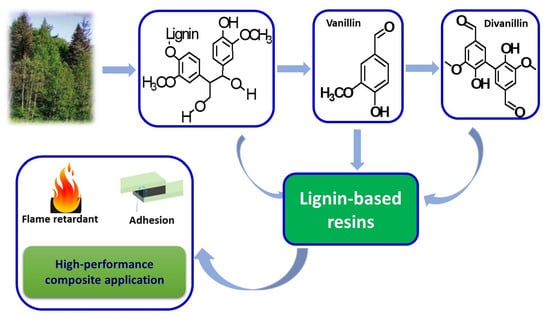
Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications
Abstract
:1. Introduction
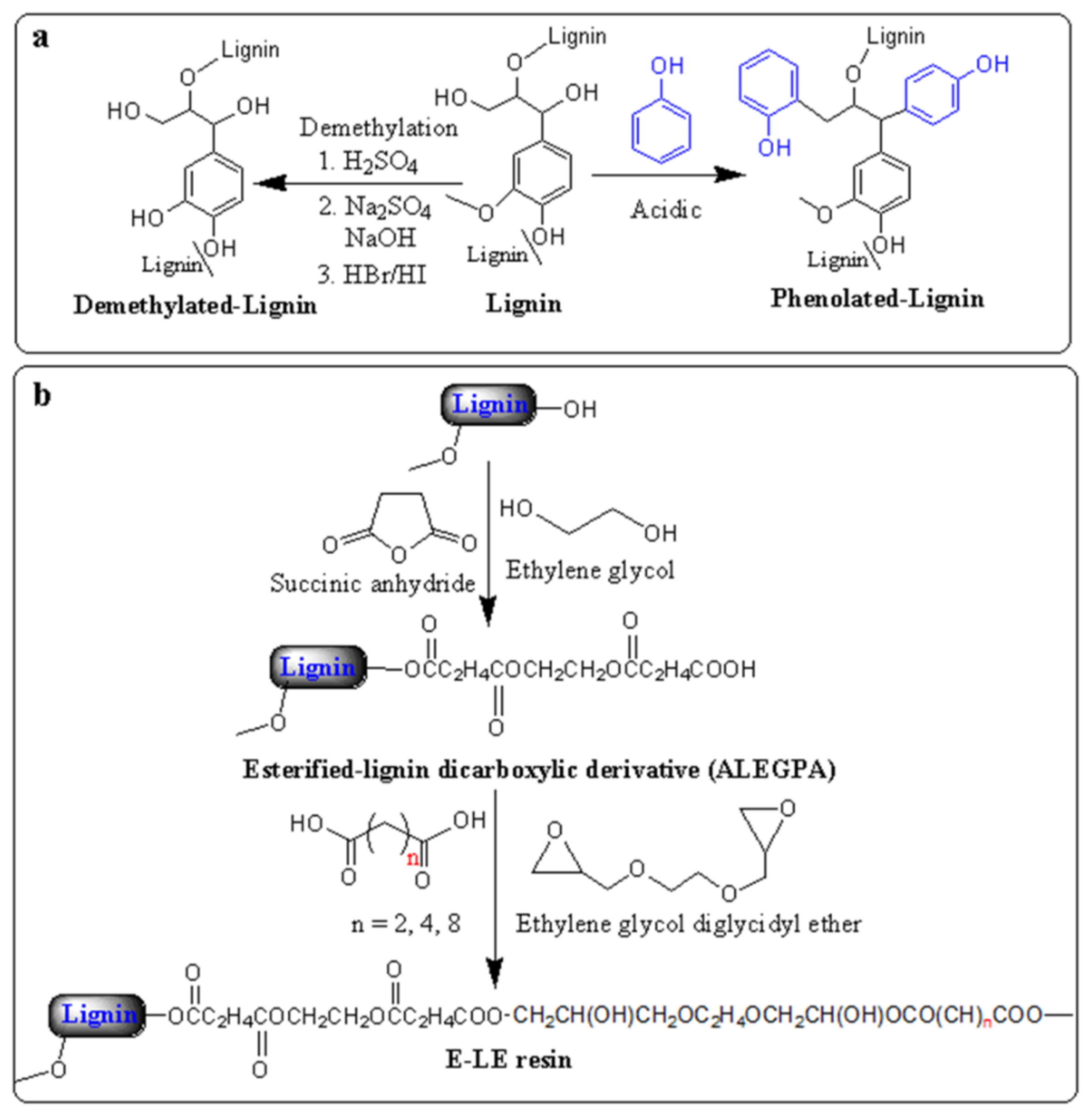
2. Lignin-Based Resins
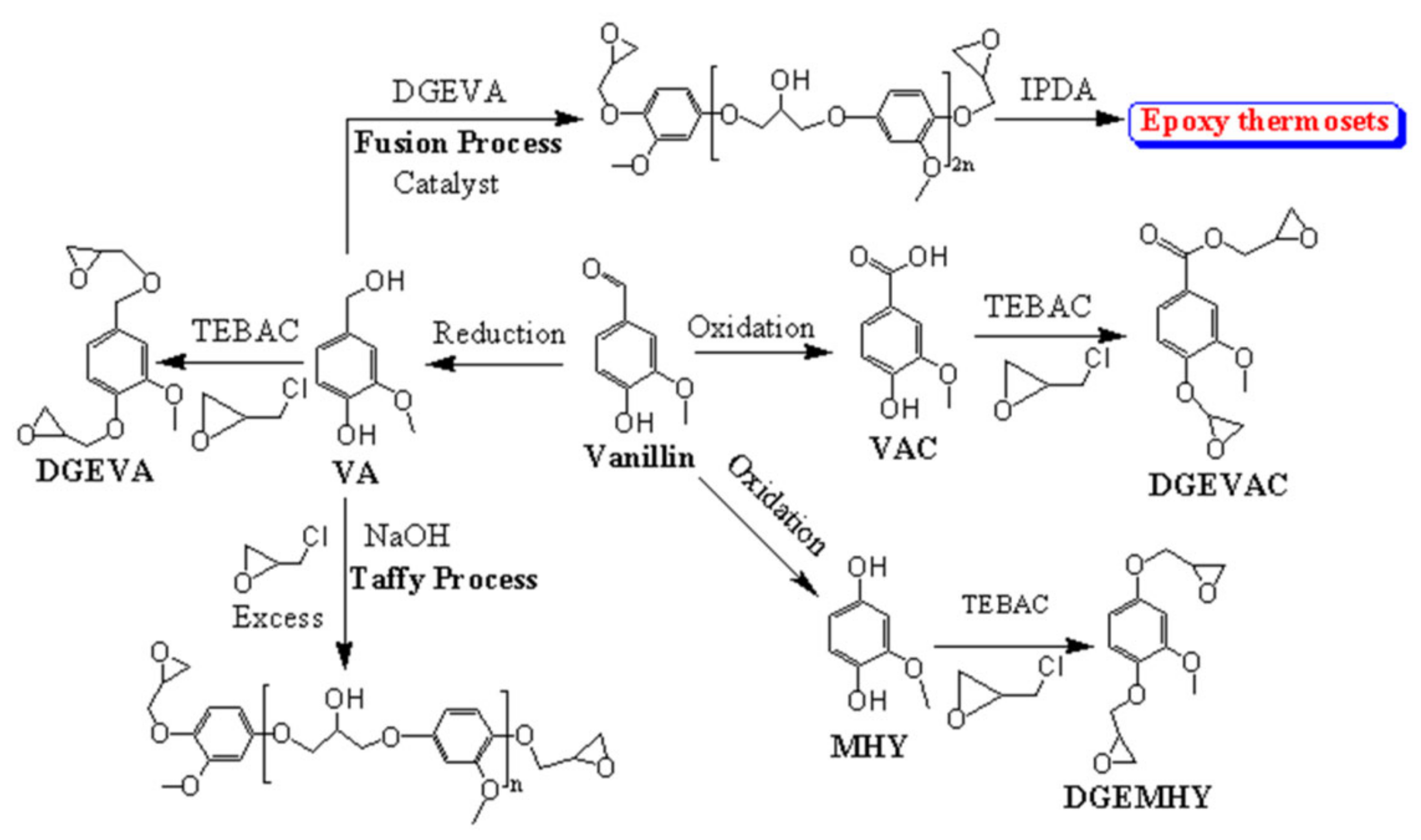
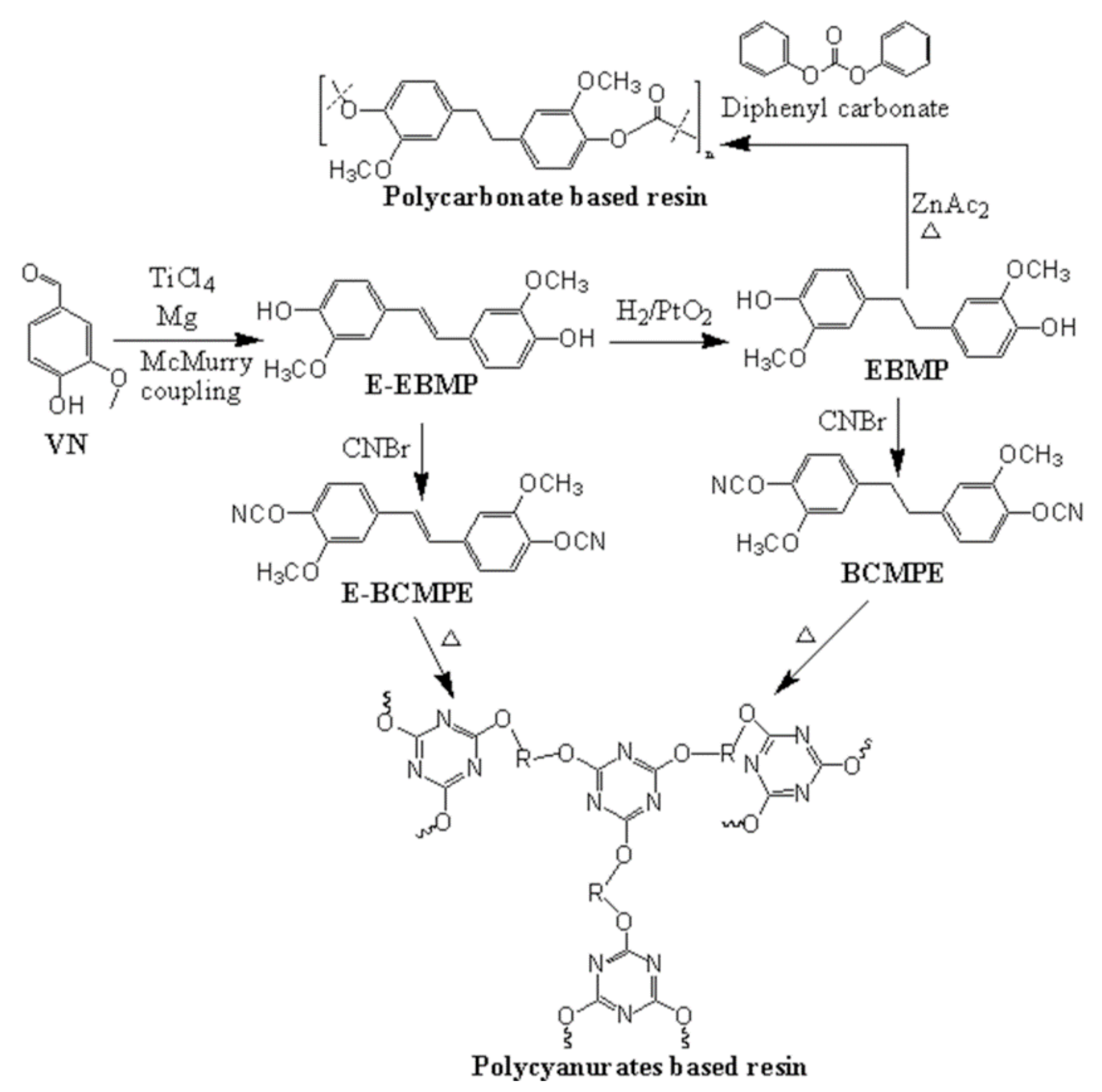
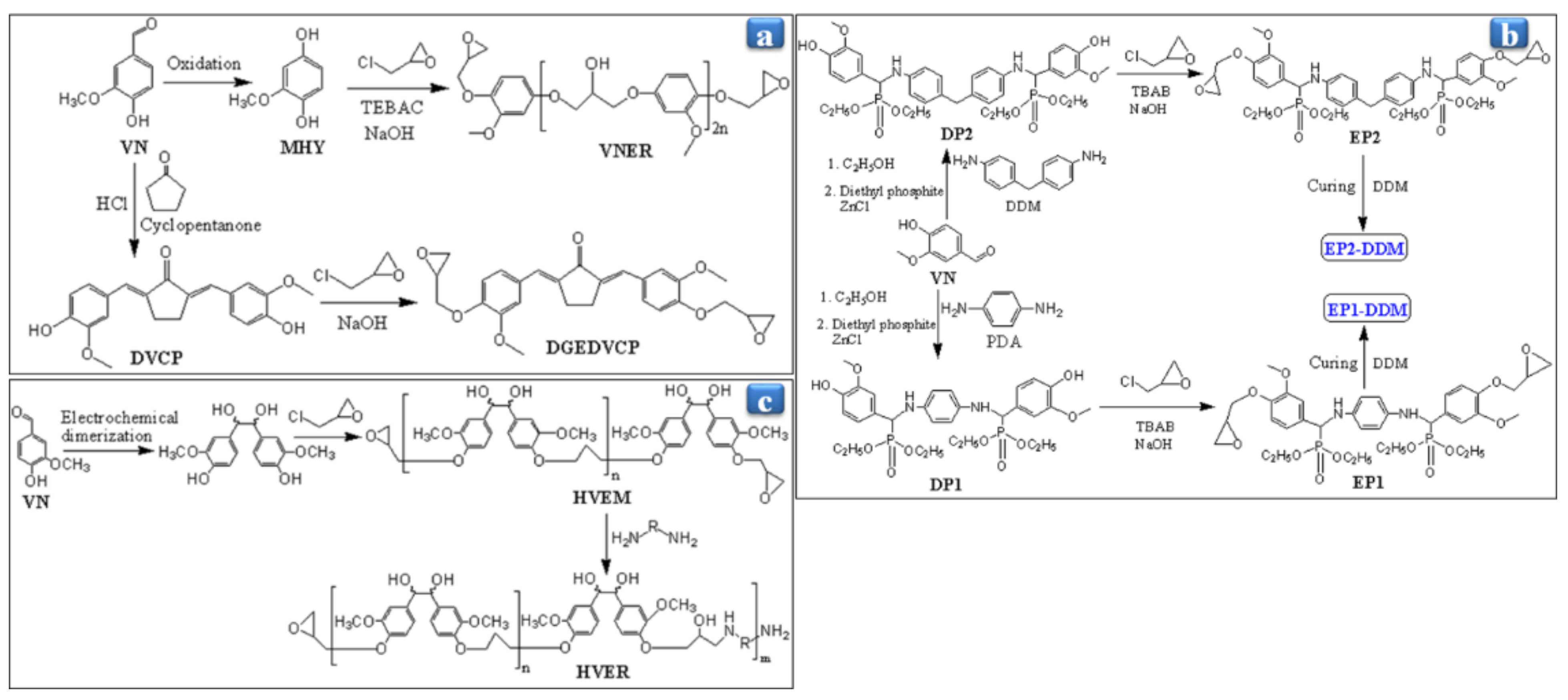
3. VN-Based Resins
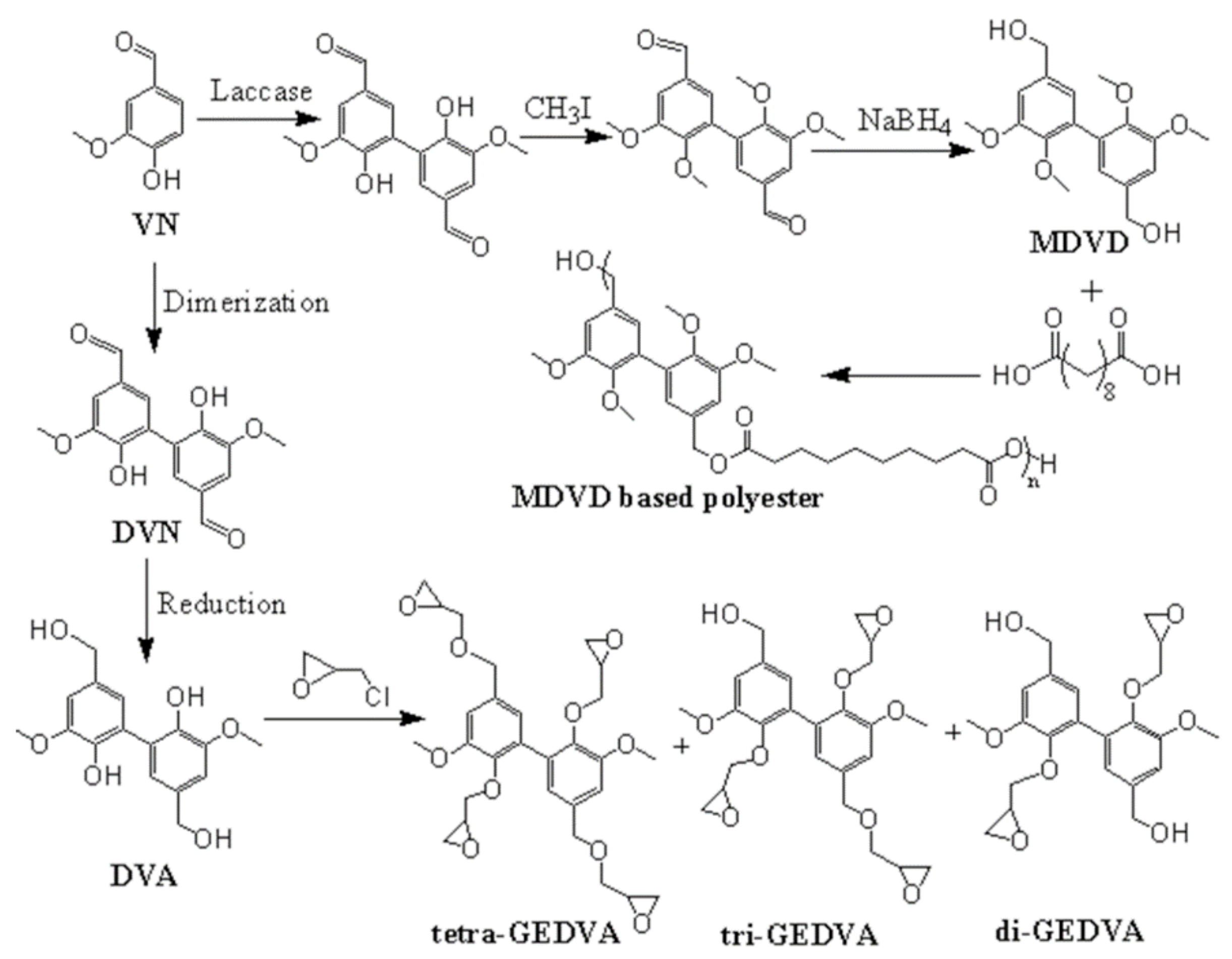
4. Divanillin-Based Resins
5. Applications of Lignin-Based Resins
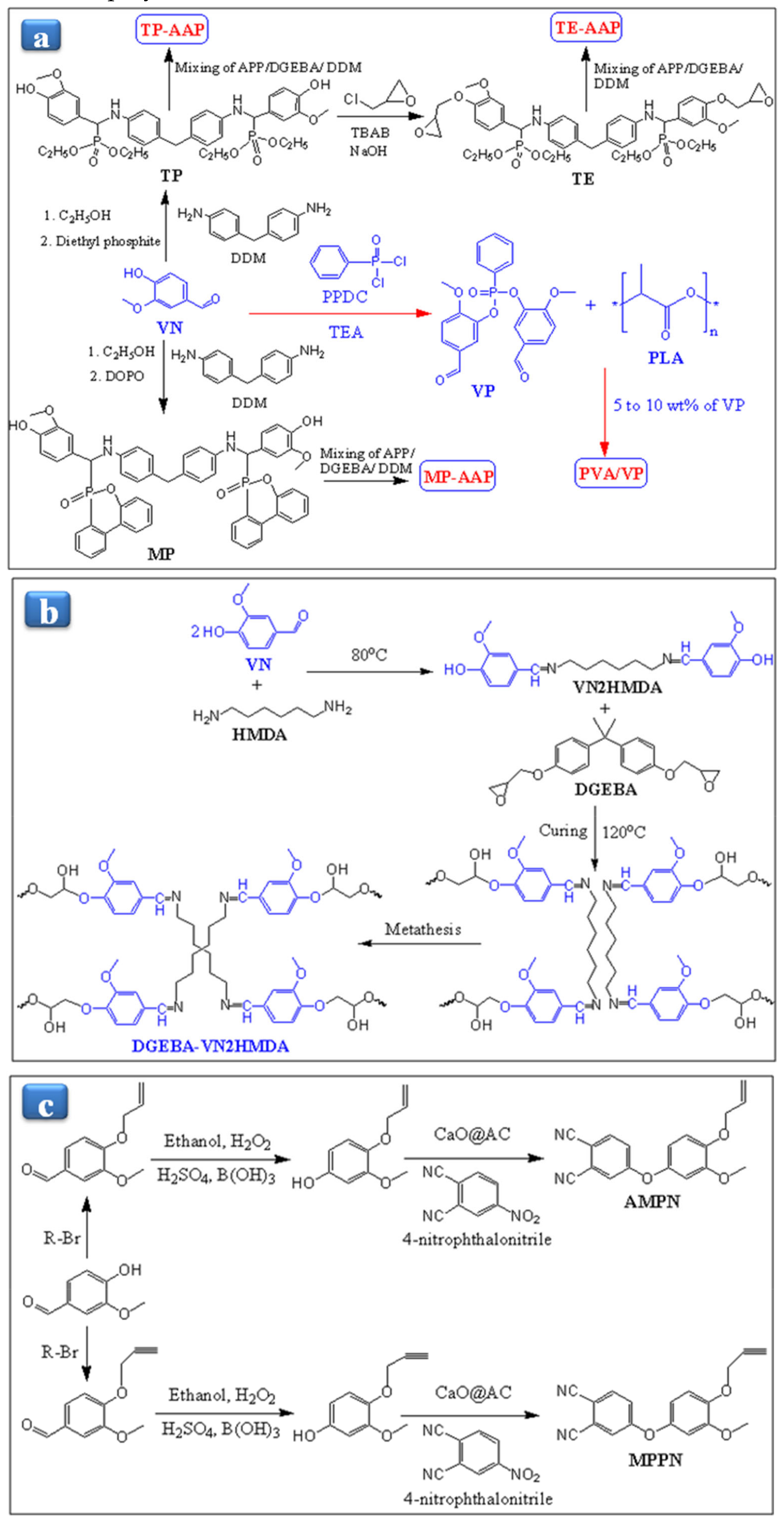
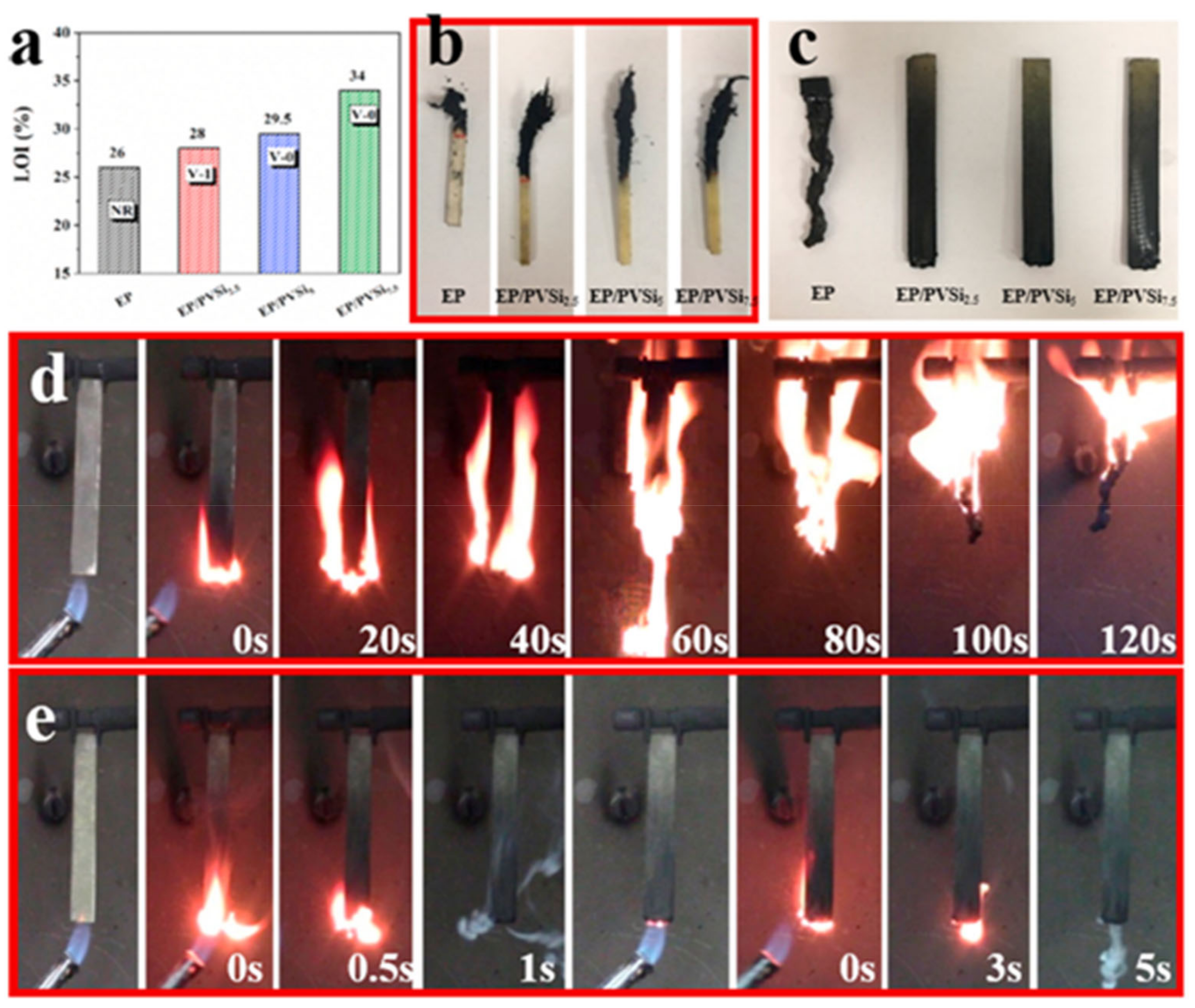
6. Applications of Vanillin-Based Resins
7. Applications of Divanillin-Based Resin
8. Conclusions
9. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, J.; Wang, W.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins. RSC Adv. 2016, 6, 67435–67443. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin biopolymers in the age of controlled polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [Green Version]
- Dastpak, A.; Lourençon, T.V.; Balakshin, M.; Farhan Hashmi, S.; Lundström, M.; Wilson, B.P. Solubility study of lignin in industrial organic solvents and investigation of electrochemical properties of spray-coated solutions. Ind. Crops Prod. 2020, 148, 112310. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular Lignin Solubility and Structure in Organic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 17839–17850. [Google Scholar] [CrossRef]
- Toh, K.; Yokoyama, H.; Noda, H.; Yuguchi, Y. Antioxidant capacity of lignin from green tea waste. J. Food Biochem. 2010, 34, 192–206. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Applicability of lignins from different sources as antioxidants based on the protective effects on lipid peroxidation induced by oxygen radicals. Ind. Crops Prod. 2009, 30, 184–187. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Varanasi, P.; Singh, P.; Auer, M.; Adams, P.D.; Simmons, B.A.; Singh, S. Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.N.; De Sousa, D.P. Overview of the role of vanillin on redox status and cancer development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef] [Green Version]
- Barghini, P.; Di Gioia, D.; Fava, F.; Ruzzi, M. Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb. Cell Fact. 2007, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Xia, H.; Yu, T.; Jin, L.; Li, X.; Zhang, Y.; Shu, L.; Zeng, L.; He, Z. A colorimetric assay for vanillin detection by determination of the luminescence of o-toluidine condensates. PLoS ONE 2018, 13, e0194010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Shimasaki, T.; Ogihara, M. High-performance bio-based bismaleimide resins using succinic acid and eugenol. Polym. J. 2011, 43, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-based polymers with potential for biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Kint, D.; Muñoz-Guerra, S. A review on the potential biodegradability of poly(ethylene terephthalate). Polym. Int. 1999, 48, 346–352. [Google Scholar] [CrossRef]
