Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application
Abstract
:1. Introduction
2. Chemical and Methods
2.1. Synthesis of SA/PVA Biopolymer
2.2. Synthesis of SA/PVA-MMT Blended Membrane
2.3. SA/PVA-MMT External Cross-Linking
2.4. Membrane Characterization
2.5. Membrane Performance Tests
2.5.1. Fluid Intake and Swelling Ratio
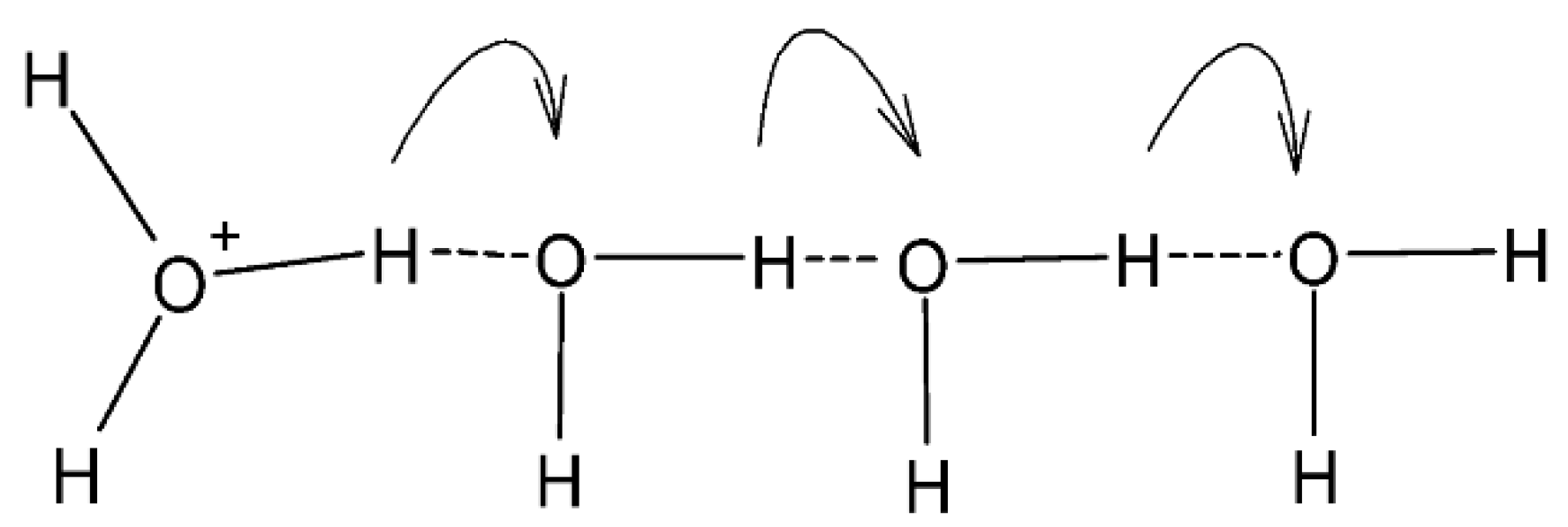
2.5.2. Proton Conductivity
2.5.3. Methanol Permeability
2.5.4. Membrane Selectivity
3. Results and Discussion
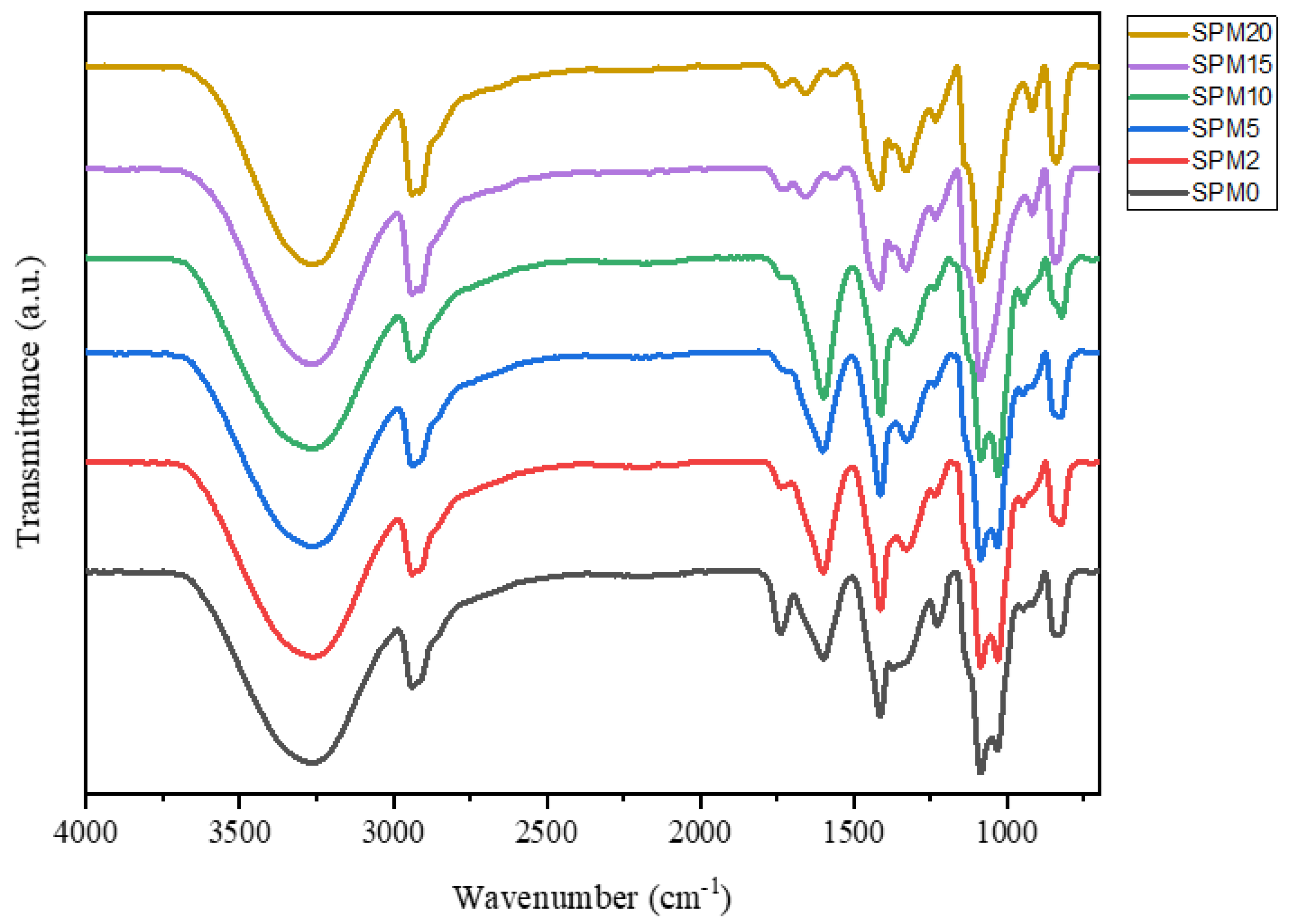
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
| Functional Groups | Wavenumbers (cm−1) | References | |||||
|---|---|---|---|---|---|---|---|
| SA/PVA | SPM2 | SPM5 | SPM10 | SPM15 | SPM20 | ||
| Vibration O-H SA/PVA | 3260 | 3259 | 3257 | 3264 | 3262 | 3265 | [19] |
| Vibration C-H SA/PVA | 2939 | 2938 | 2938 | 2938 | 2939 | 2938 | [29] |
| C=O | 1739 | ||||||
| -COO asymmetrical & symmetrical SA/PVA | 1599, 1414 | 1600, 1413 | 1600, 1413 | 1598, 1412 | 1657, 1418 | 1657, 1418 | [19] |
| C-O alcohol/ether | 1230 | 1328 | 1327 | 1325 | 1330, 1234 | 1329, 1234 | |
| Polysaccharide bending C-C, C-H | 1085, 828 | 1086, 825 | 1086, 827 | 1085 | 1085 | 1085 | [29] |
| C-O-C | 1032 | 1031 | 1032 | 1029 | - | - | [25] |
| Si-O, Al-O MMT | - | - | - | 947, 822 | 918, 843 | 917, 843 | [31] |
3.2. Raman Spectroscopy
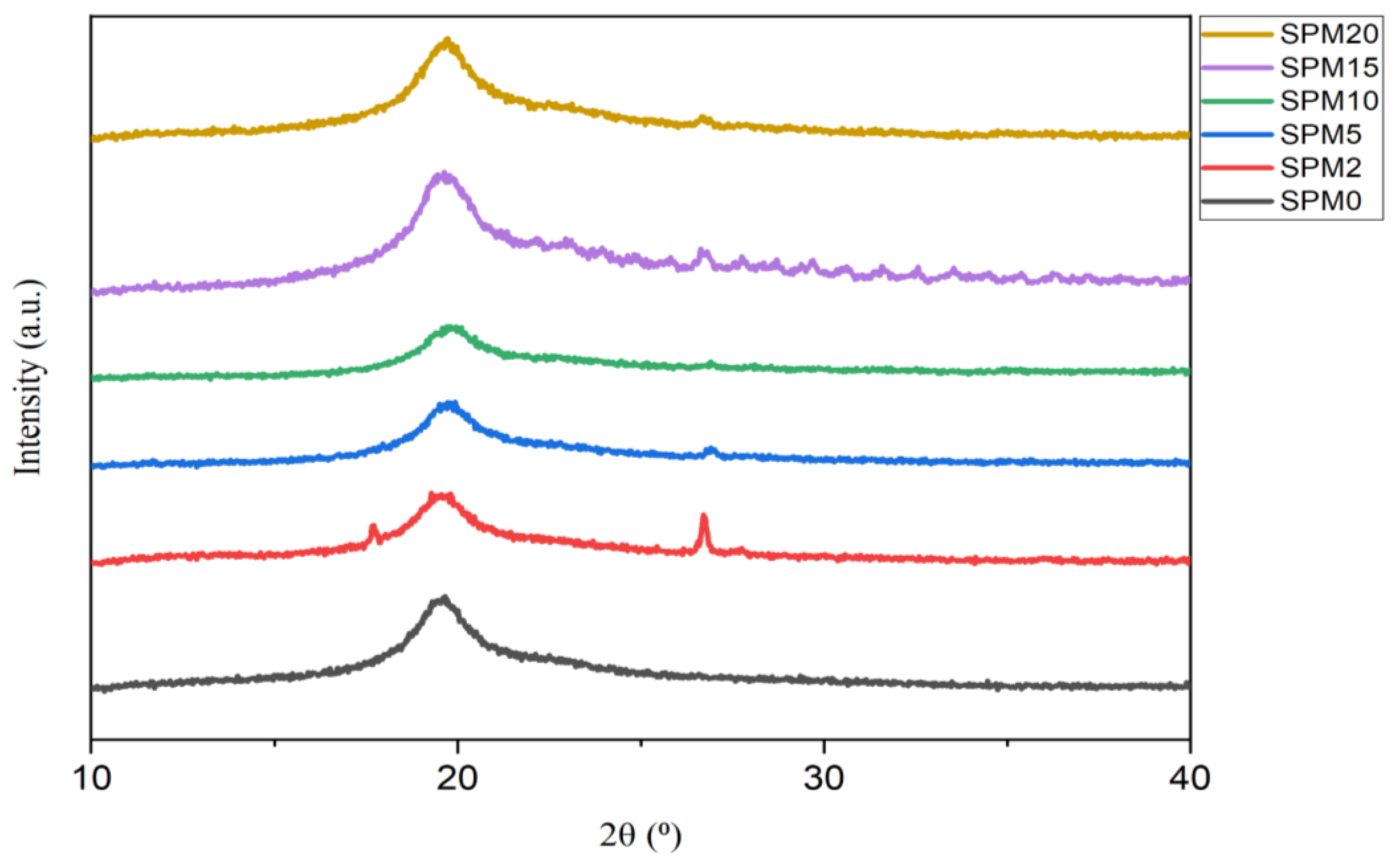
3.3. X-ray Powder Diffraction (XRD)
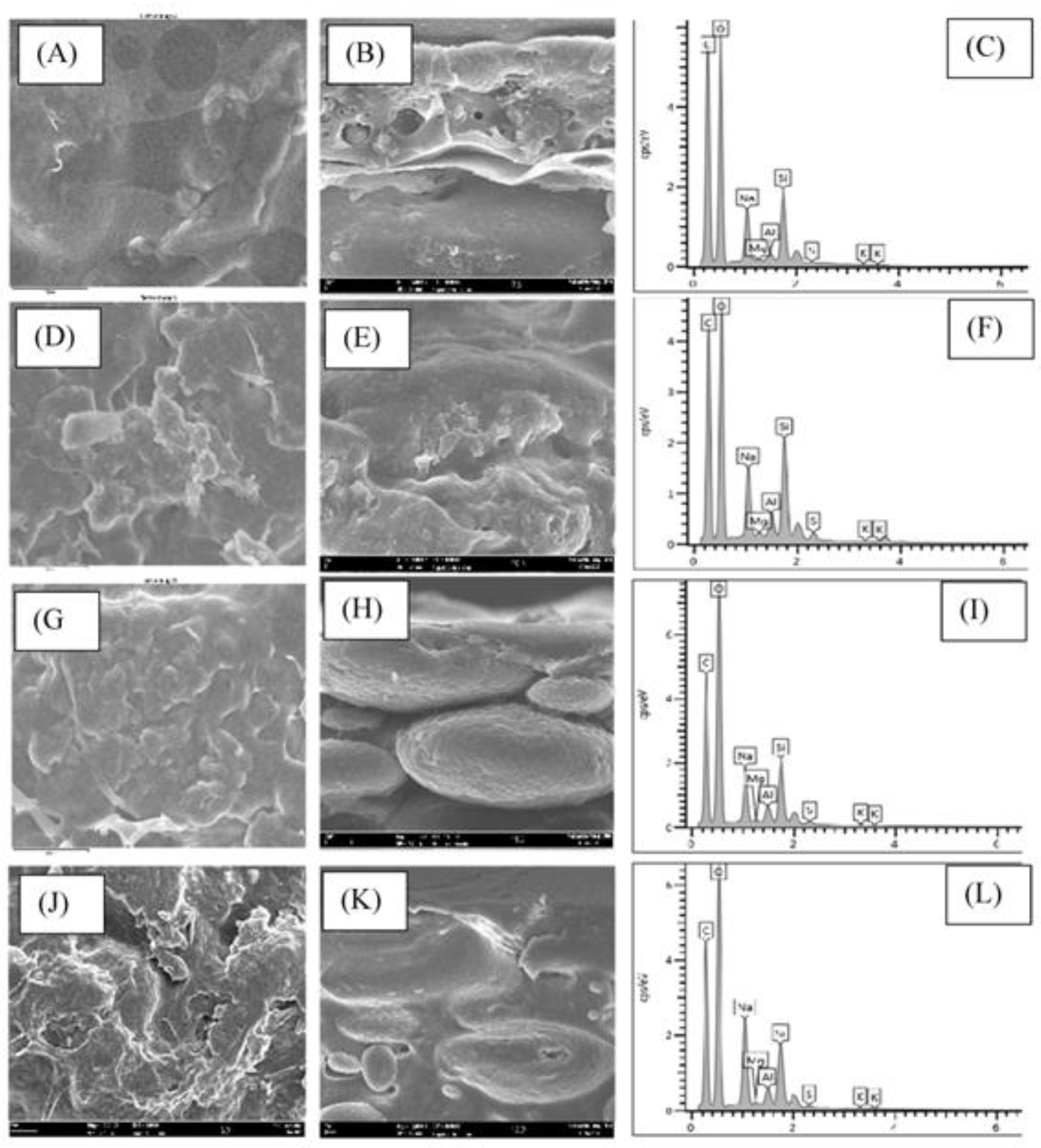
