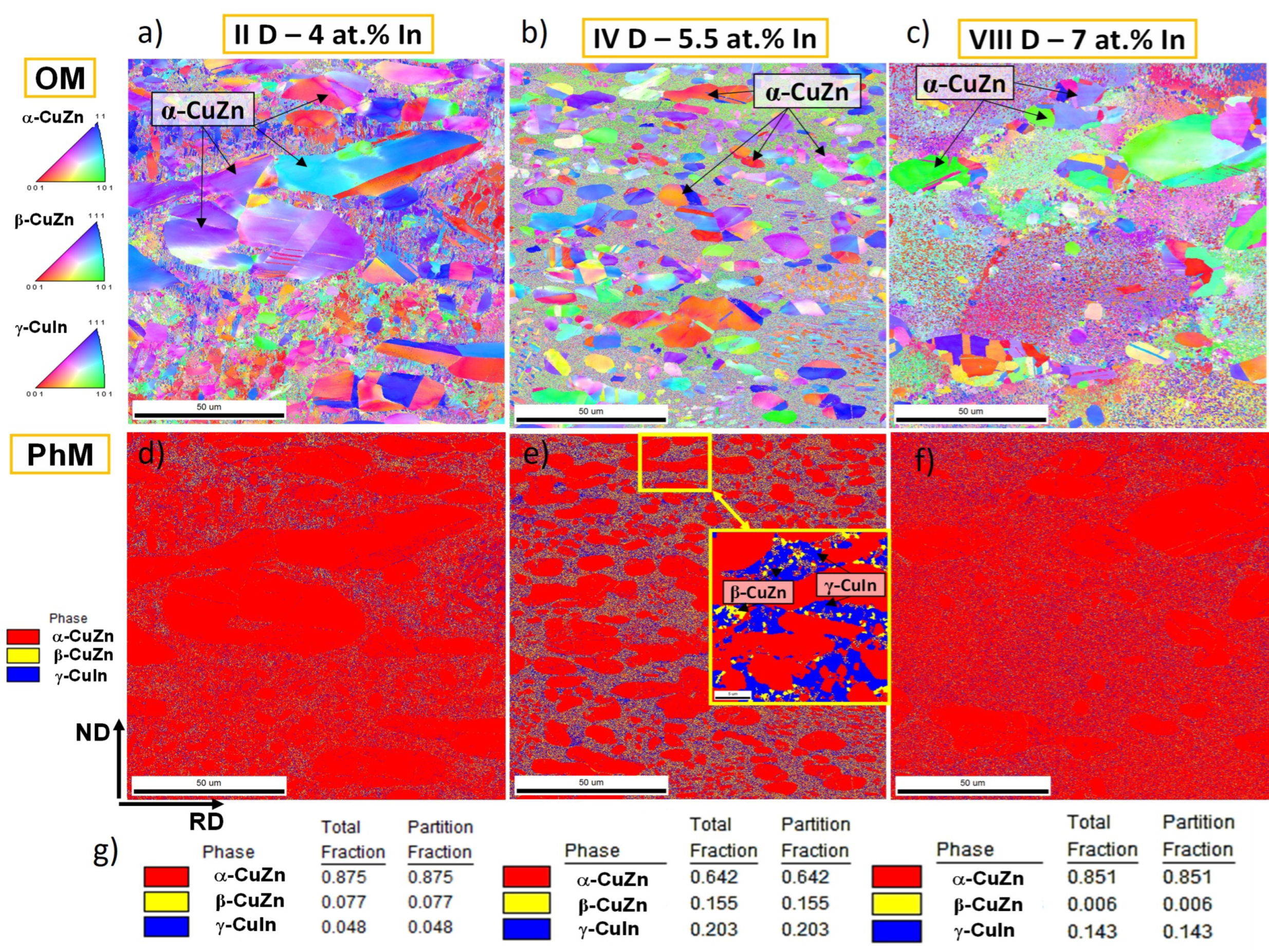
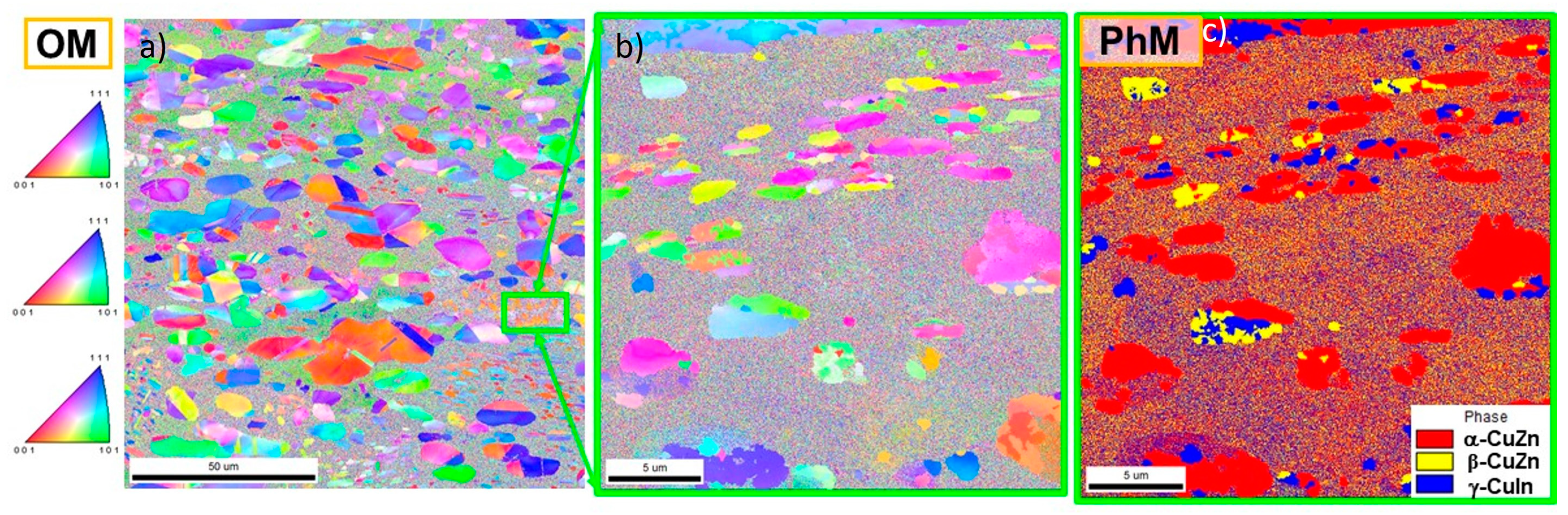
- The microstructural characterization and determination of chemical composition were carried out on the samples’ cross-sections using the FEI QUANTA 3D FEGSEM scanning electron microscope (SEM), ThermoFisher Sci., Brno, Czech Republic equipped with the EDAX-EDXS energy-dispersive X-ray spectrometer. Qualitative changes in composition were observed in the backscattered electron (BSE) mode. The investigations were conducted in high vacuum conditions (106 mbar) at the accelerating voltage of 20 keV. Microstructural evolution, due to the annealing at 200 °C, was examined using electron backscatter diffraction (EBSD) with the TSL EBSD system. The composition maps were obtained in the plane perpendicular to the transverse rolling direction (ND/RD). Samples’ surfaces were prepared by vibratory polishing using a Master Met2 silicon suspension. The following parameters were used for the EBSD measurements: tilt angle = 70°, voltage = 20 kV, working distance = 10.0 mm and mapping step size = 0.27 µm.