- Huang, X.; Li, C.; Zheng, L.; Zhang, D.; Guan, G.; Xiao, Y. Synthesis, characterization and properties of biodegradable poly(butylene succinate)-block-poly(propylene glycol)segmented copolyesters. Polym. Int. 2009, 58, 893–899. [Google Scholar] [CrossRef]
- Woolnough, C.A.; Yee, L.H.; Charlton, T.; Foster, L.J.R. Environmental degradation and biofouling of “green” plastics including short and medium chain length polyhydroxyalkanoates. Polym. Int. 2010, 59, 658–667. [Google Scholar] [CrossRef]
- Woolnough, C.A.; Charlton, T.; Yee, L.H.; Sarris, M.; Foster, L.J.R. Surface changes in polyhydroxyalkanoate films during biodegradation and biofouling. Polym. Int. 2008, 57, 1042–1051. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.G.; Dubois, P. Modification of Poly(ethylene 2,5-furandicarboxylate) with Biobased 1,5-Pentanediol: Significantly Toughened Copolyesters Retaining High Tensile Strength and O2 Barrier Property. Biomacromolecules 2019, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Shibata, M.; Itakura, Y.; Watanabe, H. Bio-based thermosetting resins composed of cardanol novolac and bismaleimide. Polym. J. 2013, 45, 758–765. [Google Scholar] [CrossRef]
- Jian, X.Y.; An, X.P.; Li, Y.D.; Chen, J.H.; Wang, M.; Zeng, J.B. All Plant Oil Derived Epoxy Thermosets with Excellent Comprehensive Properties. Macromolecules 2017, 50, 5729–5738. [Google Scholar] [CrossRef]
- Kamjornsupamitr, T.; Hunt, A.J.; Supanchaiyamat, N. Development of hyperbranched crosslinkers from bio-derived platform molecules for the synthesis of epoxidised soybean oil based thermosets. RSC Adv. 2018, 8, 37267–37276. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Tung, S.H.; Jeng, R.J.; Abu-Omar, M.M.; Lin, C.H. A facile strategy to achieve fully bio-based epoxy thermosets from eugenol. Green Chem. 2019, 21, 4475–4488. [Google Scholar] [CrossRef]
- Nameer, S.; Johansson, M. Fully bio-based aliphatic thermoset polyesters via self-catalyzed self-condensation of multifunctional epoxy monomers directly extracted from natural sources. J. Coat. Technol. Res. 2017, 14, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Jayaramudu, T.; Ko, H.; Chan, H.; Woong, J.; Sik, E.; Kim, J. Adhesion properties of poly (ethylene oxide) -lignin blend for nanocellulose composites. Compos. Part B 2019, 156, 43–50. [Google Scholar] [CrossRef]
- Marotta, A.; Ambrogi, V.; Cerruti, P.; Mija, A. Green approaches in the synthesis of furan-based diepoxy monomers. RSC Adv. 2018, 8, 16330–16335. [Google Scholar] [CrossRef] [Green Version]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.U.; Kim, J.W.; Kim, H.C.; Zhai, L.; Kim, J. Esterified PVA-lignin resin by maleic acid applicable for natural fiber reinforced composites. J. Appl. Polym. Sci. 2019, 137, 48836. [Google Scholar] [CrossRef]
- Ko, H.U.; Zhai, L.; Park, J.H.; Lee, J.Y.; Kim, D.; Kim, J. Poly(vinyl alcohol)–lignin blended resin for cellulose-based composites. J. Appl. Polym. Sci. 2018, 135, 46655. [Google Scholar] [CrossRef]
