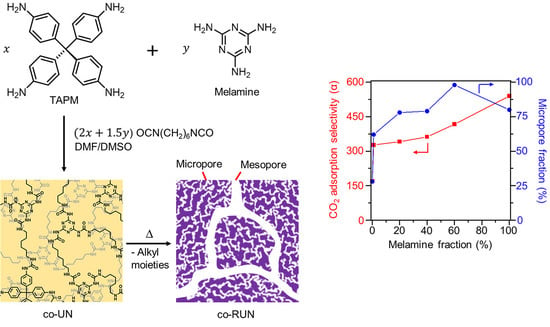
Rearranged Copolyurea Networks for Selective Carbon Dioxide Adsorption at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rearranged Copolyurea Networks (co-RUNs)
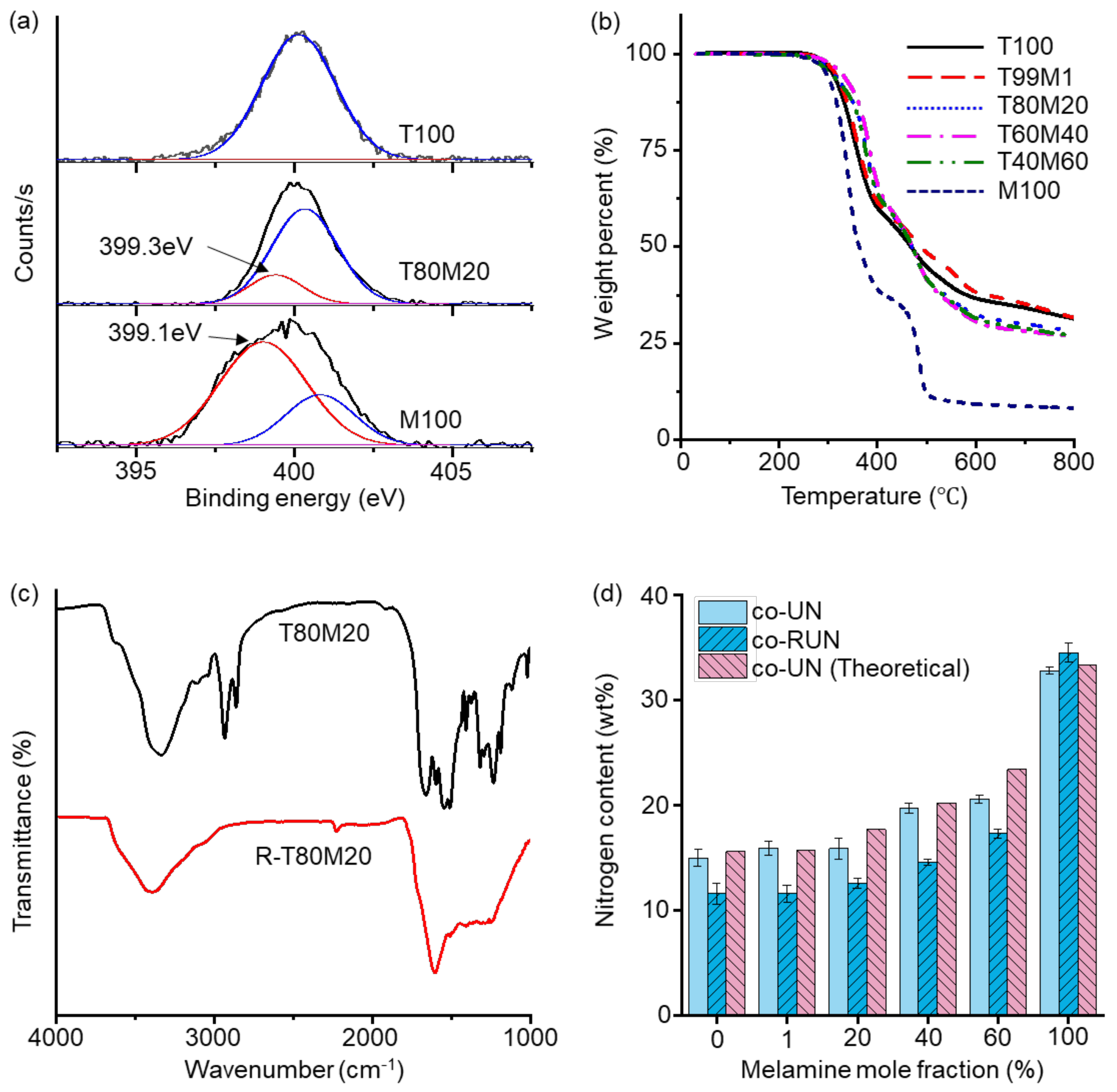
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ben, T.; Zhang, B.; Fu, Y.; Qiu, S. Ultrahigh Gas Storage both at Low and High Pressures in KOH-Activated Carbonized Porous Aromatic Frameworks. Sci. Rep. 2013, 3, 2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.; Le, V.-D.; Maiti, U.N.; Hwang, J.O.; Park, W.J.; Lim, J.; Lee, K.E.; Bae, Y.-S.; Kim, Y.-H.; Kim, S.O. Selective and Regenerative Carbon Dioxide Capture by Highly Polarizing Porous Carbon Nitride. ACS Nano 2015, 9, 9148–9157. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.-F.; Zhong, J.-J.; Qian, X.; Song, S.-L.; Zhang, Y.-G.; Li, D.-H. Nitrogen-Enriched Porous Polyacrylonitrile-Based Carbon Fibers for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hung, W.-S.; El-Mahdy, A.F.M.; Ahmed, M.M.M.; Dai, L.; Chen, T.; Kuo, S.-W. High-Molecular-Weight PLA-b-PEO-b-PLA Triblock Copolymer Templated Large Mesoporous Carbons for Supercapacitors and CO2 Capture. Polymers 2020, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chin, W.-T.; Huang, C.-C. The Application of Hollow Carbon Nanofibers Prepared by Electrospinning to Carbon Dioxide Capture. Polymers 2021, 13, 3275. [Google Scholar] [CrossRef] [PubMed]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Estevez, L.; Barpaga, D.; Zheng, J.; Sabale, S.; Patel, R.L.; Zhang, J.-G.; McGrail, B.P.; Motkuri, R.K. Hierarchically Porous Carbon Materials for CO2 Capture: The Role of Pore Structure. Ind. Eng. Chem. Res. 2018, 57, 1262–1268. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.; Choi, W.; Kim, C.; Choi, M. Oxidation-stable amine-containing adsorbents for carbon dioxide capture. Nat. Commun. 2018, 9, 726. [Google Scholar] [CrossRef] [Green Version]
- Politakos, N.; Barbarin, I.; Cordero-Lanzac, T.; Gonzalez, A.; Zangi, R.; Tomovska, R. Reduced Graphene Oxide/Polymer Monolithic Materials for Selective CO2 Capture. Polymers 2020, 12, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, J.W.F.; He, J.; Mei, J.; Haghpanah, R.; Chen, Z.; Kurosawa, T.; Chen, S.; Bae, W.