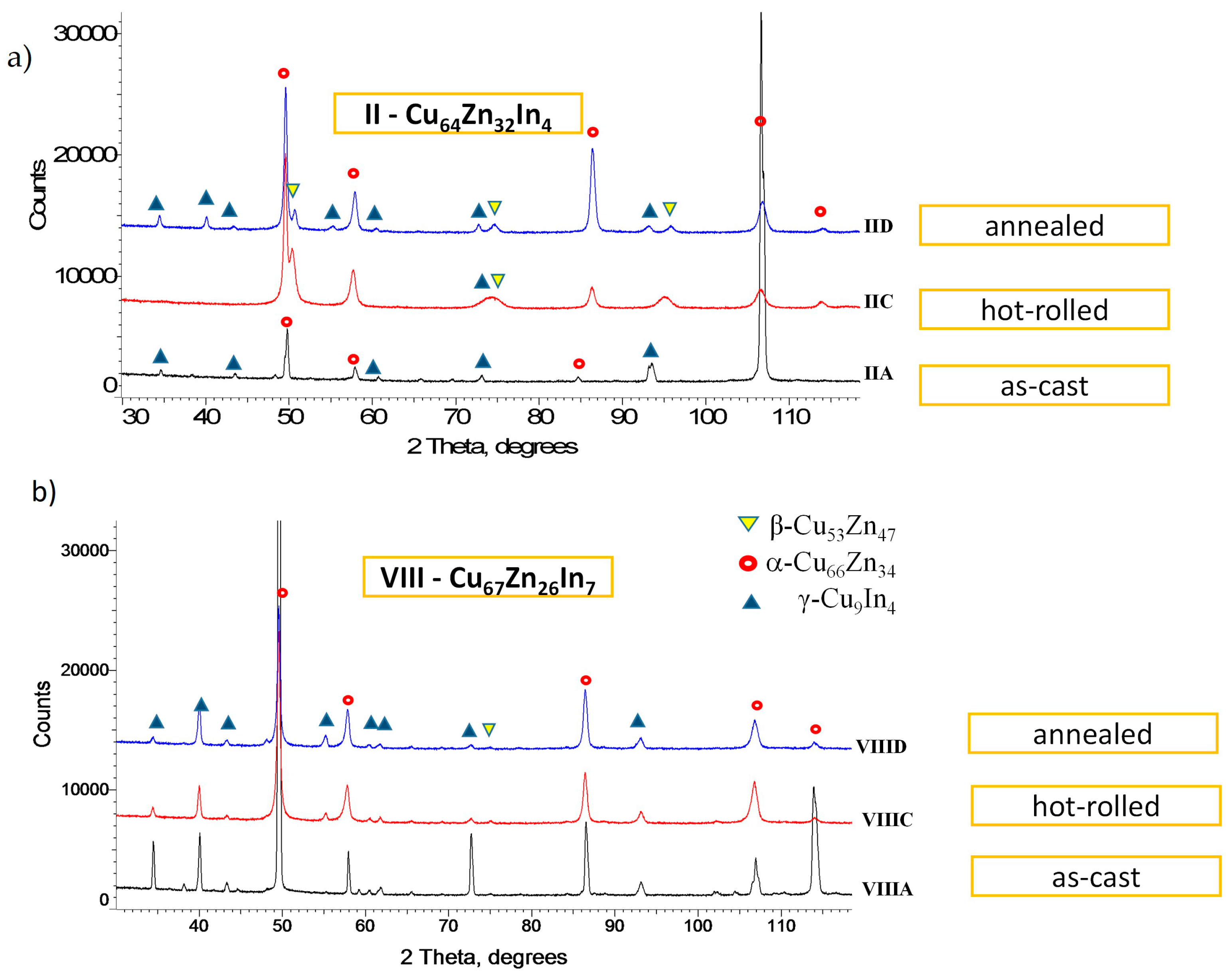
- An X-ray structural analysis of the alloys was performed with use of the D8 Bruker diffractometer, Germany with Co Kα radiation, operated at 40 kV/45 mA, with a standard holder. Measurements were conducted in the 2θ range of 20–100°, with the scanning step of 0.02°. For the phase identification, the PDF4+(ver. 2023) crystallographic database and the program Diffrac.EVA V.3.0 was applied. Investigations were conducted both on cross-sectional and longitudinal sample sections.
- The thermomechanical analysis (TMA) of the hot-rolled samples was performed with the use of the Netzsch TMA 402 F1 Hyperion instrument, Selb, Germany. The analysis was conducted using the tensile mode with a constant tensile loading (2.5 N) and heating rate of 5 °C/min, in the temperature range RT-650 °C. The ceramic active measuring elements used were made from alumina, and a high-purity helium protective atmosphere was used. The samples for the tensile experiment in TMA were prepared in the form of rectangles 20.5 mm × 1.0 mm with the thickness 0.6 mm. The surface was polished with sand paper and polishing grazes to the mirror state.