- Bobade, S.K.; Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Bio-Based Thermosetting Resins for Future Generation: A Review. Polym. Plast. Technol. Eng. 2016, 55, 1863–1896. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A Review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N.; Capricho, J.C. Multifunctionality in Epoxy Resins Multifunctionality in Epoxy Resins. Polym. Rev. 2020, 60, 1–41. [Google Scholar] [CrossRef]
- Farmer, T.J.; Comerford, J.W.; Robert, T. Post-polymerization modification of bio-based polymers: Maximizing the high functionality of polymers derived from biomass. Polym. Int. 2018, 67, 775–789. [Google Scholar] [CrossRef]
- Park, Y.; Doherty, W.O.S.; Halley, P.J. Developing lignin-based resin coatings and composites. Ind. Crops Prod. 2008, 27, 163–167. [Google Scholar] [CrossRef]
- Rico-Garc, D.; Ruiz-Rubio, L.; Leyre, P. Lignin-Based Hydrogels: Synthesis and Applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Yang, L.; Seshan, K.; Li, Y. A review on thermal chemical reactions of lignin model compounds. Catal. Today 2017, 298, 276–297. [Google Scholar] [CrossRef]
- Sawamura, K.; Tobimatsu, Y.; Kamitakahara, H.; Takano, T. Lignin Functionalization through Chemical Demethylation: Preparation and Tannin-Like Properties of Demethylated Guaiacyl-Type Synthetic Lignins. ACS Sustain. Chem. Eng. 2017, 5, 5424–5431. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Liu, W.; Yao, Y.; Fu, O.; Jiang, S.; Fang, Y.; Wei, Y.; Lu, X. Lignin-derived carbon nanosheets for high-capacitance supercapacitors. RSC Adv. 2017, 7, 48537–48543. [Google Scholar] [CrossRef]
- Kumari, S.; Chauhan, G.S.; Monga, S.; Kaushik, A.; Ahn, J.H. New lignin-based polyurethane foam for wastewater treatment. RSC Adv. 2016, 6, 77768–77776. [Google Scholar] [CrossRef]
- Wang, H.; Eberhardt, T.L.; Wang, C.; Gao, S.; Pan, H. Demethylation of alkali lignin with halogen acids and its application to phenolic resins. Polymers 2019, 11, 1771. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Fast curing bio-based phenolic resins via lignin demethylated under mild reaction condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Sheng, X.; Yu, S.; Li, H.; Lu, J.; Zhou, J.; Wang, H. Preparation and characterization of thermo-sensitive gel with phenolated alkali lignin. Sci. Rep. 2018, 8, 14450. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Jiang, X.; Lin, J.; Zhao, G.; Chang, H.M.; Jameel, H. Reactivity improvement by phenolation of wheat straw lignin isolated from a biorefinery process. New J. Chem. 2019, 43, 2238–2246. [Google Scholar] [CrossRef]
- Podschun, J.; Saake, B.; Lehnen, R. Reactivity enhancement of organosolv lignin by phenolation for improved bio-based thermosets. Eur. Polym. J. 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Lignin in straw and its applications as an adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. [Google Scholar] [CrossRef]