-G.; Pan, L.; Tok, J.B.H.; et al. Hierarchical N-Doped Carbon as CO2 Adsorbent with High CO2 Selectivity from Rationally Designed Polypyrrole Precursor. J. Am. Chem. Soc. 2016, 138, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Perna, L.; Nogalska, A.; Ambrogi, V.; Cerruti, P.; Tylkowski, B.; García-Valls, R.; Giamberini, M. Polymer Blends for Improved CO2 Capture Membranes. Polymers 2019, 11, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Wang, T.-T.; Liu, X.-H.; Zou, K.; Deng, W.-Q. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, Y.-C.; Yeh, C.-Y.; Weng, C.-H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef] [Green Version]
- Mehio, N.; Dai, S.; Jiang, D.-e. Quantum Mechanical Basis for Kinetic Diameters of Small Gaseous Molecules. J. Phys. Chem. A 2014, 118, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Zhou, A.; Dong, S.; Shi, K.; Li, B.; Han, J.; O’Hare, D. High-efficiency CO2 separation using hybrid LDH-polymer membranes. Nat. Commun. 2021, 12, 3069. [Google Scholar] [CrossRef]
- Allred, A.L.; Hensley, A.L. Electronegativities of nitrogen, phosphorus, arsenic, antimony and bismuth. J. Inorg. Nucl. Chem. 1961, 17, 43–54. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-Y.; Jeon, E.; Bae, J.-S.; Park, M.-K.; Kim, C.; Noh, D.Y.; Lee, E.; Park, J.-W. Thermo-processable covalent scaffolds with reticular hierarchical porosity and their high efficiency capture of carbon dioxide. J. Mater. Chem. A 2015, 3, 14871–14875. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Li, S.; Chung, J.J.; Nommeots-Nomm, A.; Solanki, A.K.; Stevens, M.M.; Jones, J.R. Ductile silica/methacrylate hybrids for bone regeneration. J. Mater. Chem. B 2016, 4, 6032–6042. [Google Scholar] [CrossRef]
- John, Ł.; Janeta, M.; Szafert, S. Synthesis of cubic spherosilicates for self-assembled organic–inorganic biohybrids based on functionalized methacrylates. New J. Chem. 2018, 42, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-Y.; Bae, J.-S.; Jeon, E.; Park, J.-W. Organic Sol–Gel Synthesis: Solution-Processable Microporous Organic Networks. Angew. Chem. Int. Ed. 2010, 49, 9504–9508. [Google Scholar] [CrossRef]
- Oh, W.; Park, J.-W. Facile Synthesis of Robust and Pore-Size-Tunable Nanoporous Covalent Framework Membrane by Simultaneous Gelation and Phase Separation of Covalent Network/Poly(methyl methacrylate) Mixture. ACS Appl. Mater. Interfaces 2019, 11, 32398–32407. [Google Scholar] [CrossRef]