3.4. Field Emission Scanning Electron Microscopy (FESEM)
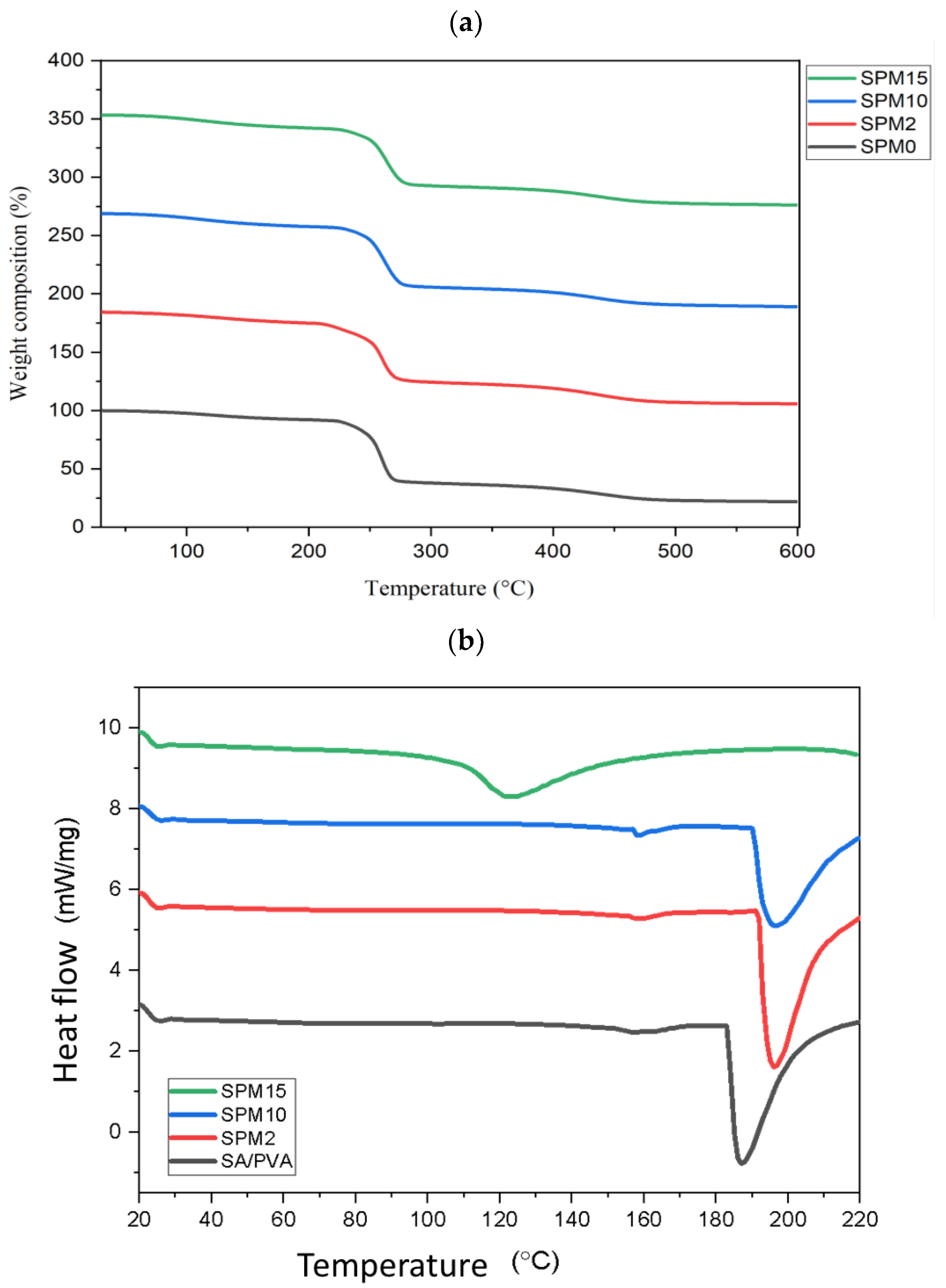
3.5. Thermogravimetric Analysis and Differential Scanning Calorimetry (TGA-DSC)
3.6. Contact Angle
3.7. Performance Test
3.7.1. Physical Insights
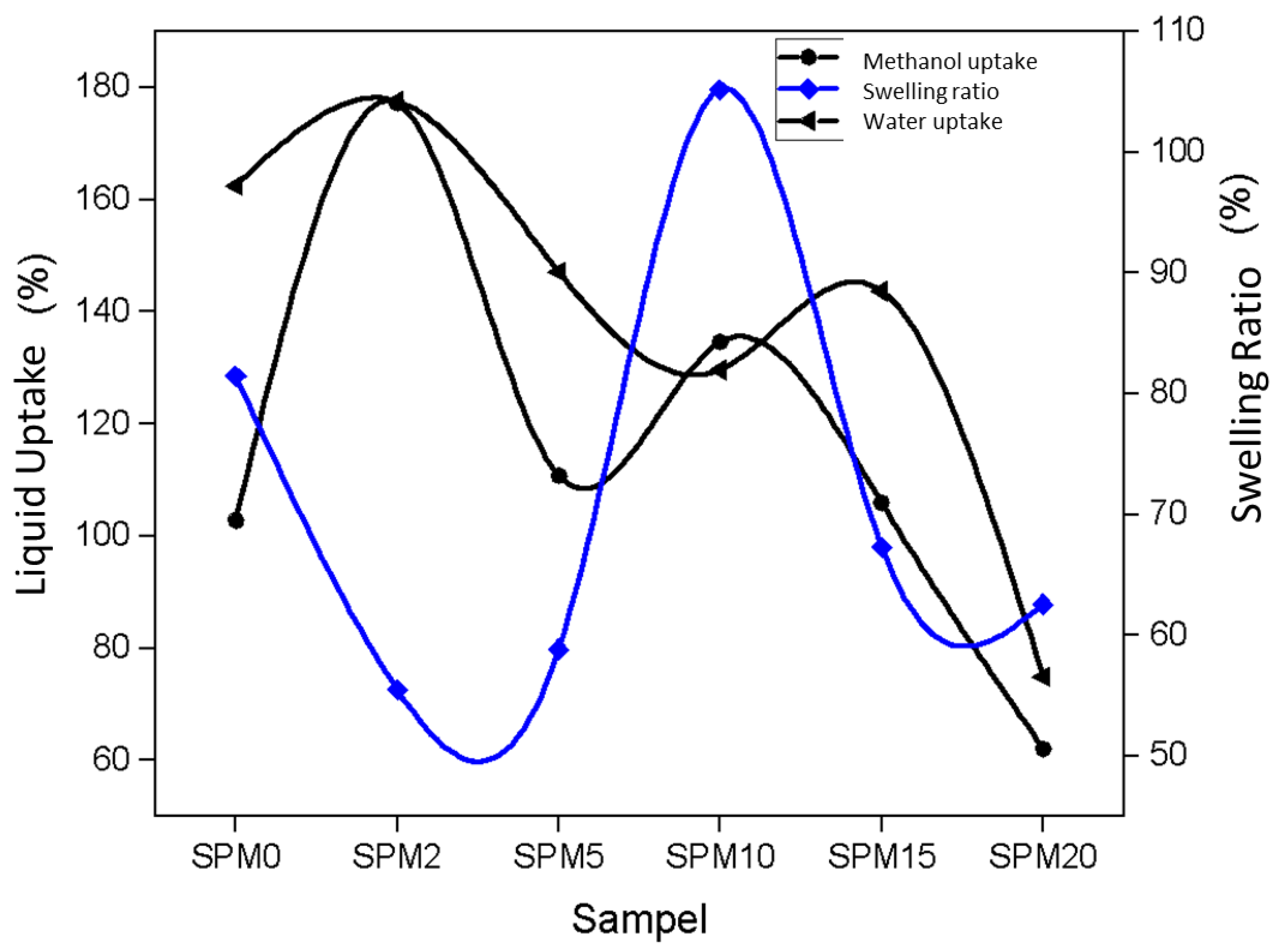
3.7.2. Fluid Intake and Swelling Ratio
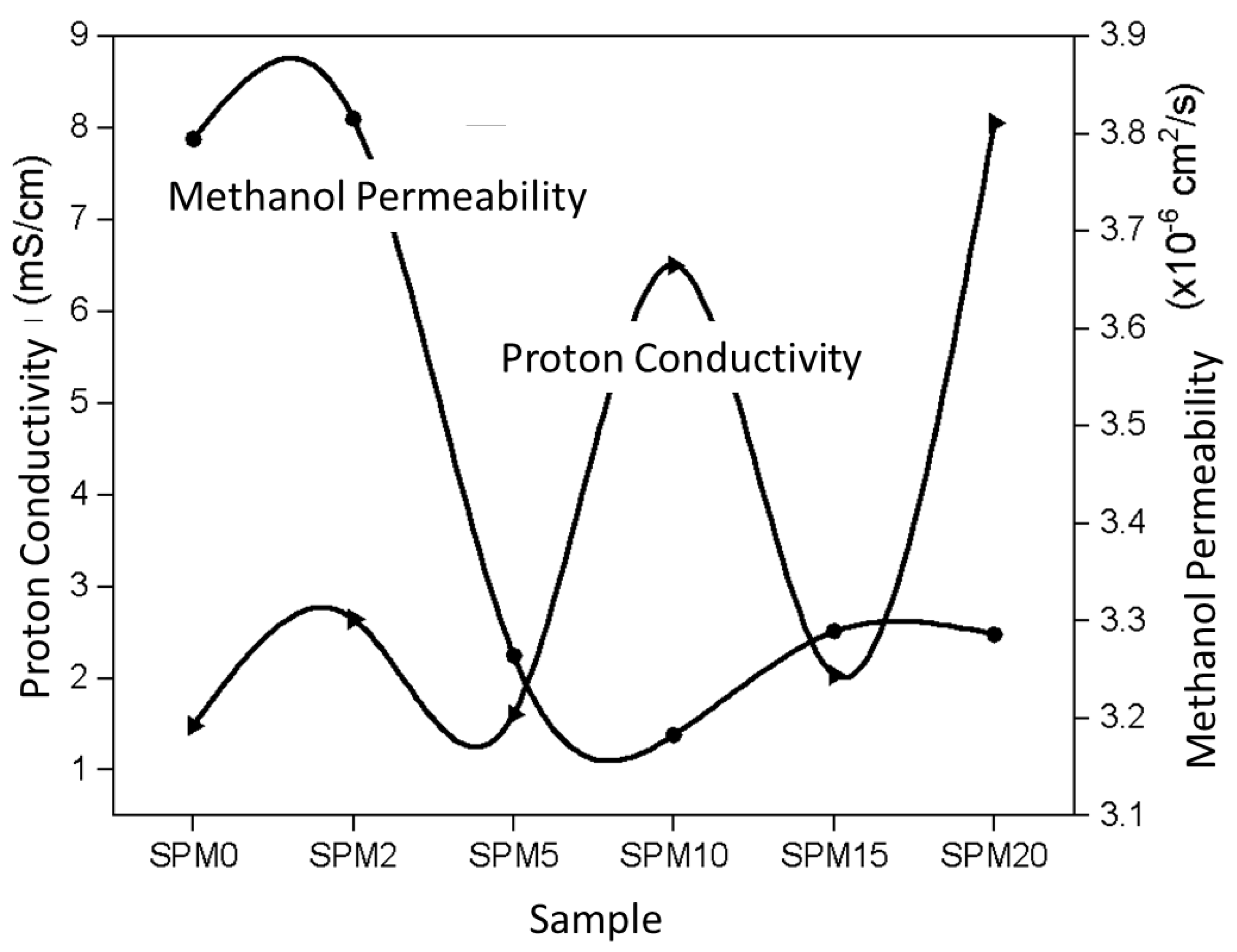
3.7.3. Proton Conductivity and Methanol Permeability
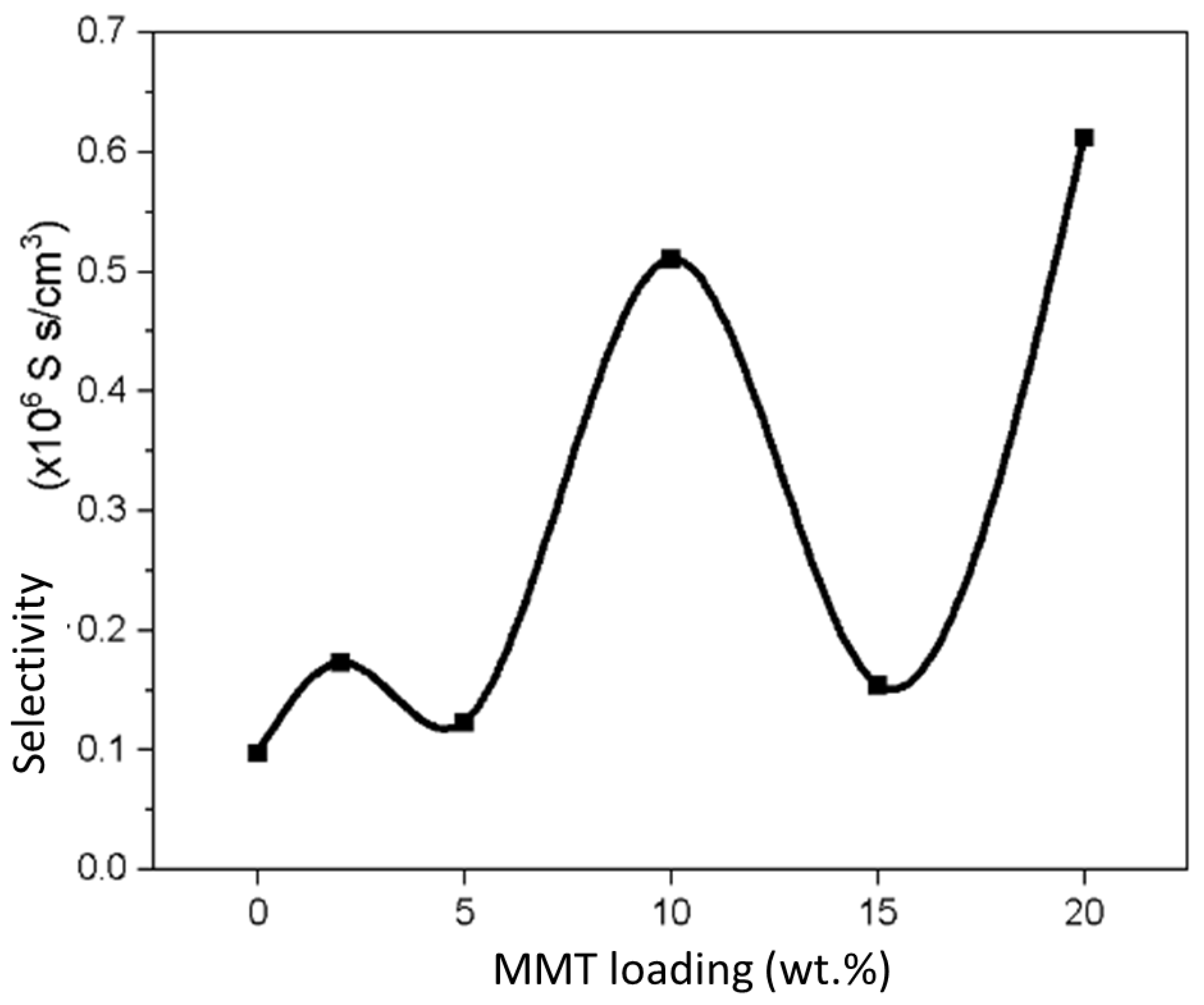
3.8. Membrane Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Shaari, N.; Saharuddin, T.S.T. Progress and major BARRIERS of nanocatalyst development in direct methanol fuel cell: A review. Int. J. Hydrog. Energy 2022, 47, 22114–22146. [Google Scholar] [CrossRef]
- Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M. An overview of nanomaterials in fuel cells: Synthesis method and application. Int. J. Hydrog. Energy 2022, 47, 18468–18495. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N. A review on sulfonated poly (ether ether ketone) based-membrane in direct borohydride fuel cell applications. Int. J. Energy Res. 2022, 46, 17873–17898. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications–A Review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.T.; Shaari, N.; Kamarudin, S.K.; Wong, W.Y. Recent biopolymers used for membrane fuel cells: Characterization analysis perspectives. Int. J. Energy Res. 2022, 46, 16178–16207. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 1–19. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Shaari, N.; Zakaria, Z.; Kamarudin, S.K. The optimization performance of cross-linked sodium alginate polymer electrolyte bio-membranes in passive direct methanol/ethanol fuel cells. Int. J. Energy Res. 2019, 43, 8275–8285. [Google Scholar] [CrossRef]
- Arefian, M.; Hojjati, M.; Tajzad, I.; Mokhtarzade, A.; Mazhar, M.; Jamavari, A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Compd. 2020, 2, 69–76. [Google Scholar]
- Smitha, B.; Sridhar, S.; Khan, A. Chitosan–sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Cabello, S.P.; Mollá, S.; Ochoa, N.A.; Marchese, J.; Giménez, E.; Compañ, V. New bio-polymeric membranes composed of alginate-carrageenan to be applied as polymer electrolyte membranes for DMFC. J. Power Sources 2014, 265, 345–355. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Performance of crosslinked sodium alginate/sulfonated graphene oxide as polymer electrolyte membrane in DMFC application: RSM optimization approach. Int. J. Hydrog. Energy 2018, 43, 22986–23003. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Sodium alginate/alumina composite biomembrane preparation and performance in DMFC application. Polym. Test. 2020, 81, 106183. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Zakaria, Z. Potential of sodium alginate/titanium oxide biomembrane nanocomposite in DMFC application. Int. J. Energy Res. 2019, 43, 8057–8069. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An Introduction to Properties and Utilization; IntechOpen: London, UK, 2018. [Google Scholar]
