Chemical Structures of Adhesive and Interphase Parts in Sucrose/Citric Acid Type Adhesive Wood-Based Molding Derived from Japanese Cedar (Cryptomeria japonica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molding Preparation
2.3. Sample Preparation in HSQC-NMR
2.4. 2D HSQC-NMR Spectroscopy
3. Results and Discussion
3.1. Adhesive Structure of the Molding: Formation of Furan Structures
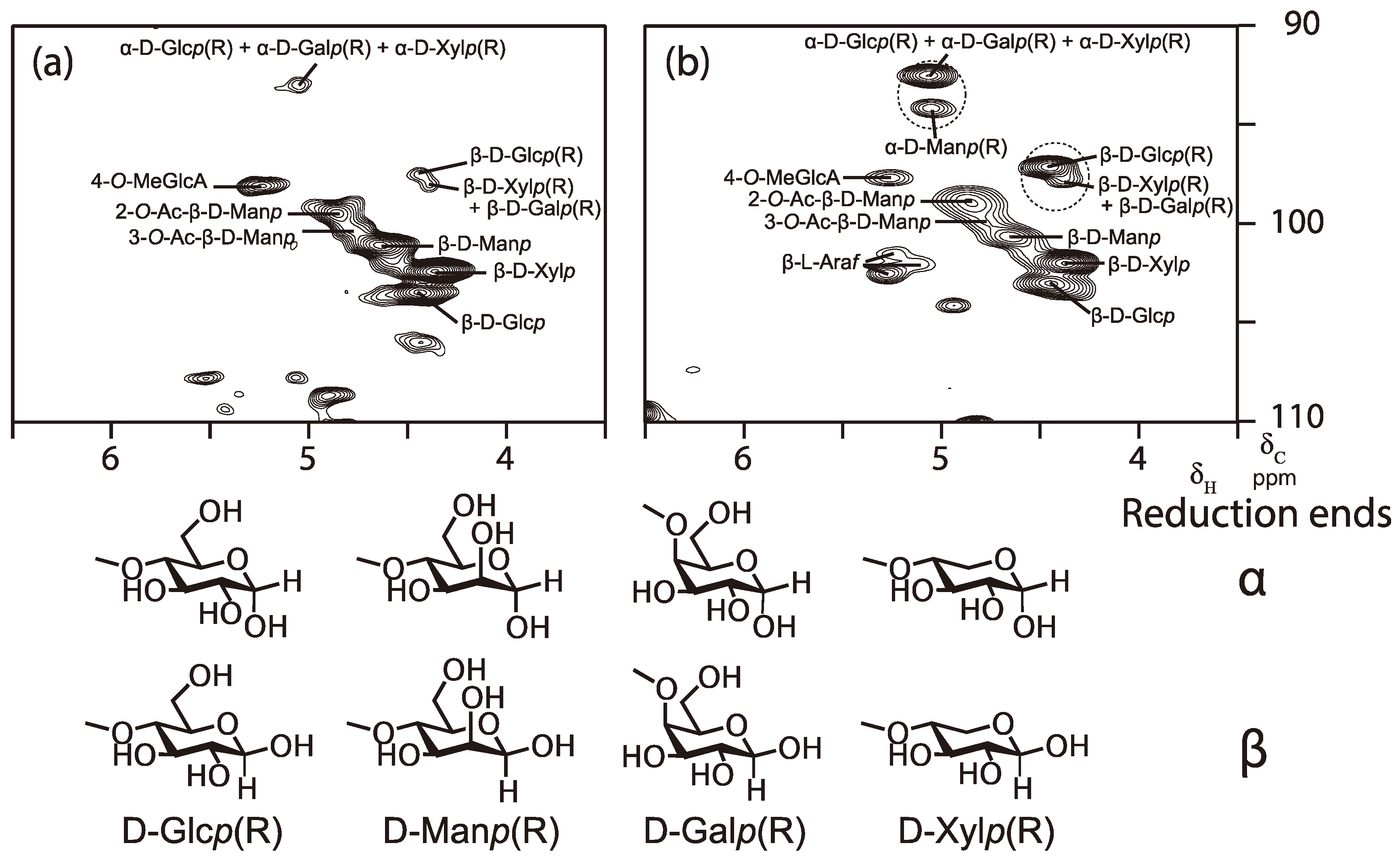
3.2. Interphase Structures between Adhesive and Wood: Esterification and Polysaccharide Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Youngquist, J.A. Wood-based composites and panel products. In Wood Handbook: Wood as an Engineering Material; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 1999; pp. 1–31. [Google Scholar]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Dunky, M. Urea–formaldehyde (UF) adhesive resins for wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Santoni, I.; Pizzo, B. Evaluation of alternative vegetable proteins as wood adhesives. Ind. Crop. Prod. 2013, 45, 148–154. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A Novel Eco-friendly Blood Meal-based Bio-adhesive: Preparation and Performance. J. Polym. Environ. 2017, 26, 607–615. [Google Scholar] [CrossRef]
- Desai, S.D.; Patel, J.V.; Sinha, V.K. Polyurethane adhesive system from biomaterial-based polyol for bonding wood. Int. J. Adhes. Adhes. 2003, 23, 393–399. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crop. Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Addis, C.C.; Koh, R.S.; Gordon, M.B. Preparation and characterization of a bio-based polymeric wood adhesive derived from linseed oil. Int. J. Adhes. Adhes. 2020, 102, 102655. [Google Scholar] [CrossRef]
- Dunky, M. Wood Adhesives Based on Natural Resources: A Critical Review Part II. Carbohydrate-Based Adhesives. Rev. Adhes. Adhes. 2020, 8, 333–378. [Google Scholar] [CrossRef]
- Yazaki, Y. Utilization of flavonoid compounds from bark and wood: A Review. Nat. Prod. Commun. 2015, 10, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Aristri, M.; Lubis, M.; Yadav, S.; Antov, P.; Papadopoulos, A.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Kawai, S. Effects of Moulding Temperature on the Physical Properties of Wood-Based Moulding Bonded with Citric Acid. For. Prod. J. 2012, 62, 63–68. [Google Scholar] [CrossRef]
- Umemura, K.; Ueda, T.; Kawai, S. Characterization of wood-based molding bonded with citric acid. J. Wood Sci. 2011, 58, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiansen, A.W.; Gillespie, R.H. Potential of carbohydrates for exterior-type adhesives. For. Prod. J. 1986, 36, 20–28. [Google Scholar]
