The Effects of Internal Electron Donors on MgCl2-Supported Ziegler–Natta Catalysts for Isotactic PP
Abstract
:1. Introduction
2. The Different Generations of Ziegler–Natta Catalyst
3. Internal Electron Donors
3.1. Ester Internal Electron Donors
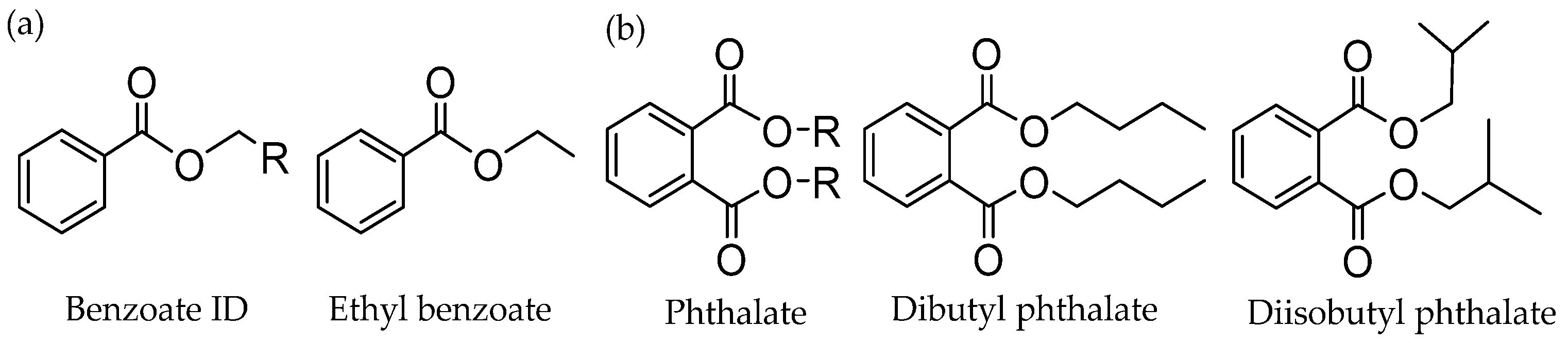
3.1.1. Monoester IEDs
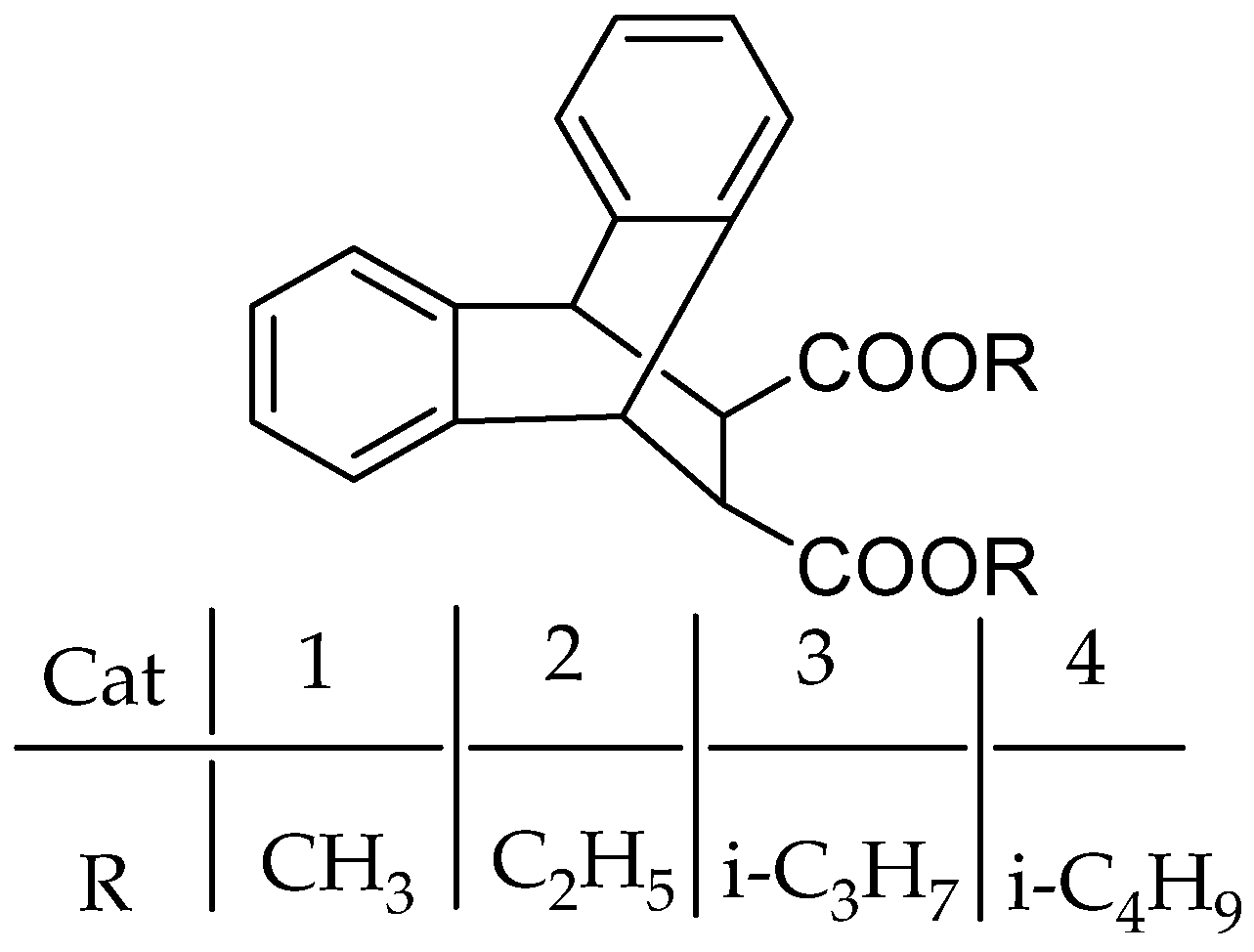
3.1.2. Diester IEDs
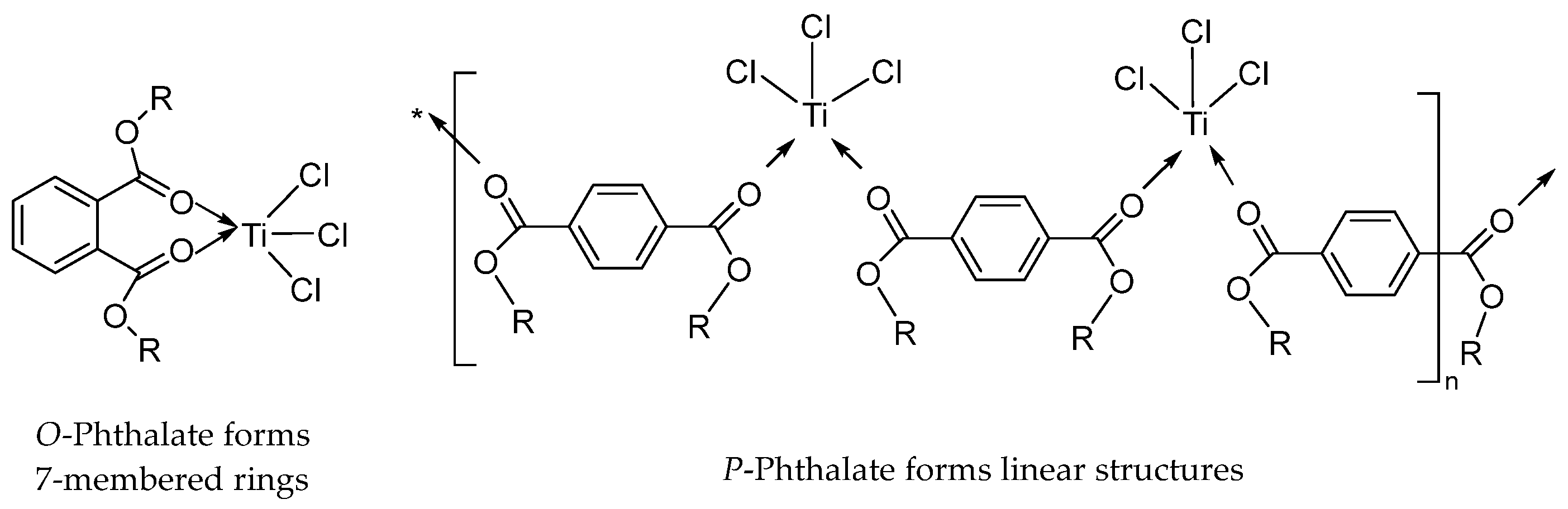