- Hirose, S.; Hatakeyama, T.; Hatakeyama, H. Glass transition and thermal decomposition of epoxy resins from the carboxylic acid system consisting of ester-carboxylic acid derivatives of alcoholysis lignin and ethylene glycol with various dicarboxylic acids. Thermochim. Acta 2005, 431, 76–80. [Google Scholar] [CrossRef]
- Hirose, S.; Hatakeyama, T.; Hatakeyama, H. Synthesis and thermal properties of epoxy resins from ester-carboxylic acid derivative of alcoholysis lignin. Macromol. Symp. 2003, 197, 157–169. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties. Thermochim. Acta 2015, 618, 48–55. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2020, 22, 178–183. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S. Is the Kissinger Equation Applicable to the Processes that Occur on Cooling? Macromol. Rapid Commun. 2002, 23, 771–775. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Mititelu-mija, A.; Vincent, L.; Alzina, C. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim. Acta 2006, 447, 167–177. [Google Scholar] [CrossRef]
- Ngo, T.; Hoa, S.V.; Cole, K.C. Curing Kinetics and Mechanical Properties of Epoxy Nanocomposites Based on Different Organoclays. Polym. Eng. Sci. 2007, 47, 649–661. [Google Scholar] [CrossRef]
- Cai, H.; Li, P.; Sui, G.; Yu, Y.; Li, G. Thermochimica Acta Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim. Acta 2008, 473, 101–105. [Google Scholar] [CrossRef]
- Mititelu, A.; Sladic, C.; Vincent, L. A Study of Epoxy—Amine Cure Kinetics by Combining Isoconversional Analysis with Temperature Modulated DSC and Dynamic Rheometry. Macromol. Chem. Phys. 2003, 204, 1815–1821. [Google Scholar] [CrossRef]
- Perrin, F.-X.; Nguyen, T.M.H.; Vernet, J.-L. Kinetic Analysis of Isothermal and Nonisothermal Epoxy—Amine Cures by Model—Free Isoconversional Methods. Macromol. Chem. Phys. 2007, 208, 718–729. [Google Scholar] [CrossRef]
- Chen, H.Z.; Li, Z.Y.; Liu, X.Y.; Tian, Y.M.; Yang, L.; Wang, Z.C. Depolymerization of renewable resources-lignin by sodium hydroxide as a catalyst and its applications to epoxy resin. J. Appl. Polym. Sci. 2015, 132, 42176. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Synthesis of Renewable Thermoset Polymers through Successive Lignin Modification Using Lignin-Derived Phenols. ACS Sustain. Chem. Eng. 2017, 5, 5059–5066. [Google Scholar] [CrossRef]
- Koike, T. Progress in Development of Epoxy Resin Systems Based on Wood Biomass in Japan. Polym. Eng. Sci. 2006, 52, 701–717. [Google Scholar] [CrossRef]
- Wu, S.; Guo, Q.; Kraska, M.; Mai, Y. Toughening Epoxy Thermosets with Block Ionomers: The Role of Phase Domain Size. Macromolecules 2013, 46, 8190–8202. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Zhang, J.; Li, S.; Li, M.; Xia, J.; Zhou, Y. Epoxy Monomers Derived from Tung Oil Fatty Acids and Its Regulable Thermosets Cured in Two Synergistic Ways. Biomacromolecules 2014, 15, 837–843. [Google Scholar] [CrossRef]
- Garcia, F.G.; Soares, B.G.; Pita, V.J.R.R.; Rieumont, J. Mechanical Properties of Epoxy Networks Based on DGEBA and Aliphatic Amines. J. Appl. Polym. Sci. 2007, 106, 2047–2055. [Google Scholar] [CrossRef]