- Conner, A.H.; River, B.H.; Lorenz, L.F. Carbohydrate Modfied Phenol-Formaldehyde Resins. J. Wood Chem. Technol. 1986, 6, 591–613. [Google Scholar] [CrossRef]
- Trosa, A.; Pizzi, A. Industrial hardboard and other panels binder from waste lignocellulosic liquors/phenol-formaldehyde resins. Holz Roh Werkst. 1998, 56, 229–233. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-Free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Basta, A.; El-Saied, H.; Winandy, J.E.; Sabo, R. Preformed Amide-containing biopolymer for Improving the Environmental Performance of Synthesized Urea–formaldehyde in Agro-fiber Composites. J. Polym. Environ. 2011, 19, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Belgacem, M.N.; Gandini, A. Furan-based adhesives. In Handbook of Adhesive Technology, 2nd ed.; Pizzi, A., Mittal, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 615–634. [Google Scholar]
- Zhang, D.; Zhang, A.; Xue, L. A review of preparation of binderless fiberboards and its self-bonding mechanism. Wood Sci. Technol. 2015, 49, 661–679. [Google Scholar] [CrossRef]
- Kawamoto, H. Review of Reactions and Molecular Mechanisms in Cellulose Pyrolysis. Curr. Org. Chem. 2016, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, R.-J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Jeong, J.; Antonyraj, C.A.; Shin, S.; Kim, S.; Kim, B.; Lee, K.-Y.; Cho, J.K. Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup. J. Ind. Eng. Chem. 2012, 19, 1106–1111. [Google Scholar] [CrossRef]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard. J. Wood Sci. 2013, 59, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Umemura, K.; Sugihara, O.; Kawai, S. Investigation of a new natural adhesive composed of citric acid and sucrose for particleboard II: Effects of board density and pressing temperature. J. Wood Sci. 2014, 61, 40–44. [Google Scholar] [CrossRef]
- Widyorini, R.; Nugraha, P.A.; Rahman, M.Z.A.; Prayitno, T.A. Bonding Ability of a New Adhesive Composed of Citric Acid-Sucrose for Particleboard. BioResources 2016, 11, 4526–4535. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Kusumah, S.S.; Subyakto; Umemura, K. Modification of novel bio-based adhesive made from citric acid and sucrose by ZnCl2. Int. J. Adhes. Adhes. 2021, 108, 102866. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of Sucrose-Citric Acid Adhesive: Synthesis and Application on Plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further Exploration of Sucrose–Citric Acid Adhesive: Investigation of Optimal Hot-Pressing Conditions for Plywood and Curing Behavior. Polymers 2019, 11, 1996. [Google Scholar] [CrossRef] [Green Version]
