Effects of Particle Size and Surface Chemistry on the Dispersion of Graphite Nanoplates in Polypropylene Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
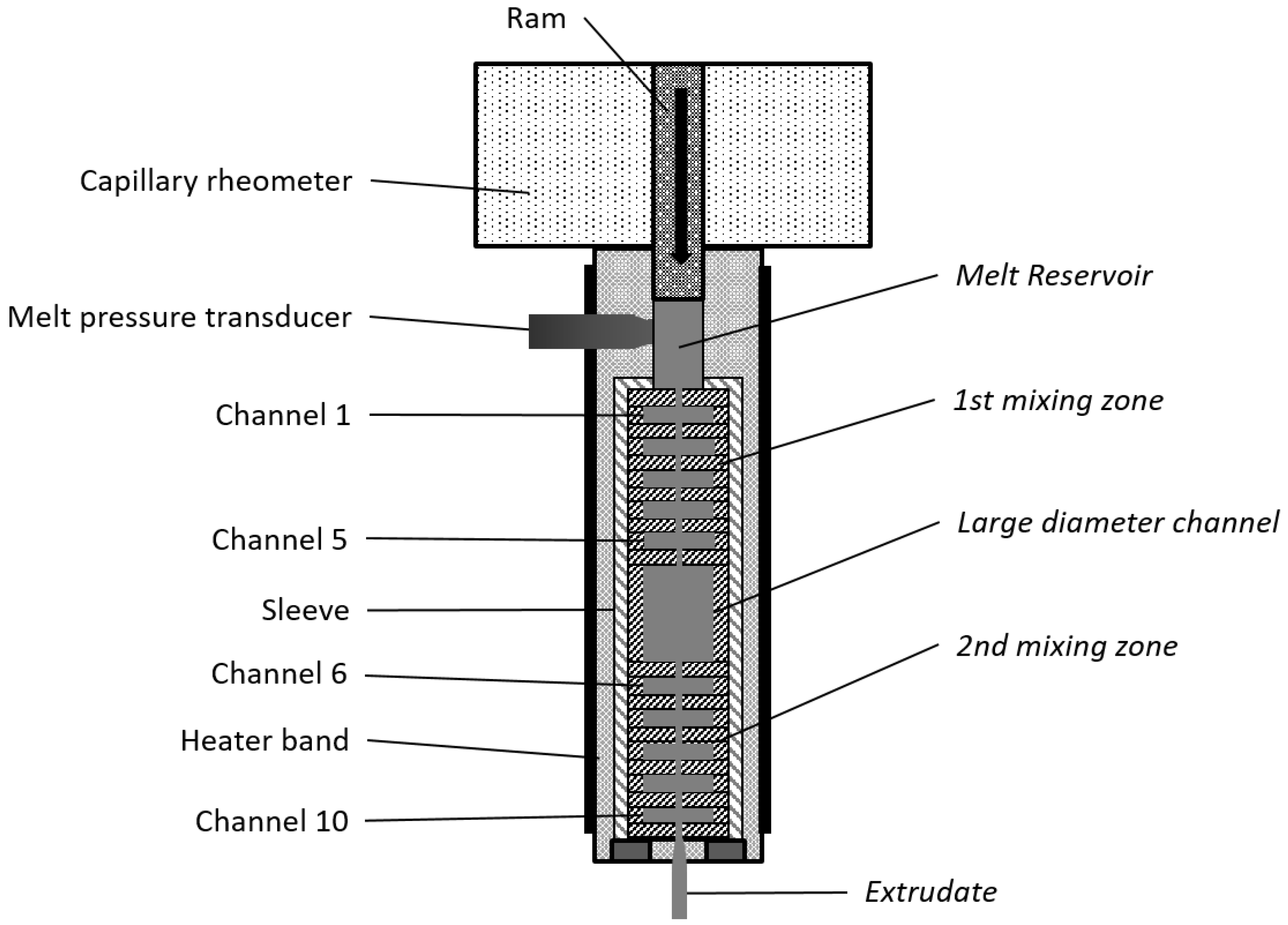
2.2. Processing of Nanocomposites
2.3. Characterization of Graphite Nanoplates
2.4. Characterization of Nanocomposites
3. Results and Discussion
3.1. Graphite Nanoplates Size and Morphology
3.2. Dispersion of the Graphite Nanoplates in Polypropylene
3.3. Electrical Conductivity of the Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sur, U.K. Graphene: A rising star on the horizon of materials science. Int. J. Electrochem. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Santos, R.M.; Vilaverde, C.; Cunha, E.; Paiva, M.C.; Covas, J.A. Probing dispersion and re-agglomeration phenomena upon melt-mixing of polymer-functionalized graphite nanoplates. Soft Matter 2015, 12, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Itkis, M.E.; Bekyarova, E.B.; Haddon, R.C. Anisotropic thermal and electrical properties of thin thermal interface layers of graphite nanoplatelet-based composites. Sci. Rep. 2013, 3, 1710. [Google Scholar] [CrossRef]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Jang, B.Z.; Zhamu, A. Processing of nanographene platelets (NGPs) and NGP nanocomposites: A review. J. Mater. Sci. 2008, 43, 5092–5101. [Google Scholar] [CrossRef]
- Bousmina, M. Study of intercalation and exfoliation processes in polymer nanocomposites. macromolecules 2006, 39, 4259–4263. [Google Scholar] [CrossRef]
- Steurer, P.; Wissert, R.; Thomann, R.; Mülhaupt, R. Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide. Macromol. Rapid Commun. 2009, 30, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.D.L. Exfoliation of graphite. J. Mater. Sci. 1987, 22, 4190–4198. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family–A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Vilaverde, C.; Santos, R.M.; Paiva, M.C.; Covas, J.A. Dispersion and re-agglomeration of graphite nanoplates in polypropylene melts under controlled flow conditions. Compos. A 2015, 78, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Poh, H.L.; Sanek, F.L.; Sanek, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by staudenmaier, hofmann and hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Paiva, M.C.; Simon, F.; Novais, R.M.; Ferreira, T.; Proença, M.F.; Xu, W.; Besenbacher, F. Controlled functionalization of carbon nanotubes by a solvent-free multicomponent approach. ACS Nano 2010, 4, 7379–7386. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Fernandes, F.M.; Proença, M.F.; Silva, C.J.R.; Paiva, M.C. The 1,3-dipolar cycloaddition reaction in the functionalization of carbon nanofibers. J. Nanosci. Nanotechnol. 2007, 7, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, M.; Gartner, T.; Tzounis, L.; Micusik, M.; Pötschke, P.; Stamm, M.; Heinrich, G.; Voit, B. Influence of the MWCNT surface functionalization on the thermoelectric properties of melt-mixed polycarbonate composites. Compos. Sci. Technol. 2014, 101, 133–138. [Google Scholar] [CrossRef]
- Novais, R.M.; Covas, J.A.; Paiva, M.C. The effect of flow type and chemical functionalization on the dispersion of carbon nanofiber agglomerates in polypropylene. Compos. A 2012, 43, 833–841. [Google Scholar] [CrossRef]
- Pegel, S.; Pötschke, P.; Petzold, G.; Alig, I.; Dudkin, S.M.; Lellinger, D. Dispersion, agglomeration, and network formation of multiwalled carbon nanotubes in polycarbonate melts. Polymer 2008, 49, 974–984. [Google Scholar] [CrossRef]
- Krause, B.; Mende, M.; Pötschke, P.; Petzold, G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon 2010, 48, 2746–2754. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nafezarefi, F.; Tai, N.H.; Schlagenhauf, L.; Nuesch, F.A.; Chu, B.T.T. Size and synergy effects of nanofiller hybrids including graphene nanoplatelets and carbon nanotubes in mechanical properties of epoxy composites. Carbon 2012, 50, 5380–5386. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Prada-Silvy, R.; Tan, Y.; Azad, S.; Krause, B.; Pötschke, P.; Grady, B.P. Aspect ratio effects of multi-walled carbon nanotubes on electrical, mechanical, and thermal properties of polycarbonate/MWCNT composites. J. Polym. Sci. B 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Morcom, M.; Atkinson, K.; Simon, G.P. The effect of carbon nanotube properties on the degree of dispersion and reinforcement of high density polyethylene. Polymer 2010, 51, 3540–3550. [Google Scholar] [CrossRef]
- Versavaud, S.; Régnier, G.; Gouadec, G.; Vincent, M. Influence of injection molding on the electrical properties of polyamide 12 filled with multi-walled carbon nanotubes. Polymer 2014, 55, 6811–6818. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Kasaliwal, G.R.; Villmow, T.; Pegel, S.; Pötschke, P. Polymer-Carbon Nanotube Composites, 1st ed.; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Cambridge, UK, 2011; pp. 92–132. ISBN 978-1-84569-761-7. [Google Scholar]