Phthalate IEDs
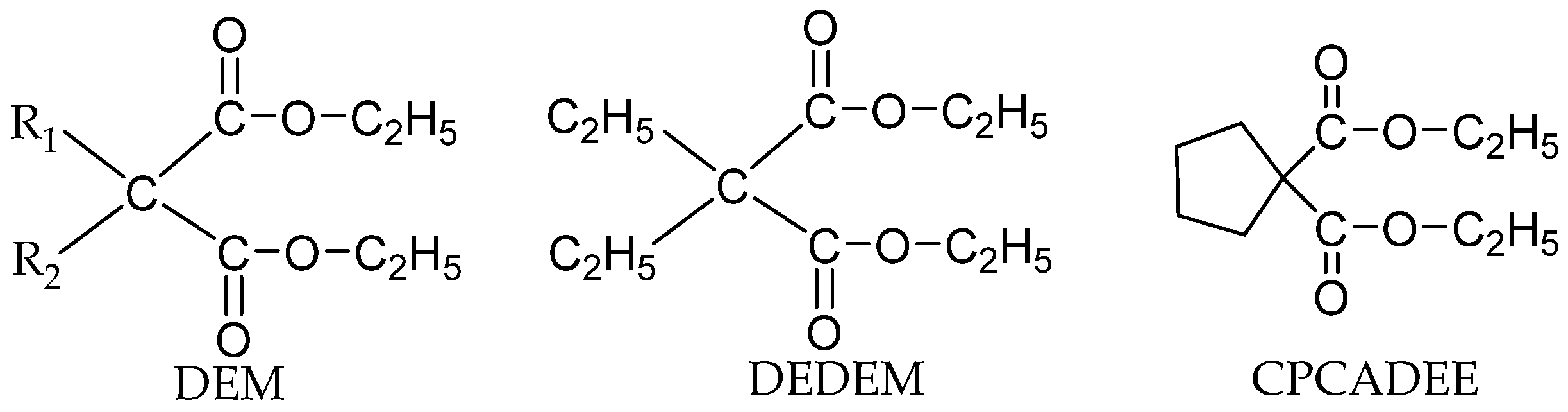
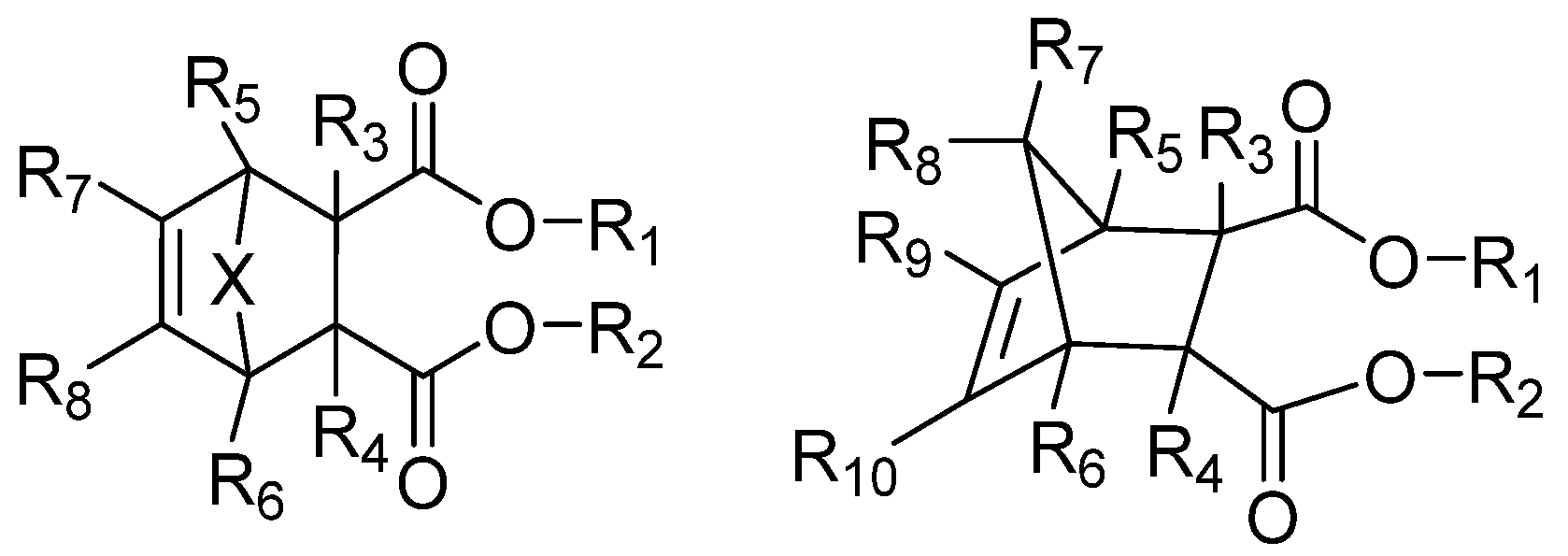
Malonate IEDs