- For the transmission electron microscopy (TEM/HRTEM) studies, the FEI TECNAI G2 FEG/200 kV microscope, equipped with the high-angle annular dark-field detector (HAADF) and the EDAX Phoenix EDS spectrometer, was used. Thin foils for TEM were prepared by electrochemical polishing in the HNO3/methanol solution, supplemented with the ion milling.
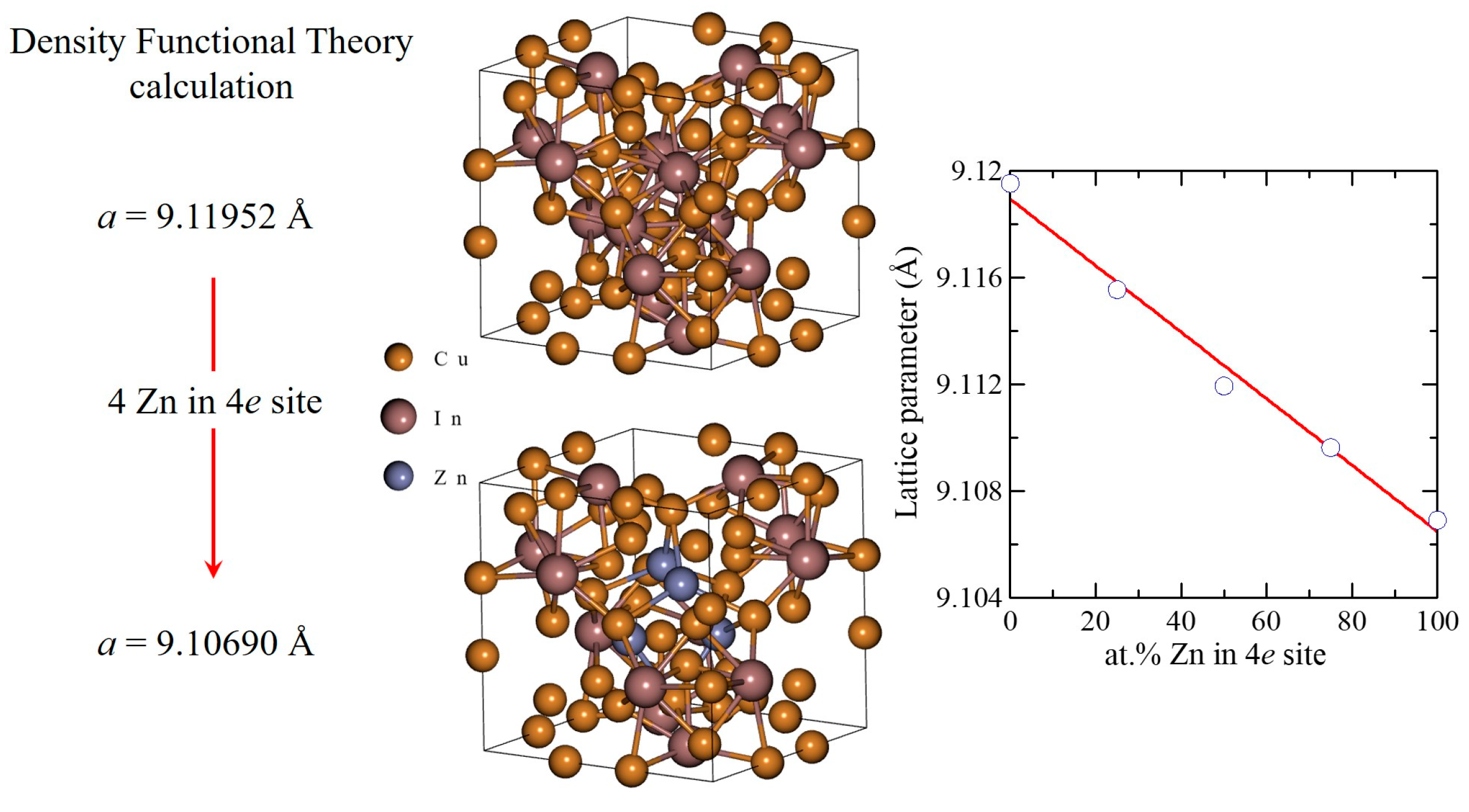
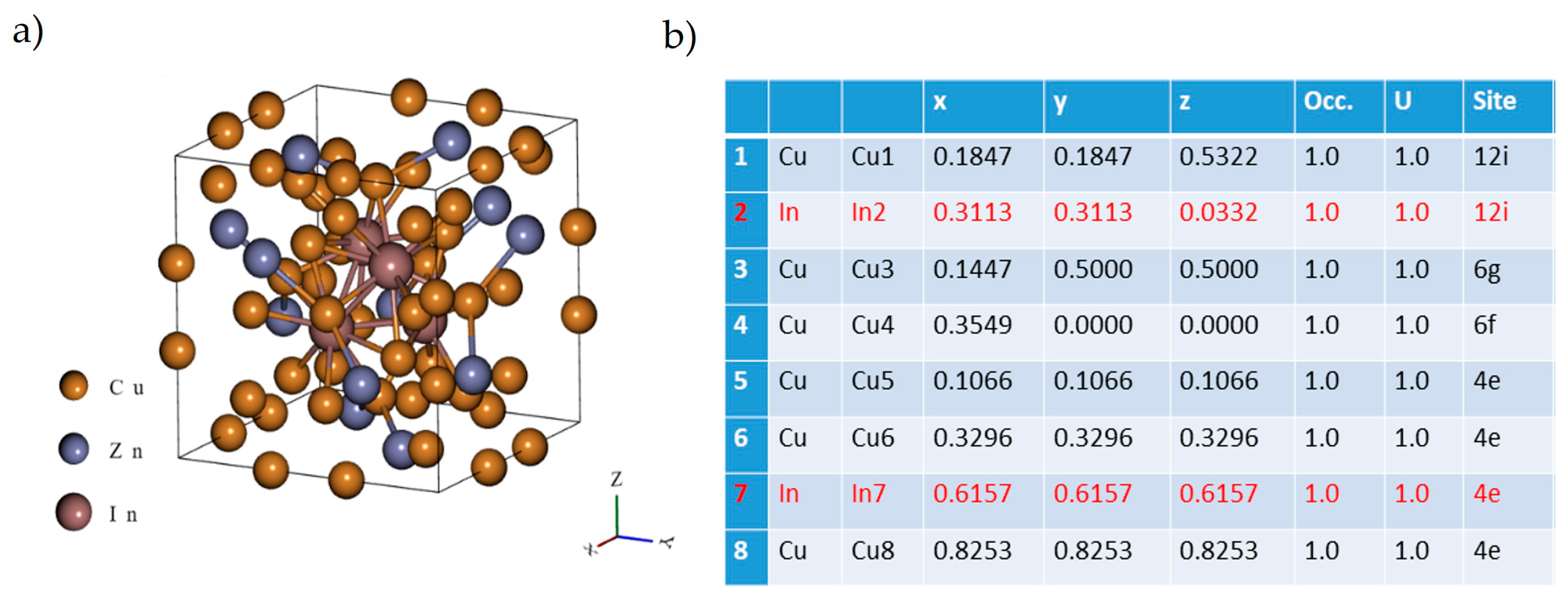
- The electronic structure of the Cu9In4-type ternary phase was determined by applying density functional theory (DFT). DFT calculations were performed using Quantum Espresso (ver. 2023) software at the computing center HPC “ACK Cyfronet AGH” on the Ares Supercomputer, under computational grant no. PLG/2023/016473. The cell parameters of the samples, investigated on their cross-sectional and longitudinal sections, were calculated using the Nelson–Riley extrapolation method.