- Kasaliwal, G.R.; Göldel, A.; Pötschke, P.; Heinrich, G. Influences of polymer matrix melt viscosity and molecular weight on MWCNT agglomerate dispersion. Polymer 2011, 52, 1027–1036. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Müller, M.T.; Boldt, R.; Pötschke, P. The influence of matrix viscosity on MWCNT dispersion and electrical properties in different thermoplastic nanocomposites. Polymer 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Bai, J.B.; Allaoui, A. Effect of the length and the aggregate size of MWNTs on the improvement efficiency of the mechanical and electrical properties of nanocomposites—Experimental investigation. Compos. A 2003, 34, 689–694. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Askeland, P.; Drzal, L.T. The nucleating effect of exfoliated graphite nanoplatelets and their influence on the crystal structure and electrical conductivity of polypropylene nanocomposites. J. Mater. Sci. 2007, 43, 2895–2907. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite-polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon 2007, 45, 1446–1452. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Rodrigues, P.; Santos, R.M.; Paiva, M.C.; Covas, J.A. Development of dispersion during compounding and extrusion of Polypropylene/Graphite Nanoplates Composites. Int. Polym. Proc. 2017, 32, 614–622. [Google Scholar] [CrossRef]
- Jamali, S.; Paiva, M.C.; Covas, J.A. Dispersion and re-agglomeration phenomena during melt mixing of polypropylene with multi-wall carbon nanotubes. Polym. Test. 2013, 32, 701–707. [Google Scholar] [CrossRef]
- Scurati, A.; Feke, D.L.; Manas-Zloczower, I. Analysis of the kinetics of agglomerate erosion in simple shear flows. Chem. Eng. Sci. 2005, 60, 6564–6573. [Google Scholar] [CrossRef]
- Domingues, N.; Gaspar-Cunha, A.; Covas, J.A.; Camesasca, M.; Kaufman, M.; Manas-Zloczower, I. Modeling of agglomerate dispersion in single screw extruders. Model. Int. Polym. Process. 2010, 25, 188–198. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]






| xGnP | Carbon purity (%) | Bulk density | Density | Width (µm) | Thickness (nm) | Surface area (m2·g−1) | Electrical conductivity in plane/perpendicular to surface (S·m−1) |
|---|---|---|---|---|---|---|---|
| xGnP Grade C® | 98.0 | 0.20–0.40 | 2.0–2.25 | 1–2 | 2 | 750 | Not available |
| xGnP Grade M® | >99.5 | 0.03–0.10 | 2.2 | 15 | 6-8 | 120–150 | 107/102 |
| xGnP Grade H® | >99.5 | 0.03–0.10 | 2.2 | 5 | 15 | 60–80 | 107/102 |
| GnP powder | Average area of the GnP powders (µm2) |
|---|---|
| GnPC | 45 ± 4 |
| fGnPC-PP | 71 ± 12 |
| GnPM | 326 ± 34 |