- Ando, D.; Umemura, K. Bond Structures between Wood Components and Citric Acid in Wood-Based Molding. Polymers 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J.; Akiyama, T. Solution-state 2D NMR of Ball-milled Plant Cell Wall Gels in DMSO-d 6. BioEnergy Res. 2008, 1, 56–66. [Google Scholar] [CrossRef]
- Kim, H.; Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5. Org. Biomol. Chem. 2009, 8, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Kishimoto, T.; Kishino, M.; Hamada, M.; Nakajima, N. Heteronuclear Single-Quantum Coherence Nuclear Magnetic Resonance (HSQC NMR) Characterization of Acetylated Fir (Abies sachallnensis MAST) Wood Regenerated from Ionic Liquid. J. Agric. Food Chem. 2011, 59, 5382–5389. [Google Scholar] [CrossRef] [PubMed]
- Ando, D.; Nakatsubo, F.; Yano, H. Acetylation of Ground Pulp: Monitoring Acetylation via HSQC-NMR Spectroscopy. ACS Sustain. Chem. Eng. 2016, 5, 1755–1762. [Google Scholar] [CrossRef]
- Locas, C.P.; Yaylayan, V.A. Isotope Labeling Studies on the Formation of 5-(Hydroxymethyl)-2-furaldehyde (HMF) from Sucrose by Pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef]
- Woo, K.S.; Hwang, I.G.; Lee, Y.R.; Lee, J.; Jeong, H.S. Characteristics of Sucrose Thermal Degradation with High Temperature and High Pressure Treatment. Food Sci. Biotechnol. 2009, 18, 717–723. [Google Scholar]
- Lee, J.W.; Thomas, L.C.; Jerrell, J.; Feng, H.; Cadwallader, K.R.; Schmidt, S.J. Investigation of Thermal Decomposition as the Kinetic Process That Causes the Loss of Crystalline Structure in Sucrose Using a Chemical Analysis Approach (Part II). J. Agric. Food Chem. 2010, 59, 702–712. [Google Scholar] [CrossRef]
- Chuda, Y.; Ono, H.; Ohnishi-Kameyama, M.; Matsumoto, K.; Nagata, T.; Kikuchi, Y. Mumefural, Citric Acid Derivative Improving Blood Fluidity from Fruit-Juice Concentrate of Japanese Apricot (Prunus mume Sieb. et Zucc). J. Agric. Food Chem. 1999, 47, 828–831. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, D.; Umemura, K. Chemical Structures of Adhesive and Interphase Parts in Sucrose/Citric Acid Type Adhesive Wood-Based Molding Derived from Japanese Cedar (Cryptomeria japonica). Polymers 2021, 13, 4224. https://doi.org/10.3390/polym13234224
Ando D, Umemura K. Chemical Structures of Adhesive and Interphase Parts in Sucrose/Citric Acid Type Adhesive Wood-Based Molding Derived from Japanese Cedar (Cryptomeria japonica). Polymers. 2021; 13(23):4224. https://doi.org/10.3390/polym13234224
Chicago/Turabian StyleAndo, Daisuke, and Kenji Umemura. 2021. "Chemical Structures of Adhesive and Interphase Parts in Sucrose/Citric Acid Type Adhesive Wood-Based Molding Derived from Japanese Cedar (Cryptomeria japonica)" Polymers 13, no. 23: 4224. https://doi.org/10.3390/polym13234224