3. Results and Discussion
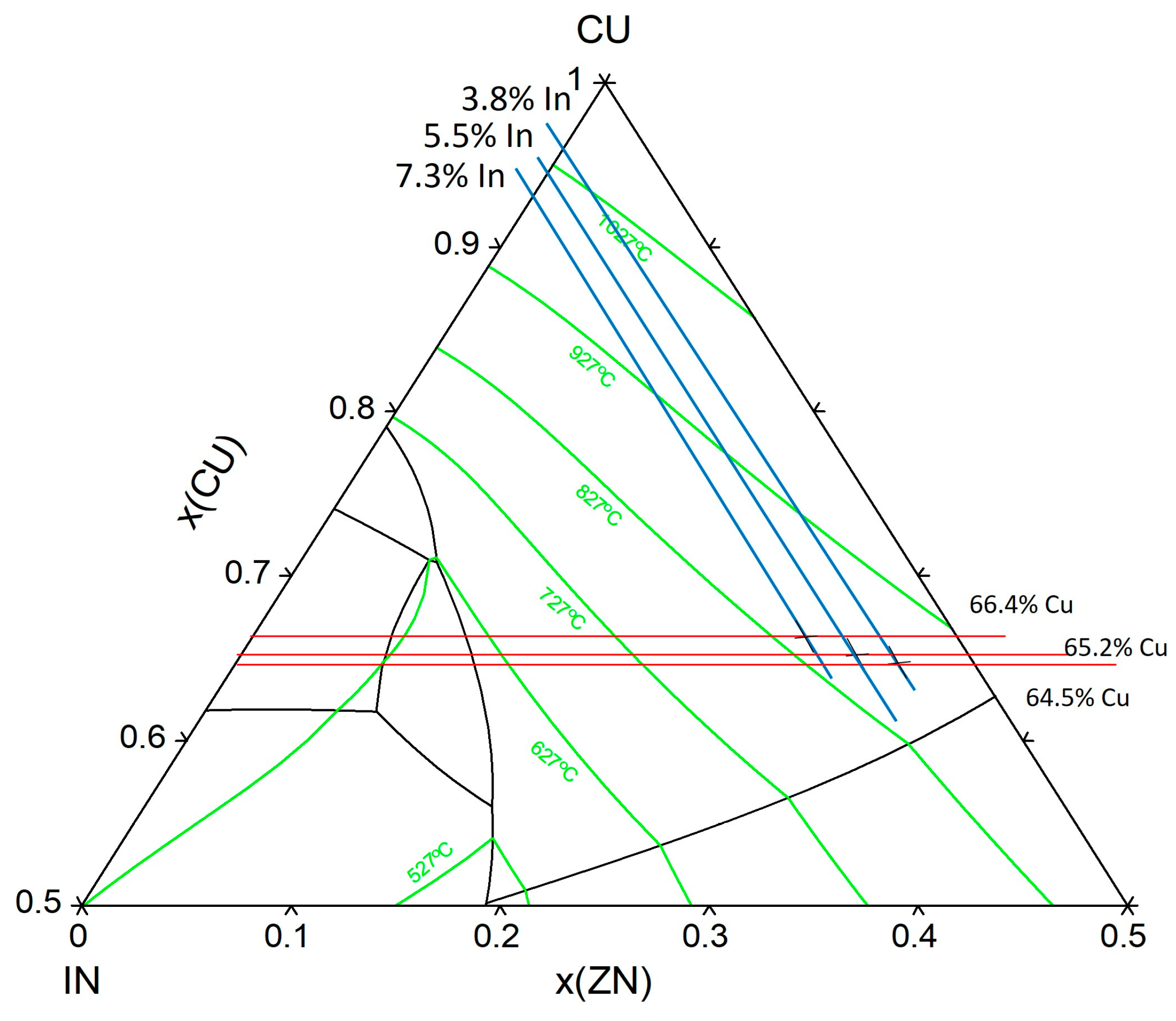
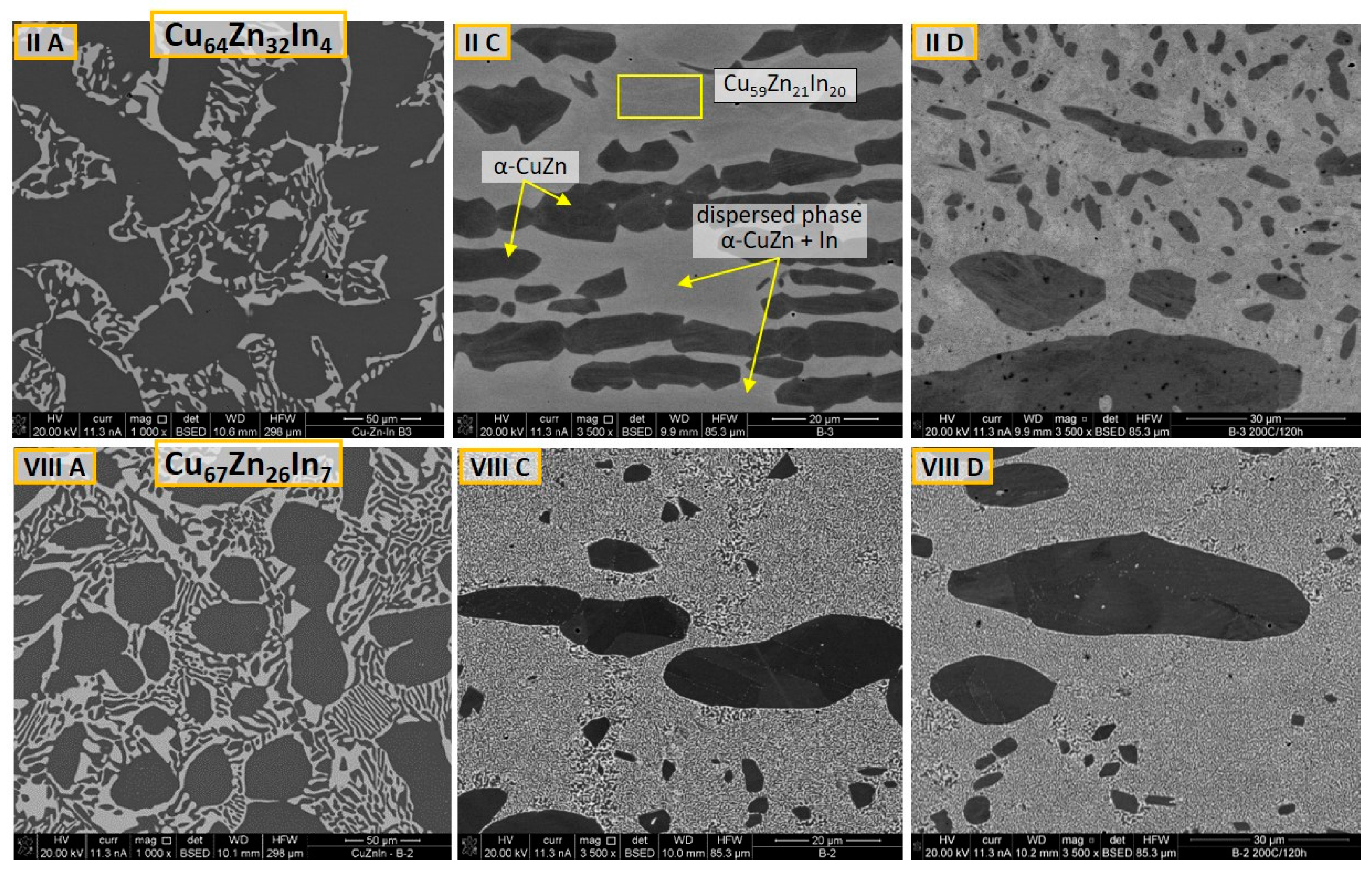
3.1. Microstructure and Phase Composition
3.1.1. As-Cast Alloys
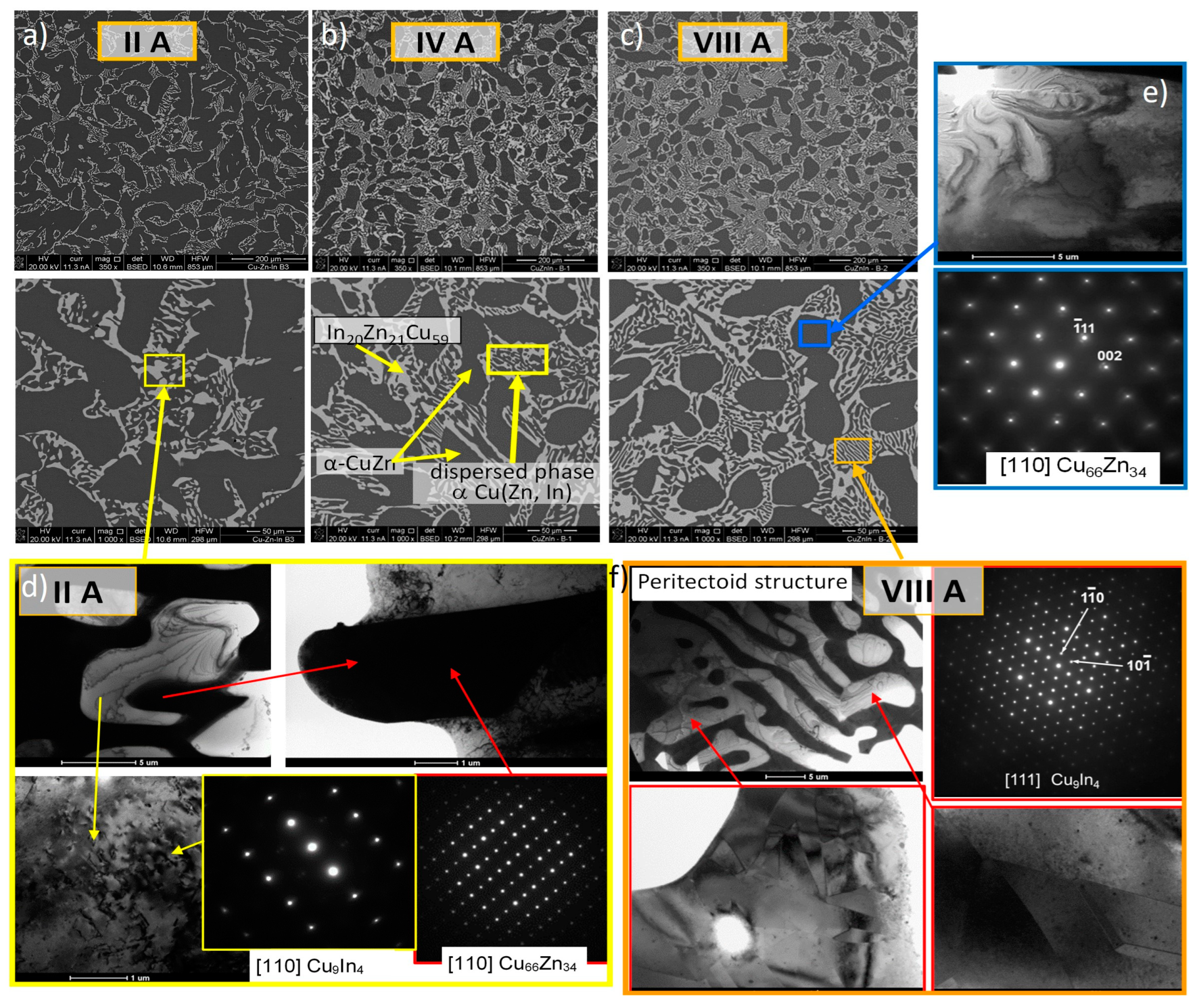
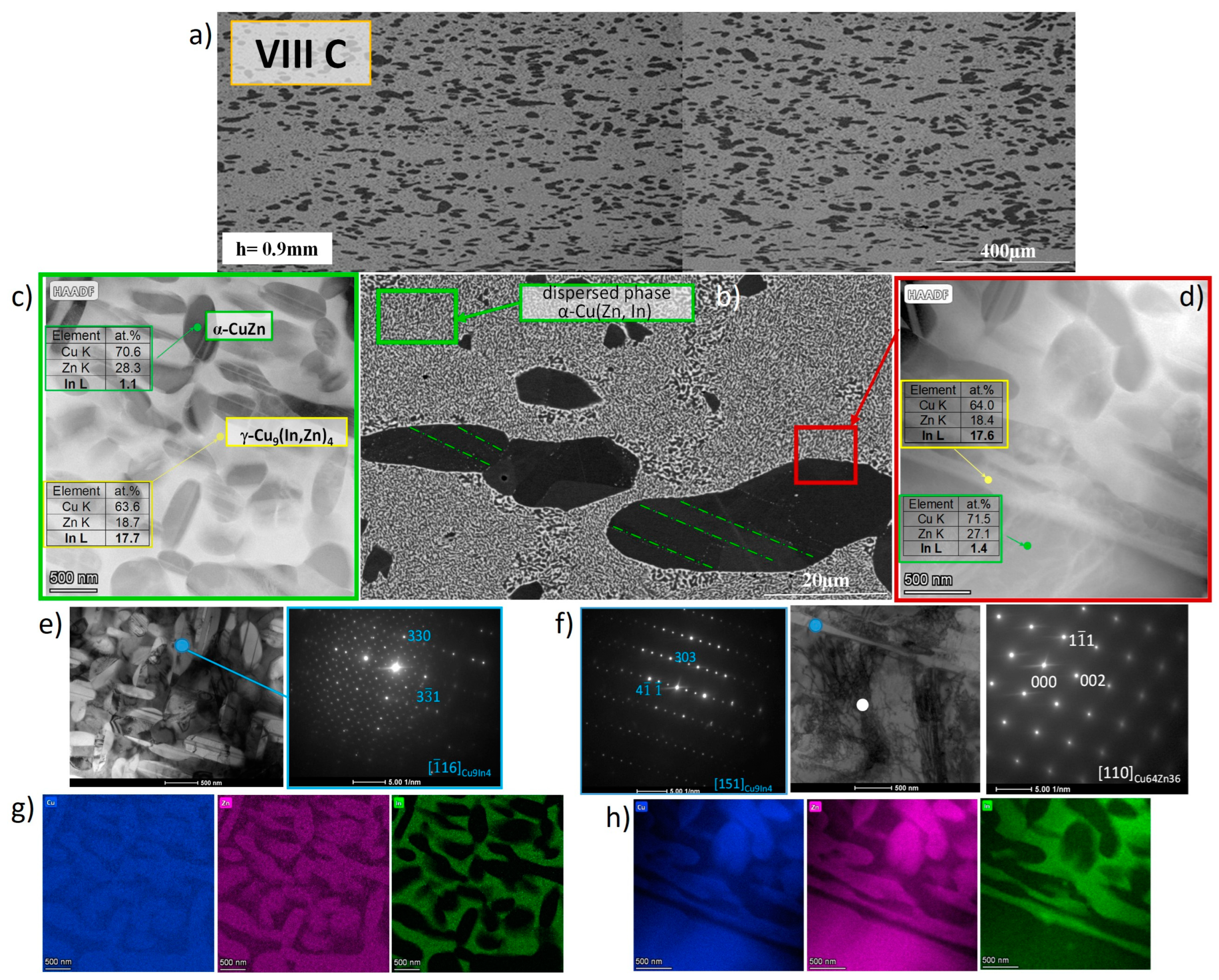
3.1.2. Hot-Rolled Alloys
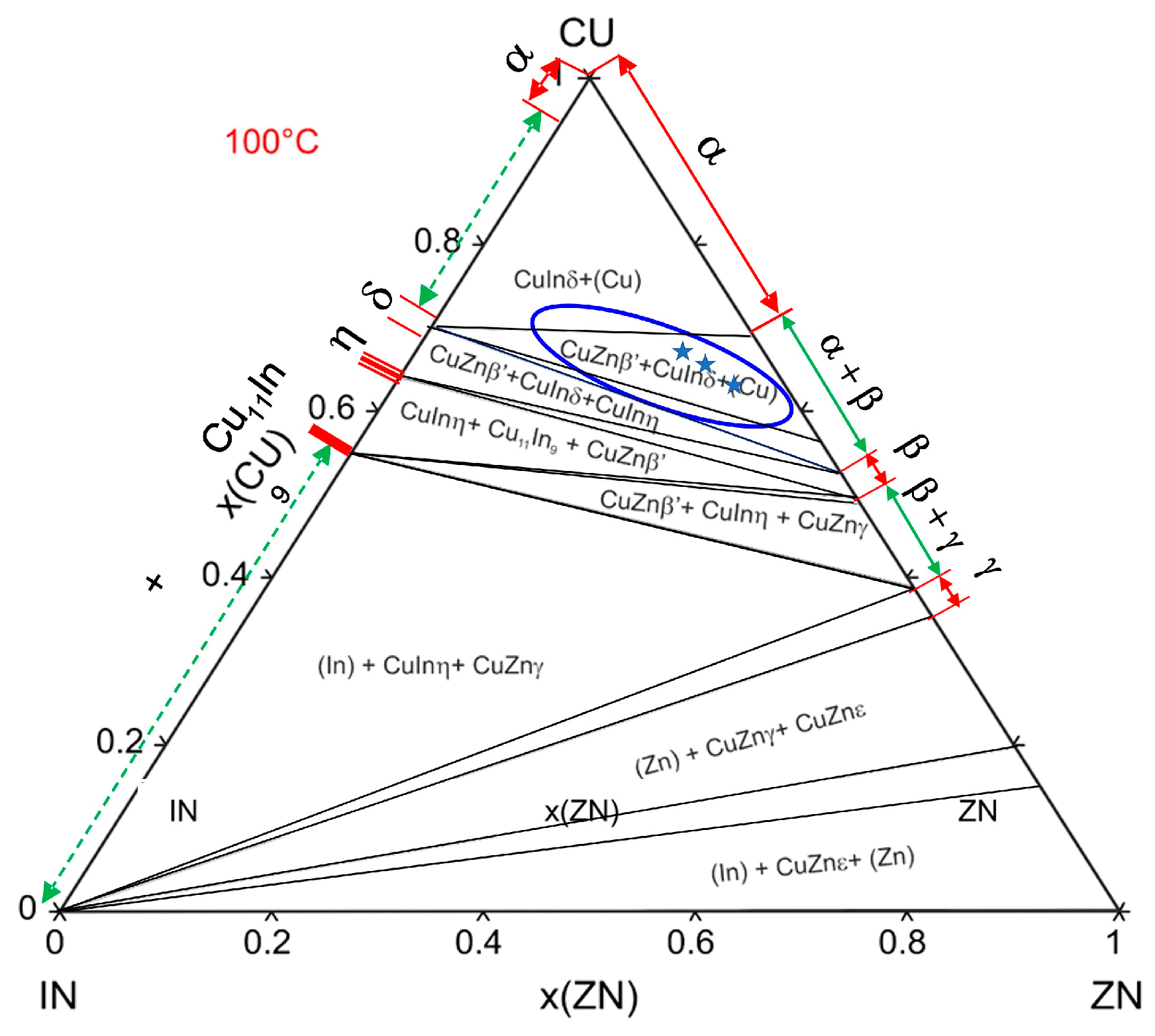
3.1.3. Evolution of Microstructure after Annealing
3.2. XRD Phase Composition Analysis
3.3. Modeling of the Structures of γ* Cu(In,Zn)
3.4. Thermomechanical Analysis of Hot-Rolled Alloys
4. Summary
5. Conclusions
- A ternary phase with an approximate composition of Cu8(In,Zn)4,5 and a structure analogous to the high-temperature γ-Cu9In4 phase remains stable at low temperatures. This behavior results from the absence of a peritectoidal decomposition and the absence of the β-CuIn phase,
- A modification of the structure of the γ-Cu9In4 phase has been identified, characterized by a reduced unit cell and altered electronic structure, where zinc atoms replace indium atoms in a one-to-one ratio, and this phase has been labeled as γ* Cu(In,Zn),
- The α-CuZn solution phase, with the minor additions of indium (1–2% at.), was the primary phase crystallized during casting and remained stable at low temperatures.
- The hot-rolling process preserves the phase composition and leads to the formation of a composite-like structure, with a matrix composition of γ* and large ellipsoidal α-CuZn solution particles,
- On a microscale, the γ* matrix exhibits a specific structure resulting from the segregation processes and is composed of micrometer-sized α-CuZn(In) solution particles and nano-sized β-CuZn phase precipitates.