| fGnPM-PP | 758 ± 123 |
| GnPH | 194 ± 11 |
| fGnPH-PP | 289 ± 17 |
| Location in extensional mixer | Aav (µm2) * | NA (mm−2) | N | Aav (µm2) * | NA (mm−2) | N |
|---|---|---|---|---|---|---|
| PP/GnPC | PP/fGnPC-PP | |||||
| Reservoir | 15.0 ± 0.9 | 240.7 | 4289 | 24 ± 8 | 2000.3 | 1828 |
| Channel 3 | 14 ± 2 | 152.2 | 862 | 12 ± 2 | 275.4 | 1722 |
| Channel 5 | 10.7 ± 0.7 | 74.93 | 1650 | 10 ± 1 | 131.5 | 1237 |
| Large channel | 14.6 ± 0.8 | 167.4 | 3989 | 14 ± 4 | 634.0 | 1170 |
| Channel 8 | 18 ± 2 | 139.5 | 1046 | 12 ± 2 | 279.1 | 1436 |
| Channel 10 | 14.6 ± 0.9 | 88.48 | 1298 | 10 ± 1 | 191.3 | 1335 |
| PP/GnPM | PP/fGnPM-PP | |||||
| Reservoir | 131 ± 19 | 1895.1 | 827 | 120 ± 19 | 1510.4 | 744 |
| Channel 3 | 63 ± 6 | 644.8 | 1243 | 72 ± 8 | 571.2 | 880 |
| Channel 5 | 51 ± 4 | 367.4 | 1268 | 56 ± 6 | 530.1 | 920 |
| Large channel | 83 ± 4 | 1712.2 | 1288 | 110 ± 14 | 1113.0 | 800 |
| Channel 8 | 47 ± 4 | 543.4 | 1626 | 64 ± 8 | 745.2 | 951 |
| Channel 10 | 40 ± 4 | 575.1 | 1511 | 60 ± 6 | 498.0 | 805 |
| PP/GnPH | PP/fGnPH-PP | |||||
| Reservoir | 65 ± 7 | 1157.6 | 1975 | 110 ± 17 | 1814.9 | 975 |
| Channel 3 | 38 ± 3 | 297.4 | 2331 | 57 ± 6 | 476.4 | 901 |
| Channel 5 | 32 ± 2 | 267.7 | 2197 | 50 ± 5 | 388.4 | 994 |
| Large channel | 56 ± 5 | 606.0 | 2067 | 71 ± 11 | 1182.8 | 1031 |
| Channel 8 | 38 ± 3 | 384.6 | 1953 | 62 ± 8 | 823.5 | 1034 |
| Channel 10 | 34 ± 3 | 360.4 | 1582 | 55 ± 7 | 588.2 | 862 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.M.; Mould, S.T.; Formánek, P.; Paiva, M.C.; Covas, J.A. Effects of Particle Size and Surface Chemistry on the Dispersion of Graphite Nanoplates in Polypropylene Composites. Polymers 2018, 10, 222. https://doi.org/10.3390/polym10020222
Santos RM, Mould ST, Formánek P, Paiva MC, Covas JA. Effects of Particle Size and Surface Chemistry on the Dispersion of Graphite Nanoplates in Polypropylene Composites. Polymers. 2018; 10(2):222. https://doi.org/10.3390/polym10020222
Chicago/Turabian StyleSantos, Raquel M., Sacha T. Mould, Petr Formánek, Maria C. Paiva, and José A. Covas. 2018. "Effects of Particle Size and Surface Chemistry on the Dispersion of Graphite Nanoplates in Polypropylene Composites" Polymers 10, no. 2: 222. https://doi.org/10.3390/polym10020222
APA StyleSantos, R. M., Mould, S. T., Formánek, P., Paiva, M. C., & Covas, J. A. (2018). Effects of Particle Size and Surface Chemistry on the Dispersion of Graphite Nanoplates in Polypropylene Composites. Polymers, 10(2), 222. https://doi.org/10.3390/polym10020222