- Kim, T.K.; Kang, M.; Choi, Y.S.; Kim, H.K.; Lee, W.; Chang, H.; Seung, D. Preparation of Nafion-sulfonated clay nanocomposite membrane for direct menthol fuel cells via a film coating process. J. Power Sources 2007, 165, 1–8. [Google Scholar] [CrossRef]
- Musa, M.T.; Shaari, N.; Kamarudin, S.K. Carbon nanotube, graphene oxide and montmorillonite as conductive fillers in polymer electrolyte membrane for fuel cell: An overview. Int. J. Energy Res. 2021, 45, 1309–1346. [Google Scholar] [CrossRef]
- Yang, J.-M.; Wang, N.-C.; Chiu, H.-C. Preparation and characterization of poly (vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2014, 457, 139–148. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced Proton Conductivity and Methanol Permeability Reduction via Sodium Alginate Electrolyte-Sulfonated Graphene Oxide Bio-membrane. Nanoscale Res. Lett. 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of poly (vinyl alcohol)-based polymers as proton exchange membranes and challenges in fuel cell application: A review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Rhimi, A.; Zlaoui, K.; Van der Bruggen, B.; Horchani-Naifer, K.; Ennigrou, D.J. Synthesis and characterization of crosslinked membranes based on sodium alginate/polyvinyl alcohol/graphene oxide for ultrafiltration applications. Desalination Water Treat. 2021, 230, 204–218. [Google Scholar] [CrossRef]