- Low-temperature annealing intensifies the decomposition process of the γ* matrix through the precipitation of binary α and β phases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miodownik, A.P. Cu-Zn Phase Diagram. In Binary Alloy Phase Diagrams; ASM International: Detroit, MI, USA, 1989; p. 1509. [Google Scholar]
- Kowalski, M.; Spencer, P.J. Thermodynamic Reevaluation of the Cu-Zn System. J. Phase Equilibria 1993, 14, 432–438. [Google Scholar] [CrossRef]
- Subramanian, P.R.; Laughlin, D.E. Cu-In Phase Diagram. In Binary Alloy Phase Diagrams; ASM International: Detroit, MI, USA, 1989; p. 1425. [Google Scholar]
- Liu, H.S.; Liu, X.J.; Cui, Y.; Wang, C.P.; Ohnuma, I.; Kainuma, R.; Jin, Z.P.; Ishida, K. Thermodynamic Assessment of the Cu-In Binary System. J. Phase Equilibria 2002, 23, 409–415. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Zakulski, W. In-Zn Phase Diagram. In Binary Alloy Phase Diagrams; ASM International: Detroit, MI, USA, 1984; p. 2318. [Google Scholar]
- Knott, S.; Chen, C.-J.; Gehringer, F.; Mikula, A. Thermodynamic properties of liquid Cu-In-Zn alloys. Inter. Mat. Res. 2006, 97, 1102–1107. [Google Scholar] [CrossRef]
- Vassilev, V.P.; Lysenko, V.A. Thermodynamic evaluation of the Cu-In-Zn system. J. Alloys Compd. 2016, 681, 606–612. [Google Scholar] [CrossRef]