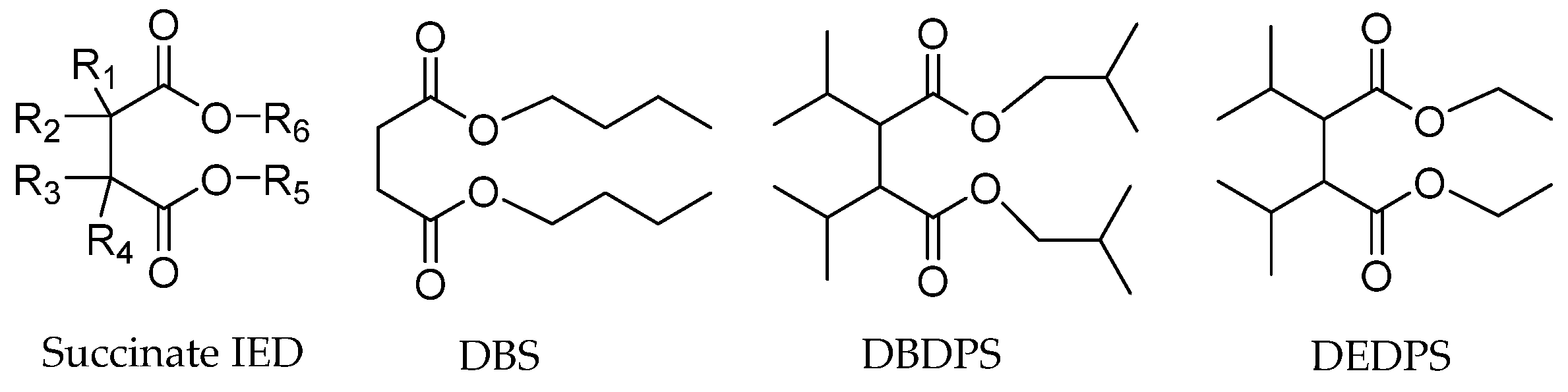
Succinate IEDs
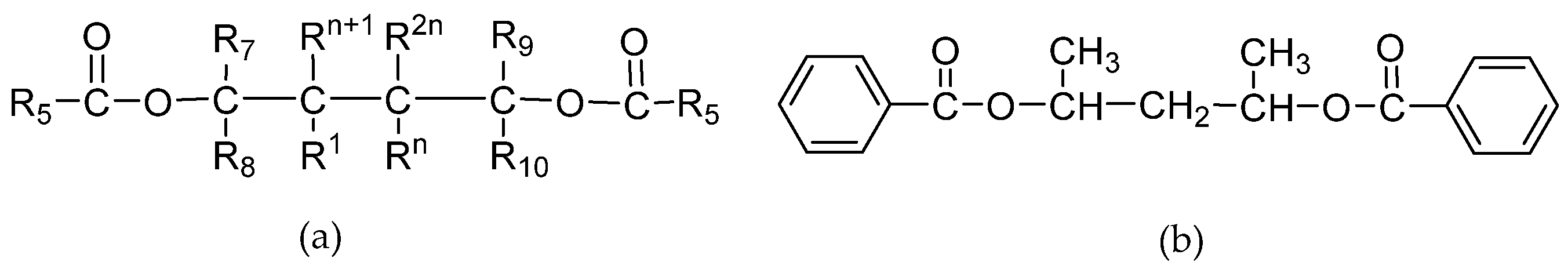
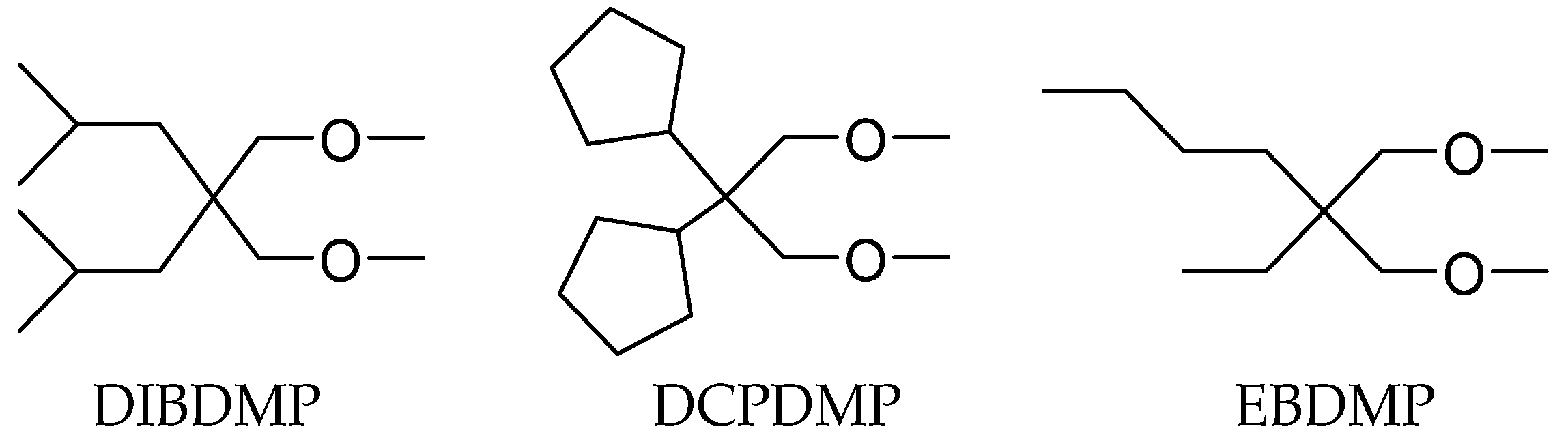