- Gri, G.; Passoni, V.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodi fi ed Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Van De Pas, D.J.; Torr, K.M. Biobased Epoxy Resins from Deconstructed Native Softwood Lignin. Biomacromolecules 2017, 18, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Van De Pas, D.J.; Nanayakkara, B.; Suckling, I.D.; Torr, K.M. Comparison of hydrogenolysis with thioacidolysis for lignin structural analysis. Holzforschung 2014, 68, 151–155. [Google Scholar] [CrossRef]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild hydrogenolysis of in-situ and isolated Pinus radiata lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Odelius, K.; Hakkarainen, M. One-pot synthesis of lignin thermosets exhibiting widely tunable mechanical properties and shape memory behavior. ACS Sustain. Chem. Eng. 2019, 7, 13456–13463. [Google Scholar] [CrossRef]
- Ono, K.; Tanaike, O.; Ishii, R.; Nakamura, T.; Shikinaka, K.; Ebina, T.; Nge, T.T.; Yamada, T. Solvent-Free Fabrication of an Elastomeric Epoxy Resin Using Glycol Lignin from Japanese Cedar. ACS Omega 2019, 4, 17251–17256. [Google Scholar] [CrossRef]
- Nge, T.T.; Takata, E.; Takahashi, S.; Yamada, T. Isolation and Thermal Characterization of Softwood-Derived Lignin with Thermal Flow Properties. ACS Sustain. Chem. Eng. 2016, 4, 2861–2868. [Google Scholar] [CrossRef]
- Nge, T.T.; Tobimatsu, Y.; Takahashi, S.; Takata, E.; Yamamura, M.; Miyagawa, Y.; Ikeda, T.; Umezawa, T.; Yamada, T. Isolation and Characterization of Polyethylene Glycol (PEG)-Modified Glycol Lignin via PEG Solvolysis of Softwood Biomass in a Large-Scale Batch Reactor. ACS Sustain. Chem. Eng. 2018, 6, 7841–7848. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Rodrigues, A.E. Chemical Engineering Research and Design An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Mai, V.-D.; Shin, S.-R.; Lee, D.-S.; Kang, I. Thermal Healing, Reshaping and Ecofriendly Recycling of Epoxy Resin Crosslinked with Schiff Base of Vanillin and Hexane-1,6-Diamine. Polymers 2019, 11, 293. [Google Scholar] [CrossRef] [Green Version]
- Brazinha, C.; Barbosa, S.; Crespo, G. Green Chemistry Sustainable recovery of pure natural vanillin from fermentation media in a Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Zhang, R.; Maltari, R.; Guo, M.; Kontro, J.; Eronen, A.; Repo, T. Facile synthesis of vanillin from fractionated Kraft lignin. Ind. Crops Prod. 2020, 145, 112095. [Google Scholar] [CrossRef]
- Stanzione, J.F.; Sadler, J.M.; La Scala, J.; Reno, H.; Wool, R.P. Vanillin-based resin for use in composite applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J Sci Food Agric 2019, 99, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Group, I. Vanilla and Vanillin Market Projected to Reach 2969 Tons and 59,458 Tons by 2024. 2020. Available online: https://apnews.com/press-release/pr-wiredrelease/719ba67408806f97267e3c47d0a9dee1 (accessed on 13 February 2021).