- Yeo, K.E.; Oh, W.; Jeon, E.; Bae, J.-S.; Nam, J.; Park, J.-W. One-Pot Single-Step Route toward Bicontinuous Nanoporous Membranes of an Organic–Inorganic Core–Shell Network. Chem. Mater. 2020, 32, 8318–8324. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Mo, H.-R.; Ahn, M.-K.; Bae, J.-S.; Jeon, E.; Park, J.-W. Organic sol–gel synthesis of microporous molecular networks containing spirobifluorene and tetraphenylmethane nodes. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1758–1766. [Google Scholar] [CrossRef]
- Ganesan, P.; Yang, X.; Loos, J.; Savenije, T.J.; Abellon, R.D.; Zuilhof, H.; Sudhölter, E.J.R. Tetrahedral n-Type Materials: Efficient Quenching of the Excitation of p-Type Polymers in Amorphous Films. J. Am. Chem. Soc. 2005, 127, 14530–14531. [Google Scholar] [CrossRef]
- Alexey, D.; Graaf, A.; Sanden, M.C.M.; Maslakov, K.; Naumkin, A.V.; Serov, A.A. X-ray Photoelectron Spectroscopy Reference Data for Identification of the C3N4 Phase in Carbon–Nitrogen Films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar]
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S.; Zhou, N.; Ding, K.; Fan, L.; Peng, P.; Min, M.; Cheng, Y.; et al. Chapter 14—Gasification Technologies and Their Energy Potentials. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–206. [Google Scholar]
- Jang, Y.H.; Hwang, S.; Chang, S.B.; Ku, J.; Chung, D.S. Acid Dissociation Constants of Melamine Derivatives from Density Functional Theory Calculations. J. Phys. Chem. A 2009, 113, 13036–13040. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Chen, J.; Sun, J. Determination of aniline and its derivatives in environmental water by capillary electrophoresis with on-line concentration. Int. J. Mol. Sci. 2012, 13, 6863–6872. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, J.; Jeon, E.; Moon, S.-Y.; Park, J.-W. Rearranged Copolyurea Networks for Selective Carbon Dioxide Adsorption at Room Temperature. Polymers 2021, 13, 4004. https://doi.org/10.3390/polym13224004
Nam J, Jeon E, Moon S-Y, Park J-W. Rearranged Copolyurea Networks for Selective Carbon Dioxide Adsorption at Room Temperature. Polymers. 2021; 13(22):4004. https://doi.org/10.3390/polym13224004
Chicago/Turabian StyleNam, Junsik, Eunkyung Jeon, Su-Young Moon, and Ji-Woong Park. 2021. "Rearranged Copolyurea Networks for Selective Carbon Dioxide Adsorption at Room Temperature" Polymers 13, no. 22: 4004. https://doi.org/10.3390/polym13224004