- Purwanto, M.; Widiastuti, N.; Gunawan, A. Preparation and Properties of Chitosan/Montmorillonite Supported Phosphotungstic Acid Composite Membrane for Direct Methanol Fuel Cell Application. Korean J. Mater. Res. 2021, 31, 375–381. [Google Scholar] [CrossRef]
- Altaf, F.; Batool, R.; Gill, R.; Rehman, Z.U.; Majeed, H.; Ahmad, A.; Shafiq, M.; Dastan, D.; Abbas, G.; Jacob, K. Synthesis and electrochemical investigations of ABPBI grafted montmorillonite based polymer electrolyte membranes for PEMFC applications. Renew. Energy 2021, 164, 709–728. [Google Scholar] [CrossRef]
- Kalaiselvimary, J.; Selvakumar, K.; Rajendran, S.; Sowmya, G.; Ramesh Prabhu, M. Effect of surface-modified montmorillonite incorporated biopolymer membranes for PEM fuel cell applications. Polym. Compos. 2019, 40, E301–E311. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, M.; Chen, Z.; He, H.; Liu, L. Effects of montmorillonite and anatase TiO2 support on CeO2 catalysts during NH3-SCR reaction. Microporous Mesoporous Mater. 2021, 320, 111072. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Das, G.; Lee, S.H.; Yoon, Y.S. An approach of balancing the ionic conductivity and mechanical properties of PVA based nanocomposite membrane for DMFC by various crosslinking agents with ionic liquid. Int. J. Hydrog. Energy 2015, 40, 7114–7123. [Google Scholar] [CrossRef]
- Gosalawit, R.; Chirachanchai, S.; Shishatskiy, S.; Nunes, S.P. Sulfonated montmorillonite/sulfonated poly (ether ether ketone) (SMMT/SPEEK) nanocomposite membrane for direct methanol fuel cells (DMFCs). J. Membr. Sci. 2008, 323, 337–346. [Google Scholar] [CrossRef]