- Navaruckiene, A.; Skliutas, E.; Kasetaite, S.; Raudoniene, V.; Bridziuviene, D.; Malinauskas, M.; Ostrauskaite, J. Vanillin Acrylate-Based Resins for Optical 3D Printing. Polymers 2020, 12, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroescu, M.; Stoica-guzun, A.; Isopencu, G.; Jinga, S.I.; Parvulescu, O.; Dobre, T.; Vasilescu, M. Chitosan-vanillin composites with antimicrobial properties. Food Hydrocoll. 2015, 48, 62–71. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Z.; Wang, X.; Pan, Y.-T.; Kuehnert, I.; Gehde, M.; Wang, D.-Y.; Leuteritz, A. Renewable vanillin based flame retardant for poly(lactic acid): A way to enhance flame retardancy and toughness simultaneously. RSC Adv. 2018, 8, 42189–42199. [Google Scholar] [CrossRef] [Green Version]
- Fache, M.; Viola, A.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased epoxy thermosets from vanillin-derived oligomers. Eur. Polym. J. 2015, 68, 526–535. [Google Scholar] [CrossRef]
- Fache, M.; Auvergne, R.; Boutevin, B.; Caillol, S. New vanillin-derived diepoxy monomers for the synthesis of biobased thermosets. Eur. Polym. J. 2015, 67, 527–538. [Google Scholar] [CrossRef]
- Harveya, B.G.; Guenthnerb, A.J.; Meylemansa, H.A.; Hainesa, S.R.L.; Lamisonc, K.R.; Groshensa, T.J.; Cambreaa, L.R.; Davisa, M.C.; Lai, W.W. Renewable Thermosetting Resins and Thermoplastics from Vanillin. Green Chem. 2014. [Google Scholar] [CrossRef]
- Mirmohseni-Namin, A.; Mirmohseni, F. Increasing toughness and tensile strength of an epoxy–diamine system using an inorganic ultra-accelerator. RSC Adv. 2015, 5, 53025–53035. [Google Scholar] [CrossRef]
- Hong, S.; Wu, C. DSC and FTIR analysis of the curing behaviors of epoxy/DICY/solvent open systems. Thermochim. Acta 1998, 316, 167–175. [Google Scholar] [CrossRef]
- Liu, X.D.; Sudo, A.; Endo, T. Efficient Accelerating Effect of Carbonyldiimidazole on Epoxy-Dicyandiamide Curing System. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 250–256. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Bagherjeri, M.A.; Naebe, M. One-pot Synthesis of aminated multi-walled carbon nanotube 1 using thiol-ene 2 Click Chemistry for improvement of epoxy nanocomposites properties. RSC Adv. 2015, 5, 98692–98699. [Google Scholar] [CrossRef]
- Radoman, T.S.; Džunuzović, J.V.; Jeremić, K.B.; Grgur, B.N.; Miličević, D.S.; Popović, I.G.; Džunuzović, E.S. Improvement of epoxy resin properties by incorporation of TiO 2 nanoparticles surface modified with gallic acid esters. Mater. Des. 2014, 62, 158–167. [Google Scholar] [CrossRef]
- Wu, C.; Hsu, S.L. Preparation of Epoxy/Silica and Epoxy/Titania Hybrid Resists via a Sol-Gel Process for Nanoimprint Lithography. J. Phys. Chem. C 2010, 2179–2183. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully biobased epoxy resin systems composed of a vanillin-derived epoxy resin and renewable phenolic hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Liu, Y.L. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer 2001, 42, 3445–3454. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C.; Jabeen, F. Hard and Flexible, Degradable Thermosets from Renewable Bioresources with the Assistance of Water and Ethanol. Macromolecules 2016, 49, 3780–3788. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Naturally Occurring Acids as Cross-Linkers To Yield VOC-Free, High- Performance, Fully Bio-Based, Degradable Thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Liu, H.; Xu, K.; Cai, H.; Su, J.; Liu, X.; Fu, Z. Thermal properties and flame retardancy of novel epoxy based on phosphorus-modified. Polym. Adv. Technol. 2012, 23, 114–121. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Xing, W.; Lu, H.; Hu, Y. A effective flame retardant for epoxy resins based on poly (DOPO substituted dihydroxyl phenyl pentaerythritol diphosphonate). Mater. Chem. Phys. 2011, 125, 536–541. [Google Scholar] [CrossRef]
- Xu, W.; Wirasaputra, A.; Liu, S.; Yuan, Y. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym. Degrad. Stab. 2015, 122, 44–51. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Garcia-obergon, R.; Thompson, A.K. Vanillin-based polymers: IV. Hydrovanilloin epoxy resins. J. Appl. Polym. Sci. 2018, 135, 47000. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.C.; Huang, P. Effect of cure cycle on curing process and hardness for epoxy resin. Express Polym. Lett. 2009, 3, 534–541. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S. Vanillin-derived phosphorus-containing compounds and ammonium polyphosphate as green fire-resistant systems for epoxy resins with balanced properties. Polym. Adv. Technol. 2019, 30, 264–278. [Google Scholar] [CrossRef]
- Pan, X.; Webster, D.C. Impact of Structure and Functionality of Core Polyol in Highly Functional Biobased Epoxy Resins a. Macromol. Rapid Commun. 2011, 32, 1324–1330. [Google Scholar] [CrossRef]
- Yuan, B.; Fan, A.; Yang, M.; Chen, X.; Hu, Y.; Bao, C.; Jiang, S.; Niu, Y.; Zhang, Y.; He, S.; et al. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Zhang, B.; Chen, X.; Wu, Q.; Yang, L. Synthesis of a novel reactive flame retardant containing phosphaphenanthrene and piperidine groups and its application in epoxy resin. Polym. Degrad. Stab. 2017, 146, 250–259. [Google Scholar] [CrossRef]
- Wei, J.; Ma, S.; Yue, H.; Zhu, J. Comparison of Hydrogenated Bisphenol A and Bisphenol A Epoxies: Curing Behavior, Thermal and Mechanical Properties, Shape Memory Properties. Macromol. Res. 2018, 26, 529–538. [Google Scholar] [CrossRef]
- Han, Y.; Tang, D.; Wang, G.; Sun, Y.; Guo, Y.; Zhou, H.; Qiu, W. Phthalonitrile Resins Derived from Vanillin: Synthesis, Curing Behavior, and Thermal Properties. Chin. J. Polym. Sci. 2020, 38, 72–83. [Google Scholar] [CrossRef]
- Ichikawa, H.; Maruoka, K. The Claisen Rearrangement: Methods and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007; ISBN 9783527308255. [Google Scholar]
- Zeng, K.; Zhou, K.; Zhou, S.; Hong, H.; Zhou, H.; Wang, Y.; Miao, P.; Yang, G. Studies on self-promoted cure behaviors of hydroxy-containing phthalonitrile model compounds. Eur. Polym. J. 2009, 45, 1328–1335. [Google Scholar] [CrossRef]
- Sumner, M.J.; Sankarapandian, M.; Mcgrath, J.E.; Riffle, J.S.; Sorathia, U. Flame retardant novolac–bisphthalonitrile structural thermosets. Polymer 2002, 43, 5069–5076. [Google Scholar] [CrossRef]
- Augustine, D.; Mathew, D.; Nair, C.P.R. Mechanistic and kinetic aspects of the curing of phthalonitrile monomers in the presence of propargyl groups. Polymer 2015, 60, 308–317. [Google Scholar] [CrossRef]
- Valdebenito, A.; Encinas, V. Effect of solvent on the free radical polymerization of N, N -dimethylacrylamide. Polym. Int. 2010, 59, 1246–1251. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. The influence of temperature and photoinitiator concentration on photoinitiated polymerization of diacrylate monomer. Cent. Eur. J. Chem. 2005, 3, 721–730. [Google Scholar] [CrossRef]