1,3-Diol Ester IEDs
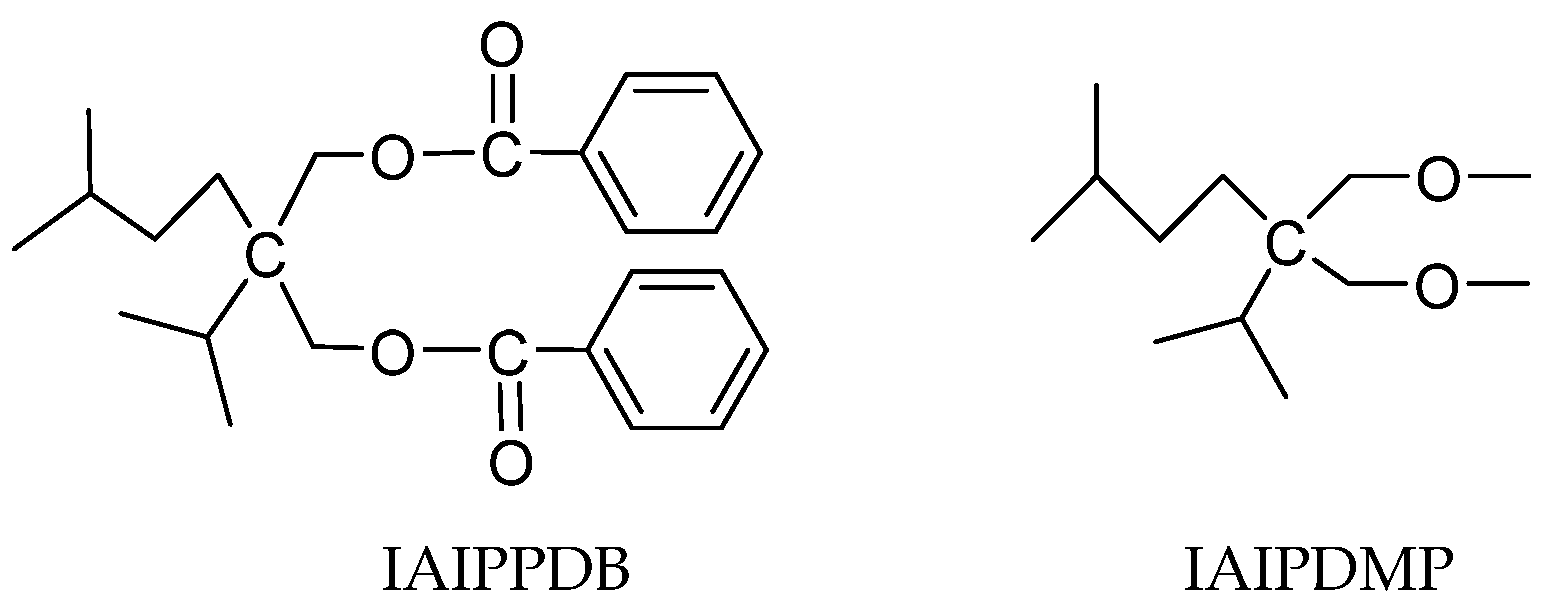
3.1.3. Other Ester IEDs
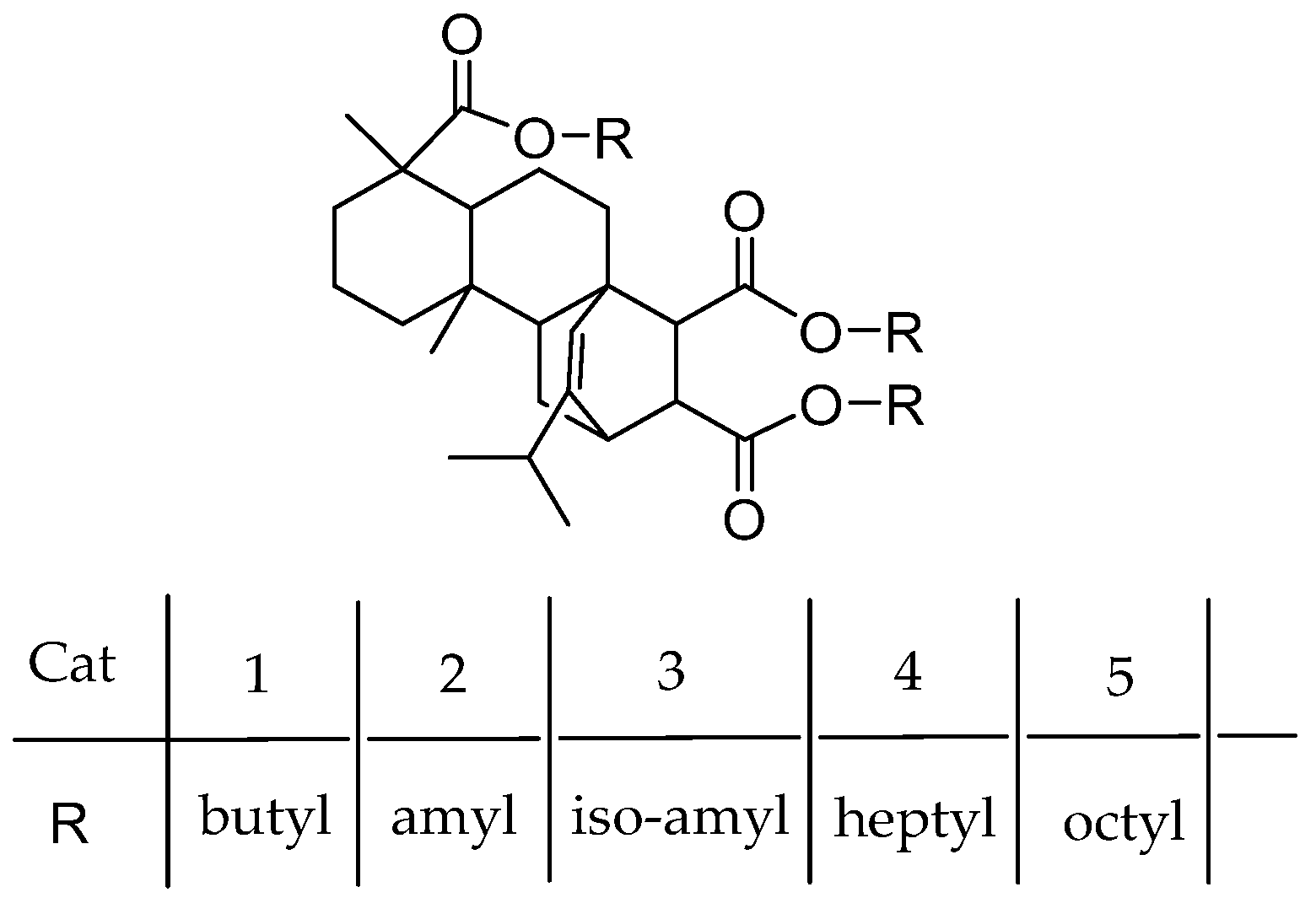