- Xing, D.; He, G.; Hou, Z.; Ming, P.; Song, S. Preparation and characterization of a modified montmorillonite/sulfonated polyphenylether sulfone/PTFE composite membrane. Int. J. Hydrog. Energy 2011, 36, 2177–2183. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lee, Y.-J. Preparation of the acidic PVA/MMT nanocomposite polymer membrane for the direct methanol fuel cell (DMFC). Thin Solid Film. 2009, 517, 4735–4740. [Google Scholar] [CrossRef]
- Wong, J.I.C.; Ramesh, S.; Jun, H.K.; Liew, C.W. Development of poly (vinyl alcohol)(PVA)-based sodium ion conductors for electric double-layer capacitors application. Mater. Sci. Eng. B 2021, 263, 114804. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.; Timmiati, S. Influence of graphene oxide on the ethanol permeability and ionic conductivity of QPVA-based membrane in passive alkaline direct ethanol fuel cells. Nanoscale Res. Lett. 2019, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Porchelvi, S.; Kannan, R.; Bahavan Palani, P.; Sainul Abidin, K.; Rajashabala, S. High conductive proton exchange membrane (SPEEK/MMT) and its characterization. Mater. Res. Innov. 2019, 23, 33–38. [Google Scholar] [CrossRef]
- Mokhtar, M.; Majlan, E.H.; Ahmad, A.; Tasirin, S.M.; Daud, W.R.W. Effect of ZnO filler on PVA-alkaline solid polymer electrolyte for aluminum-air battery applications. J. Electrochem. Soc. 2018, 165, A2483. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Effect of montmorillonite clay and biopolymer concentration on the physical and mechanical properties of alginate nanocomposite films. J. Food Eng. 2013, 117, 26–33. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, F.; Liu, Q. Sulfonated poly (2, 5-benzimidazole)(ABPBI)/MMT/ionic liquids composite membranes for high temperature PEM applications. Int. J. Hydrog. Energy 2015, 40, 16767–16774. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V. Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J. Sci. Adv. Mater. Devices 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Karim, A.K.; Omidvar, A.; Nazari, Z.; Jaberi, R. Mechanical properties and swelling behavior of acrylamide hydrogels using montmorillonite and kaolinite as clays. J. Environ. Treat. Tech. 2019, 7, 211–219. [Google Scholar]
- Junoh, H.; Jaafar, J.; Nordin, N.A.H.M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of polymer electrolyte membrane for direct methanol fuel cell application: Perspective on morphological structure. Membranes 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]

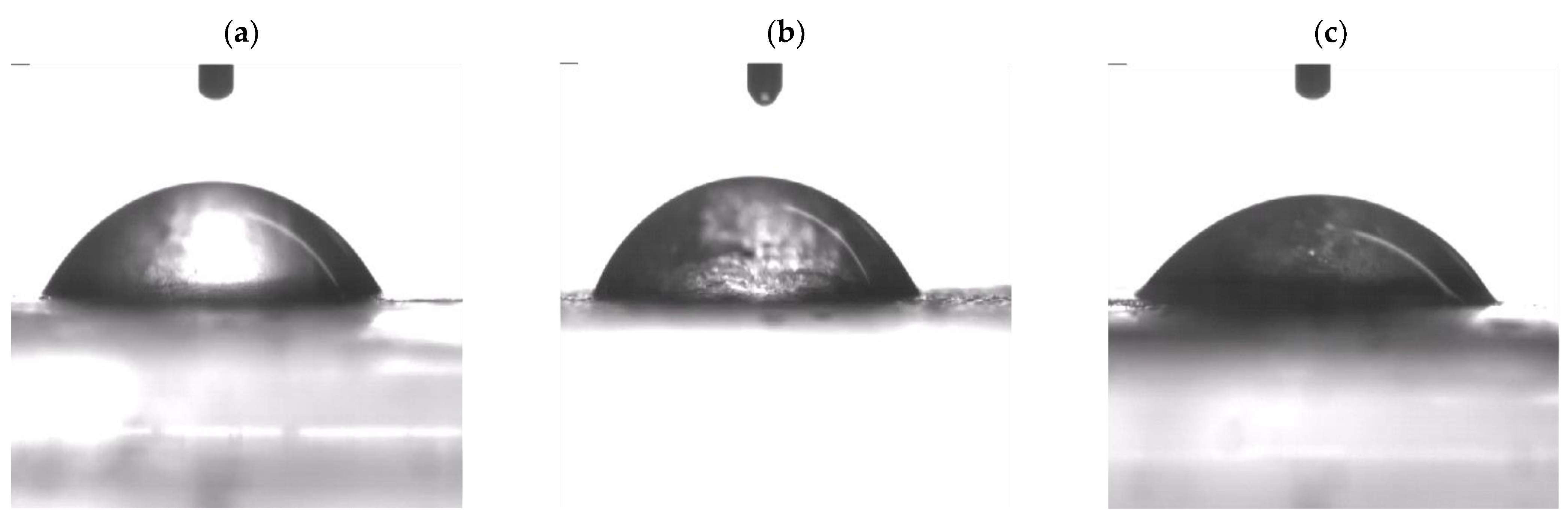

| Membrane | Ratio of SA:PVA | Content of MMT (wt%) |
|---|---|---|
| SPM0 | 40:60 | 0 |
| SPM2 | 40:60 | 2 |
| SPM5 | 40:60 | 5 |
| SPM10 | 40:60 | 10 |
| SPM15 | 40:60 | 15 |
| SPM20 | 40:60 | 20 |
| Sample | MMT Loading (wt%) | Water Uptake (%) | Methanol Uptake (%) | Swelling Ratio (%) |
|---|---|---|---|---|
| SPM0 | 0.0 | 162.46 | 98.55 | 81.45 |
| SPM2 | 2.0 | 177.50 | 203.30 | 55.51 |