- Shortall, A.C. How light source and product shade influence cure depth for a contemporary composite. J. Oral. Rehabil. 2005, 32, 906–911. [Google Scholar] [CrossRef]
- Krings, U.; Esparan, V.; Berger, R.G. The taste enhancer divanillin: A review on sources and enzymatic generation. Flavour Fragr. J. 2015, 30, 362–365. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.R.; Baker, S.C.; King, D.S.; De Silva, D.S.; Taylor, J.P. Studies of nitrile oxide cycloadditions, and the phenolic oxidative coupling of vanillin aldoxime by Geobacillus sp. DDS012 from Italian rye grass silage. Org. Biomol. Chem. 2008, 6, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Delomenède, M.; Bedos-Belval, F.; Duran, H.; Vindis, C.; Baltas, M.; Negre-Salvayre, A. Development of Novel Antiatherogenic Biaryls: Design, Synthesis, and Reactivity Me. J. Med. Chem. 2008, 51, 3171–3181. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B.; Razzaq, A. Vanillin based polymers: I. An electrochemical route to polyvanillin. Green Chem. 2012, 14, 2395–2397. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. Renewable (semi)aromatic polyesters from symmetrical vanillin-based dimers. Polym. Chem. 2015, 6, 6058–6066. [Google Scholar] [CrossRef] [Green Version]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. Selective laccase-catalyzed dimerization of phenolic compounds derived from lignin: Towards original symmetrical bio-based (bis) aromatic monomers. J. Mol. Catal. B Enzym. 2016, 125, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Antoniotti, S.; Santhanam, L.; Ahuja, D.; Hogg, M.G.; Dordick, J.S.; York, N. Structural Diversity of Peroxidase-Catalyzed Oxidation Products of o -Methoxyphenols. Org. Lett. 2004, 6, 1975–1978. [Google Scholar] [CrossRef]
- Nishimura, R.T.; Giammanco, C.H.; Vosburg, D.A. Green, Enzymatic Syntheses of Divanillin and Diapocynin for the Organic, Biochemistry, or Advanced General Chemistry Laboratory. J. Chem. Educ. 2010, 87, 526–527. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Flourat, A.L.; Cannet, M.M.M.; Ducrot, P.; Allais, F. Chemoenzymatic Total Synthesis of a Naturally Occurring (5-5 J)/(8 J-O -4 JJ) Dehydrotrimer of Ferulic Acid. Eur. J. Org. Chem 2013, 173–179. [Google Scholar] [CrossRef]
- Savonnet, E.; Grau, E.; Defoort, B.; Cramail, H. Divanillin-Based Epoxy Precursors as DGEBA Substitutes for Biobased Epoxy Thermosets. ACS Sustain. Chem. Eng. 2018, 6, 11008–11017. [Google Scholar] [CrossRef]
- Galy, J.; Sabra, A.; Pascault, J. Characterization of epoxy thermosetting systems by differential scanning calorimetry. Polym. Eng. Sci. 1986, 26, 1514–1523. [Google Scholar] [CrossRef]
- Wang, X.; Gillham, I.K. Competitive Primary Amine/Epoxy and Secondary Amine/Epoxy Reactions: Effect on the Isothermal Time-to-Vitrify. J. Appl. Polym. Sci. 1991, 43, 2267–2277. [Google Scholar] [CrossRef]
- Dušek, K.; Bleha, M.; Luňák, S. Curing of Epoxide Resins: Model Reactions of Curing with Amines. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 2393–2400. [Google Scholar] [CrossRef]
- Aouf, C.; Nouailhas, H.; Fache, M.; Caillol, S.; Boutevin, B. Multi-functionalization of gallic acid. Synthesis of a novel bio-based epoxy resin. Eur. Polym. J. 2013, 49, 1185–1195. [Google Scholar] [CrossRef]
- Fache, M.; Montérémal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy cross-linker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Savonnet, E.; Le Coz, C.; Grau, E.; Grelier, S.; Defoort, B.; Cramail, H. Divanillin-Based Aromatic Amines: Synthesis and Use as Curing Agents for Fully Vanillin-Based Epoxy Thermosets. Front. Chem. 2019, 7, 606. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Ramon, E.; Sguazzo, C.; Moreira, P.M.G.P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, D.A.; Soufen, C.A.; Orlandi, M.O.; Paulista, U.E.; Paulista, U.E. Carbon Fiber Reinforced Polymer and Epoxy Adhesive Tensile Test Failure Analysis Using Scanning Electron Microscopy. Mater. Res. 2017, 20, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Kwiecie, A.; Krajewski, P.; Hojdys, Ł.; Tekieli, M.; Słonski, M. Flexible Adhesive in Composite-to-Brick Strengthening—Experimental and Numerical Study. Polymers 2018, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Li, T.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 66, 164–173. [Google Scholar] [CrossRef]
- Heinrich, L.A.; Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.M.; Coles, S.R.; Maggs, S.; Meredith, J.; Kirwan, K. Use of lignin as a compatibiliser in hemp/epoxy composites. Compos. Sci. Technol. 2011, 71, 1804–1810. [Google Scholar] [CrossRef]
- Yu, Q.; Bahi, A.; Ko, F. Influence of Poly (ethylene oxide) (PEO) Percent and Lignin Type on the Properties of Lignin /PEO Blend Filament. Macromol. Mater. Eng. 2015, 300, 1023–1032. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Chee, J.; Yeo, C.; Yuan, D.; Li, H.; Stubbs, L.P.; He, C. Lignin Epoxy Composites: Preparation, Morphology, and Mechanical Properties. Macromol. Mater. Eng. 2016, 301, 328–336. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Yan, N.; Zhang, R.; Li, J. Demethylation of Wheat Straw Alkali Lignin for Application in Phenol Formaldehyde Adhesives. Polymers 2016, 8, 209. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.S.; Dartiailh, C.; Levin, D.B.; Yan, N. Highly Toughened and Transparent Biobased Epoxy Composites Reinforced with Cellulose Nanofibrils. Polymers 2019, 11, 612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, B.; Lilholt, H. Physical and mechanical properties of unidirectional plant fibre composites—An evaluation of the influence of porosity. Compos. Sci. Technol. 2003, 63, 1265–1272. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, R.; Li, H.; Goh, S.; Huang, S.; Lu, X. From Waste to Functional Additive: Toughening Epoxy Resin with Lignin. ACS Appl. Mater. Interfaces 2014, 6, 5810–5817. [Google Scholar] [CrossRef]
- Levita, G.; De Petris, S.; Marchetti, A.; Lazzeri, A. Crosslink density and fracture toughness of epoxy resins. J. Mater. Sci. 1991, 26, 2348–2352. [Google Scholar] [CrossRef]
- Robinson, E.J.; Douglas, E.P.; Mecholsky, J.J. The Effect of Stoichiometry on the Fracture Toughness of a Liquid Crystalline Epoxy. Polym. Eng. Sci. 2002, 42, 269–279. [Google Scholar] [CrossRef]