- Kim, B.; Lee, C.W.; Lee, D.; Kang, N. Effect of Sb Addition on Bi-26Ag-01Cu Solders for High-Temperature Applications. J. Alloys Compd. 2014, 592, 207–212. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Suganuma, K.; Takenaka, J.; Hagio, K. Interfacial Properties of Zn-Sn Alloys as High Temperature Lead-Free Solder on Cu Substrate. Mater. Trans. 2005, 46, 2413–2418. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Suganuma, K.; Inoue, M.; Izuta, G. Thermal properties and phase Stability of Zn-Sn and Zn-In Alloys as High Temperature Lead-Free Solder. Mater. Trans. 2007, 48, 584–593. [Google Scholar] [CrossRef]
- Dinsdale, A.; Watson, A.; Kroupa, A.; Vrestal, J.; Vizdal, J. Atlas of Phase Diagrams for Lead-Free Soldering; COST 531 Lead Free Solders; COST Office: Prague, Czech Republic, 2008; Volume 1, pp. 82, 107. [Google Scholar]
- Li, L.; Song, K.; Zhou, Y.W.; Liu, Q.; Wang, K.; Bao, Y.; Zhao, B.; Jian, X.D.; Ji, C.L.; Qian, P.; et al. The thermodynamic properties of disorder CuZn solid solution and nonstoichiometric CuZn alloy: Pseudo-atomic lattice inversion potential method. J. Solid. State Chem. 2020, 289, 121488. [Google Scholar] [CrossRef]
- Hong, H.L.; Wang, Q.; Dong, C.; Liaw, P.K. Understanding the Cu-Zn brass alloys using a short-range-order cluster model: Significance of specific compositions of industrial alloys. Sci. Rep. 2014, 4, 7065. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Minho, O.M.; Kajihara, M. Kinetics of Solid-State Reactive Diffusion in the Cu/Zn System. Mater. Trans. 2017, 58, 16–22. [Google Scholar] [CrossRef]