Rosinate IEDs
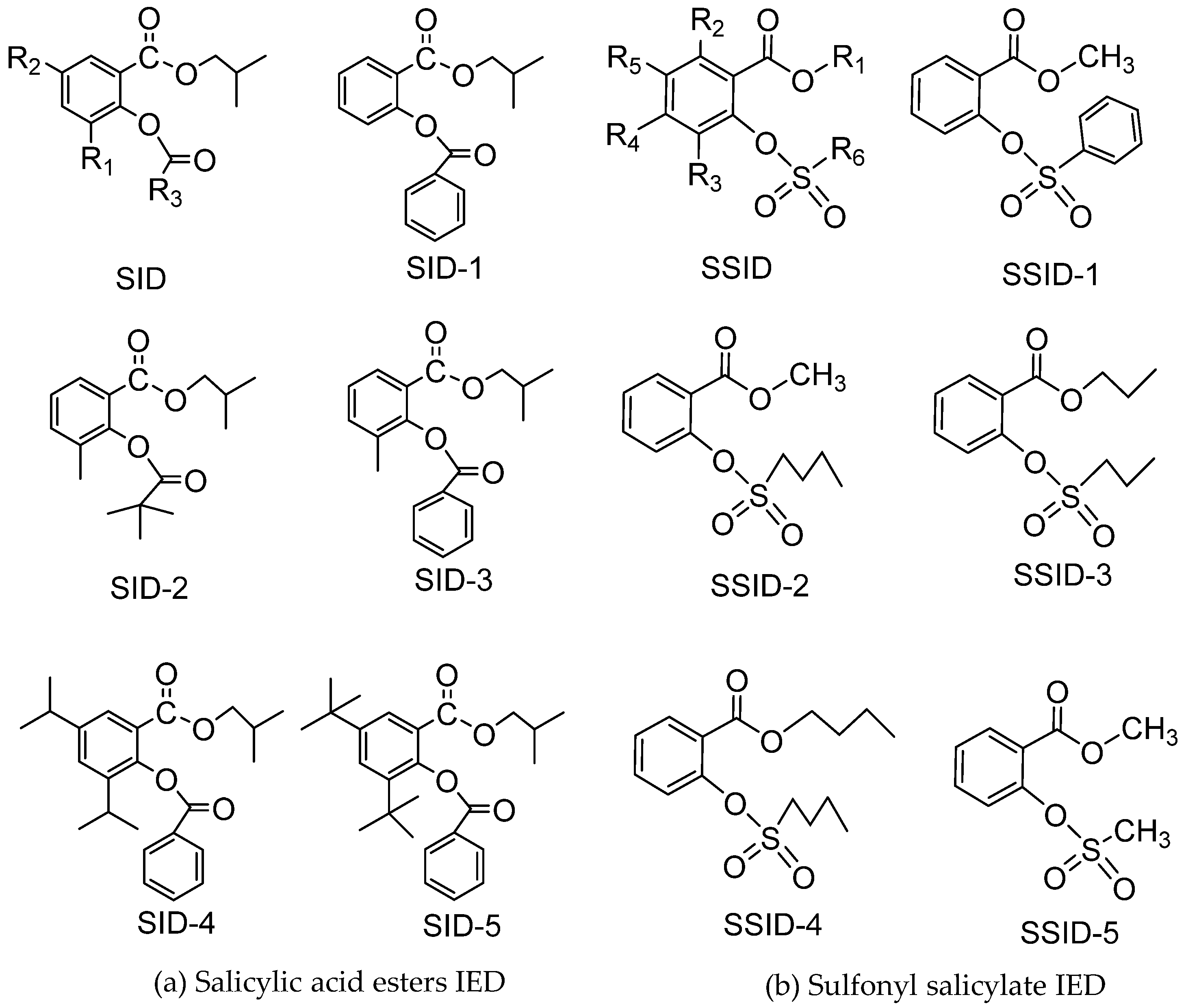
Salicylate IEDs
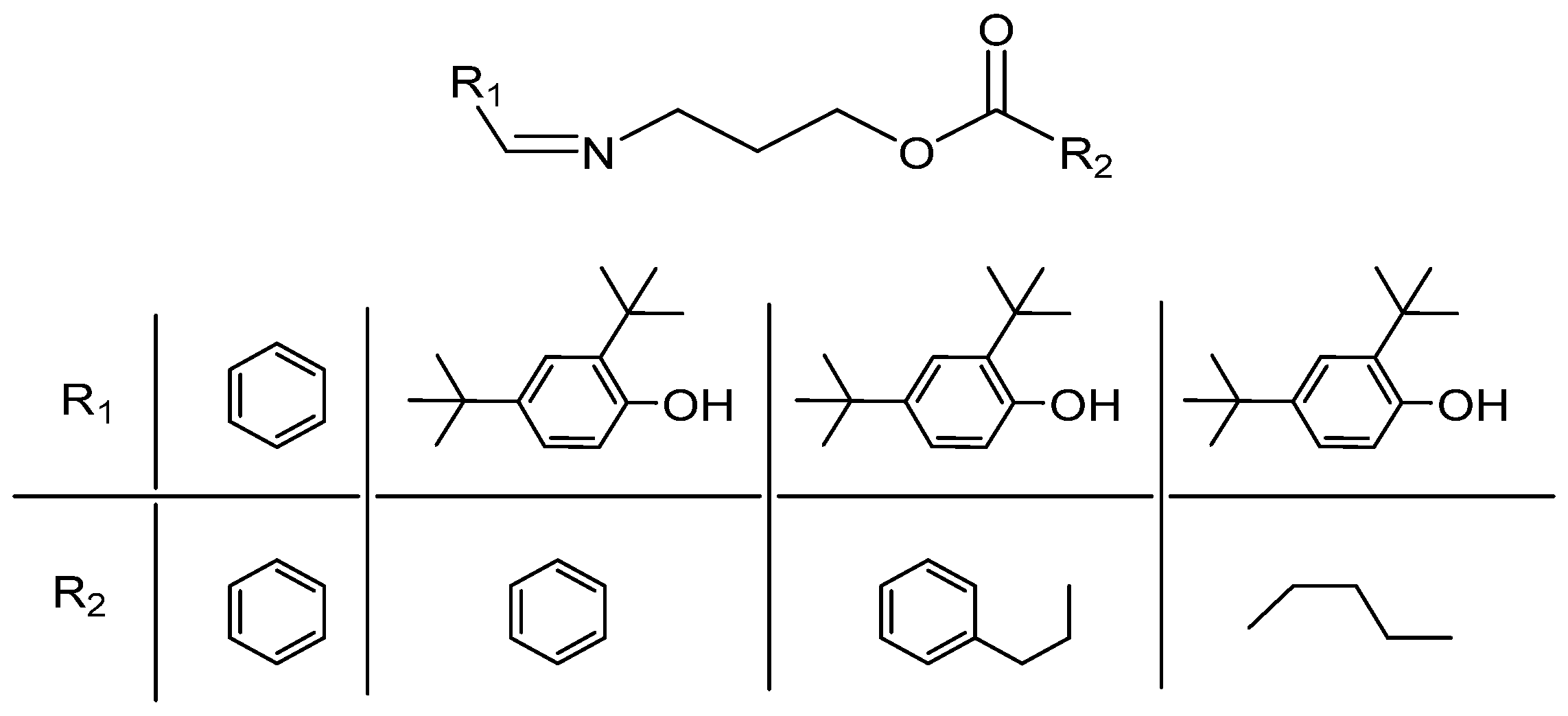
Imide Esters IEDs