- Ashrafi, B.; Martinez-rubi, Y.; Khoun, L.; Simard, B.; Johnston, A. Influence of the reaction stoichiometry on the mechanical and thermal properties of SWCNT-modified epoxy composites. Nanotechnology 2013, 26, 265701. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Nie, Y.; Chen, R.; Zhang, L.; Meng, Y.; Li, X. Hyperbranched polyether as an all-purpose epoxy modifier: Controlled synthesis and toughening mechanisms. J. Mater. Chem. A 2015, 3, 1188. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C.C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Rohart, V.; Lebel, L.L.; Dubé, M. Effects of environmental conditions on the lap shear strength of resistance-welded carbon fibre/thermoplastic composite joints. Compos. Part B Eng. 2020, 198, 108239. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Morita, M.; Otsuka, H.; Takahara, A. Molecular aggregation structure and surface properties of poly(fluoroalkyl acrylate) thin films. Macromolecules 2005, 38, 5699–5705. [Google Scholar] [CrossRef]
- Mialon, L.; Pemba, A.G.; Miller, S.A. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 2010, 12, 1704–1706. [Google Scholar] [CrossRef]
- Shimasaki, T.; Yoshihara, S.; Shibata, M. Preparation and Properties of Biocomposites Composed of Sorbitol-Based Epoxy Resin, Pyrogallol—Vanillin Calixarene, and Wood Flour. Polym. Compos. 2012, 33, 1840–1847. [Google Scholar] [CrossRef]
- Zabihi, O. Preparation and characterization of toughened composites of epoxy/poly(3,4-ethylenedioxythiophene) nanotube: Thermal, mechanical and electrical properties. Compos. Part B Eng. 2013, 45, 1480–1485. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Nal, P.; Mestry, S.; Mapari, S.; Mhaske, S. Eugenol/vanillin-derived novel triarylmethane-based crosslinking agent for epoxy coating. Iran. Polym. J. 2019, 28, 685–695. [Google Scholar] [CrossRef]
- Chu, F.; Ma, C.; Zhang, T.; Xu, Z.; Mu, X.; Cai, W.; Zhou, X.; Ma, S.; Zhou, Y.; Hu, W.; et al. Renewable vanillin-based flame retardant toughening agent with ultra-low phosphorus loading for the fabrication of high-performance epoxy thermoset. Compos. Part B 2020, 190, 107925. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.; Wang, P.; Xing, W.; Song, L.; Hu, Y. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Wang, X.; Wang, Y.; Zhao, D.; Wang, J.; Wang, X.; Wang, Y. Highly thermostable and durably flame-retardant unsaturated polyester modified by a novel polymeric flame retardant containing. Chem. Eng. J. 2018, 344, 419–430. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Y.; Wang, Y.; Jiang, G.; Liang, M.; Chen, X.; Long, G. A fractal model for kozeny-carman constant and dimensionless permeability of fibrous porous media with roughened surfaces. Fractals 2019, 27, 1950116. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, Q.; Chen, H.; Chen, X.; Long, G. A fractal model for capillary flow through a single tortuous capillary with roughened surfaces in fibrous porous media. Fractals 2021, 29, 2150017. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B 2020, 190, 107926. [Google Scholar] [CrossRef]
- Wang, Z.; Gnanasekar, P.; Sudhakaran Nair, S.; Farnood, R.; Yi, S.; Yan, N. Biobased Epoxy Synthesized from a Vanillin Derivative and Its Reinforcement Using Lignin-Containing Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2020, 8, 11215–11223. [Google Scholar] [CrossRef]
- Garbay, G.; Giraud, L.; Gali, S.M.; Hadziioannou, G.; Grau, E.; Cloutet, E.; Cramail, H.; Brochon, C. Divanillin-Based Polyazomethines: Toward Biobased and Metal-Free π-Conjugated Polymers. ACS Omega 2020, 5, 5176–5181. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Razzaq, A. Vanillin-Based Polymers—Part II: Synthesis of Schiff Base Polymers of Divanillin and Their Chelation with Metal Ions. ISRN Polym. Sci. 2012, 2012, 532171. [Google Scholar] [CrossRef] [Green Version]


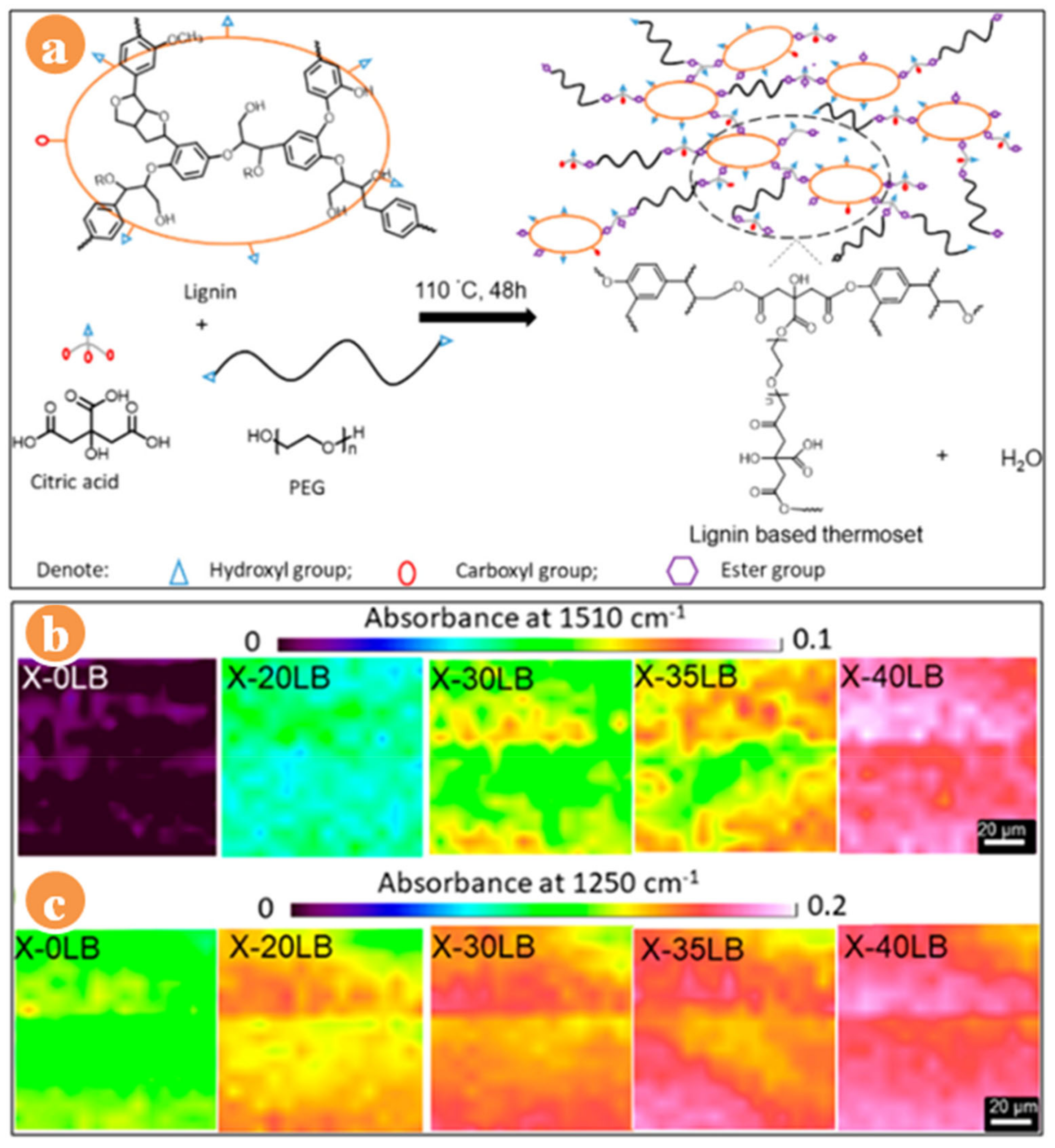




| Lignin Based Network | Solvent | Tg (°C) | Ts (°C) | IDT (°C) | Char Residue (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Toughness (kJ/m3) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DKL-DDM | - | - | 179 | 290 | 38 | - | - | - | [56] |
| DOL-DDM | - | - | 166 | 257 | 29 | - | - | - | [56] |
| DKL-DETA | - | - | 165 | 252 | 33 | - | - | - | [56] |
| DOL-DETA | - | - | 148 | 228 | 23 | - | - | - | [56] |
| DLEP | - | - | - | 258 | - | 2.66 | - | - | [65] |
| OLEP | - | - | - | 244 | - | - | - | - | [65] |
| LHEP/BADGE1:1 | - | 80 | 161 | - | - | - | - | - | [72] |
| LHOEP/BADGE1:9 | - | 111 | 169 | - | - | - | - | - | [72] |
| X-40LB | Dioxane | - | 151 | - | 30 | 34.3 ± 6.2 | - | - | [75] |
| PVA-lignin blend | DI water | - | - | 230 | - | 41.1 ± 4.2 | 2.48 ± 0.10 | 0.6 ± 3.2 | [35] |
| PEO-lignin blend | Methanol aqueous solution | - | - | 350 | 22.5 | 6.2 | 0.28 | - | [31] |
| EPLM | DI water | - | - | 200 | - | 48.5 ± 6.2 | 2.4 ± 0.2 | 0.787 | [34] |
| GL-PGEDGE | - | - | - | - | - | 5.0 | 0.08 | - | [76] |
| VN Epoxy-Based Network | Tg (°C) | Td5 (°C) | Char Residue (%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | FR | LOI (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| DGEMHQ | 132 | - | 20 | - | - | - | - | [90] |
| DGEVAC | 152 | - | 14 | - | - | - | - | [90] |
| DGEVA | 97 | - | 19 | - | - | - | - | [90] |
| VNER/EF205 | 38.3 | - | - | 14.86 ± 1.9 | 2011.63 ± 49.3 | - | - | [33] |
| VNER/EF205/2 wt% IA | 47.9 | - | - | 30.58 ± 1.7 | 959 ± 27.6 | - | - | [33] |
| DGEBA/EF205 | 52.1 | - | - | 29.63 ± 1.3 | 1770.88 ± 46.4 | - | - | [33] |
| DGEDVCP-PN | - | 394 | 59 | - | - | - | - | [98] |
| DGEDVCP-QC | - | 386 | 61 | - | - | - | - | [98] |
| DGEDVCP-GCN | - | 387 | 48 | - | - | - | - | [98] |
| EP1-DDM | 183 | 340 | 53 | 80.3 ± 5 | 2114 ± 132 | √ | 31.4 | [8] |
| EP2-DDM | 214 | 353 | 58 | 60.6 ± 3 | 2709 ± 110 | √ | 32.8 | [8] |
| DGEBA-DDM | 166 | 382 | 14 | 76.4 ± 6 | 1893 ± 140 | ˟ | 24.6 | [8] |
| TE0-APP15 | 153 | 353 | 28 | - | - | √ | 35.4 | [108] |
| TE10-APP0 | 176 | 342 | 26 | - | - | ˟ | 32 | [108] |
| MP1.5-APP3.5 | 176 | 355 | 25 | - | - | ˟ | 25 | [108] |
| MP3-APP7 | 164 | 348 | 23 | - | - | √ | 29.4 | [108] |
| PLA/5VP | - | 325 | 0.3 | 54 ± 1 | 3500 ± 100 | √ | 25.8 | [88] |
| PLA/5VP | - | 324 | 0.7 | 52 ± 1 | 3500 ± 200 | √ | 26.3 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, B.; Agumba, D.O.; Pham, D.H.; Latif, M.; Dinesh; Kim, H.C.; Alrobei, H.; Kim, J. Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications. Polymers 2021, 13, 1162. https://doi.org/10.3390/polym13071162
Kumar B, Agumba DO, Pham DH, Latif M, Dinesh, Kim HC, Alrobei H, Kim J. Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications. Polymers. 2021; 13(7):1162. https://doi.org/10.3390/polym13071162
Chicago/Turabian StyleKumar, Bijender, Dickens O. Agumba, Duc H. Pham, Muhammad Latif, Dinesh, Hyun Chan Kim, Hussein Alrobei, and Jaehwan Kim. 2021. "Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications" Polymers 13, no. 7: 1162. https://doi.org/10.3390/polym13071162
APA StyleKumar, B., Agumba, D. O., Pham, D. H., Latif, M., Dinesh, Kim, H. C., Alrobei, H., & Kim, J. (2021). Recent Research Progress on Lignin-Derived Resins for Natural Fiber Composite Applications. Polymers, 13(7), 1162. https://doi.org/10.3390/polym13071162