| SPM5 | 5.0 | 147.10 | 132.23 | 58.84 |
| SPM10 | 10.0 | 129.65 | 121.59 | 105.15 |
| SPM15 | 15.0 | 143.70 | 97.46 | 67.27 |
| SPM20 | 20.0 | 74.89 | 53.00 | 62.57 |
| Sample | Proton Conductivity (mS/cm) | Methanol Permeability (×10−8 cm2/s) | Selectivity (×105 S s/cm3) |
|---|---|---|---|
| SA/PVA | 1.4828 | 1.5179 | 0.9769 |
| SPM2 | 2.6429 | 1.5261 | 1.7318 |
| SPM5 | 1.6083 | 1.3057 | 1.2318 |
| SPM10 | 6.5025 | 1.2731 | 5.1077 |
| SPM15 | 2.0332 | 1.3155 | 1.5455 |
| SPM20 | 8.0510 | 1.3145 | 6.1250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, M.T.; Shaari, N.; Raduwan, N.F.; Kamarudin, S.K.; Wong, W.Y. Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application. Polymers 2023, 15, 2590. https://doi.org/10.3390/polym15122590
Musa MT, Shaari N, Raduwan NF, Kamarudin SK, Wong WY. Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application. Polymers. 2023; 15(12):2590. https://doi.org/10.3390/polym15122590
Chicago/Turabian StyleMusa, Maryam Taufiq, Norazuwana Shaari, Nor Fatina Raduwan, Siti Kartom Kamarudin, and Wai Yin Wong. 2023. "Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application" Polymers 15, no. 12: 2590. https://doi.org/10.3390/polym15122590
APA StyleMusa, M. T., Shaari, N., Raduwan, N. F., Kamarudin, S. K., & Wong, W. Y. (2023). Alginate/PVA Polymer Electrolyte Membrane Modified by Hydrophilic Montmorillonite for Structure and Selectivity Enhancement for DMFC Application. Polymers, 15(12), 2590. https://doi.org/10.3390/polym15122590