- Liu, K.; Ding, W.; Tao, X.; Chen, H.; Ouyang, Y.; Du, Y. Diffusional behaviors and mechanical properties of CueZn system. J. Alloys Compd. 2020, 812, 152141. [Google Scholar] [CrossRef]
- Ortner, N.; Lund, H.; Armbruster, U.; Wohlrab, S.; Kondratenko, E.V. Factors affecting primary and secondary pathways in CO2 hydrogenation to methanol over CuZnIn/MZrOx (La, Ti or Y). Catal. Today 2022, 387, 47–53. [Google Scholar] [CrossRef]
- Czeppe, T.; Sypien, A.; Wierzbicka-Miernik, A.; Garzel, G.; Kopyto, M. Phase Formation and Diffusivity in the Ternary Cu-Zn-In System. J. Mater. Eng. Perform. 2022, 31, 6962–6969. [Google Scholar] [CrossRef]





| Composition of the Alloy [%at.] | Symbol of the Sample | ||
|---|---|---|---|
| As-Cast | Two-Step Hot Rolling, up to 0.4–0.7 mm in Thickness | Annealed at 200 °C/120 h, Water Cooled | |
| Cu64Zn32In4 | II A | II C | II D |
| Cu65Zn29In5.5 | IV A | IV C | IV D |
| Cu67Zn26In7 | VIII A | VIII C | VIII D |
| (a) XRD Peaks Integration, from the Surface | ||||
| Sample | % α—Cu66Zn34 (FCC) | % γ—Cu9In4 (FCC) | % β—Cu53Zn47 (BCC) | Total |
| II D—4 at.% In | 89.4 | 5.4 | 5.2 | 100 |
| IV D—5.5 at.% In | 59.5 | 36.3 | 4.2 | 100 |
| VIII D—7 at.% In | 60.6 | 37.6 | 1.8 | 100 |
| (b) XRD Peaks Integration, from Cross-section | ||||
| Sample | % α—Cu66Zn34 (FCC) | % γ—Cu9In4 (FCC) | % β—Cu53Zn47 (BCC) | Total |
| II D—4 at.% In | 79.3 | 1.1 | 19.6 | 100 |
| IV D—5.5 at.% In | 67.6 | 21.8 | 10.6 | 100 |
| VIII D—7 at.% In | 84.8 | 13.9 | 1.2 | 100 |
| Binary Phases (PDF4+ Data Base) | Ternary Phases Experimental Results | ∆a/a [%] Relative Difference Binary/ Ternary Phases | |||
|---|---|---|---|---|---|
| Phase | Space Group | a/Å | Phase | a/Å | |
| Cu64Zn32In4—II | |||||
| α—Cu66Zn34 | 3.697 | α—Cu(Zn,In) | 3.694(1) | 0.08 | |
| β—Cu53Zn47 | 2.955 | β—(Cu,Zn,In) | 2.951(5) | 0.1 | |
| Cu65.5Zn29In5.5—IV | |||||
| α—Cu66Zn34 | 3.697 | α—Cu(Zn,In) | 3.695(2) | 0.05 | |
| β—Cu53Zn47 | 2.955 | β—(Cu,Zn,In) | 2.941(6) | 0.5 | |
| γ—Cu9In4 | 9.097 | γ—Cu9(In,Zn)4 | 9.079(8) | 0.2 | |
| Cu67Zn26In7—VIII | |||||
| α—Cu66Zn34 | 3.697 | α—Cu(Zn,In) | 3.693(1) | 0.1 | |
| γ—Cu9In4 | 9.097 | γ—Cu9(In,Zn)4 | 9.055(4) | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sypien, A.; Czeppe, T.; Wojcik, A.; Garzel, G.; Goral, A.; Kopyto, M.; Swiatek, Z. Evolution of the Microstructure, Phase Composition and Thermomechanical Properties of the CuZnIn Alloys, Achieved by Thermally Controlled Phase Transitions. Crystals 2024, 14, 669. https://doi.org/10.3390/cryst14070669
Sypien A, Czeppe T, Wojcik A, Garzel G, Goral A, Kopyto M, Swiatek Z. Evolution of the Microstructure, Phase Composition and Thermomechanical Properties of the CuZnIn Alloys, Achieved by Thermally Controlled Phase Transitions. Crystals. 2024; 14(7):669. https://doi.org/10.3390/cryst14070669
Chicago/Turabian StyleSypien, Anna, Tomasz Czeppe, Anna Wojcik, Grzegorz Garzel, Anna Goral, Marek Kopyto, and Zbigniew Swiatek. 2024. "Evolution of the Microstructure, Phase Composition and Thermomechanical Properties of the CuZnIn Alloys, Achieved by Thermally Controlled Phase Transitions" Crystals 14, no. 7: 669. https://doi.org/10.3390/cryst14070669