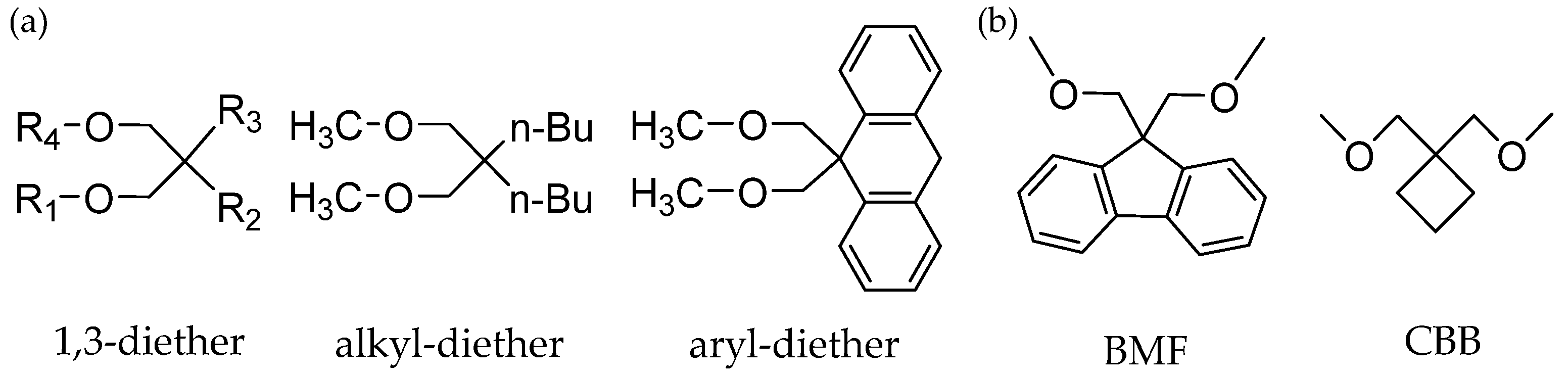
3.2. Diether IEDs
3.3. Other Types of IEDs
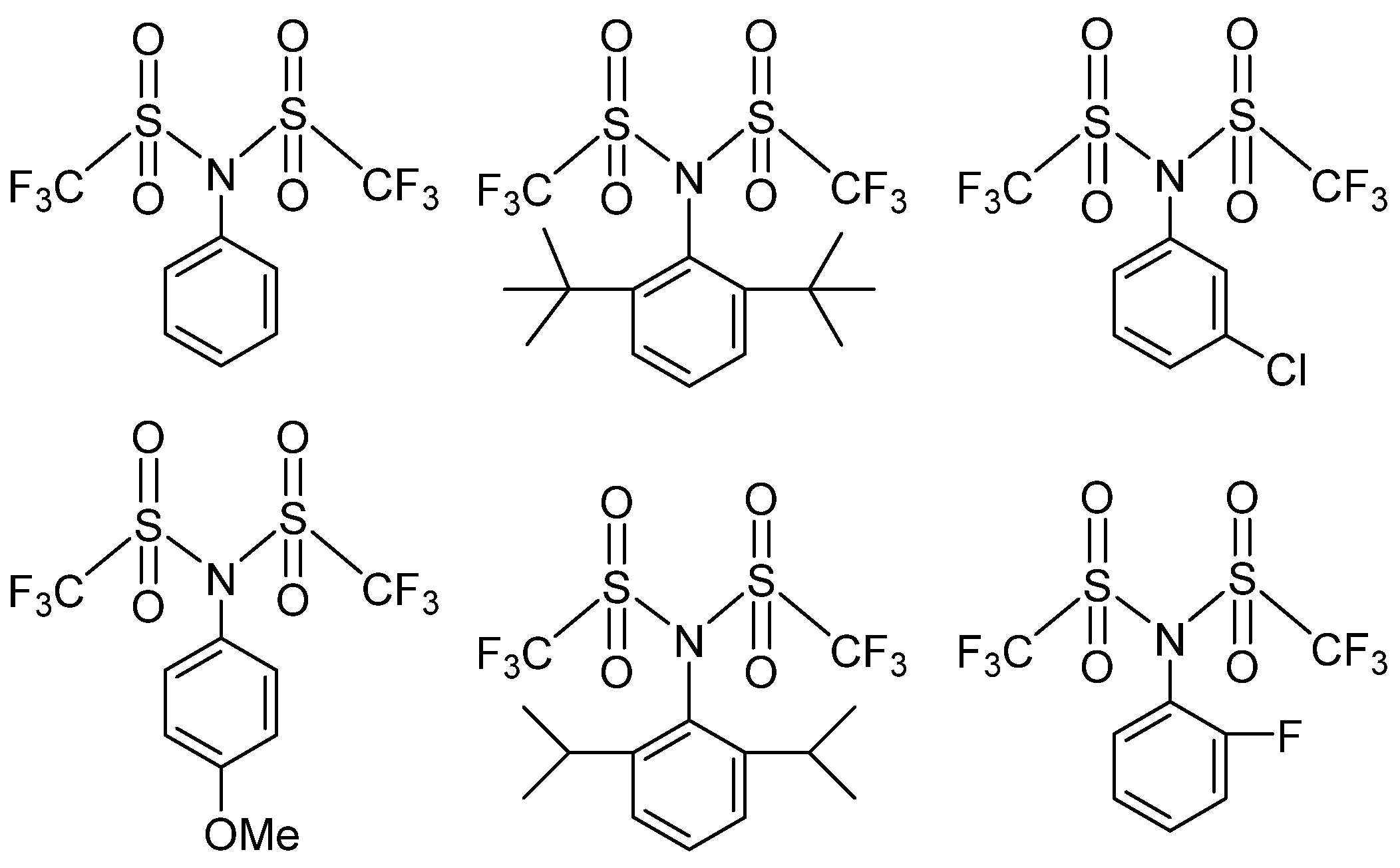
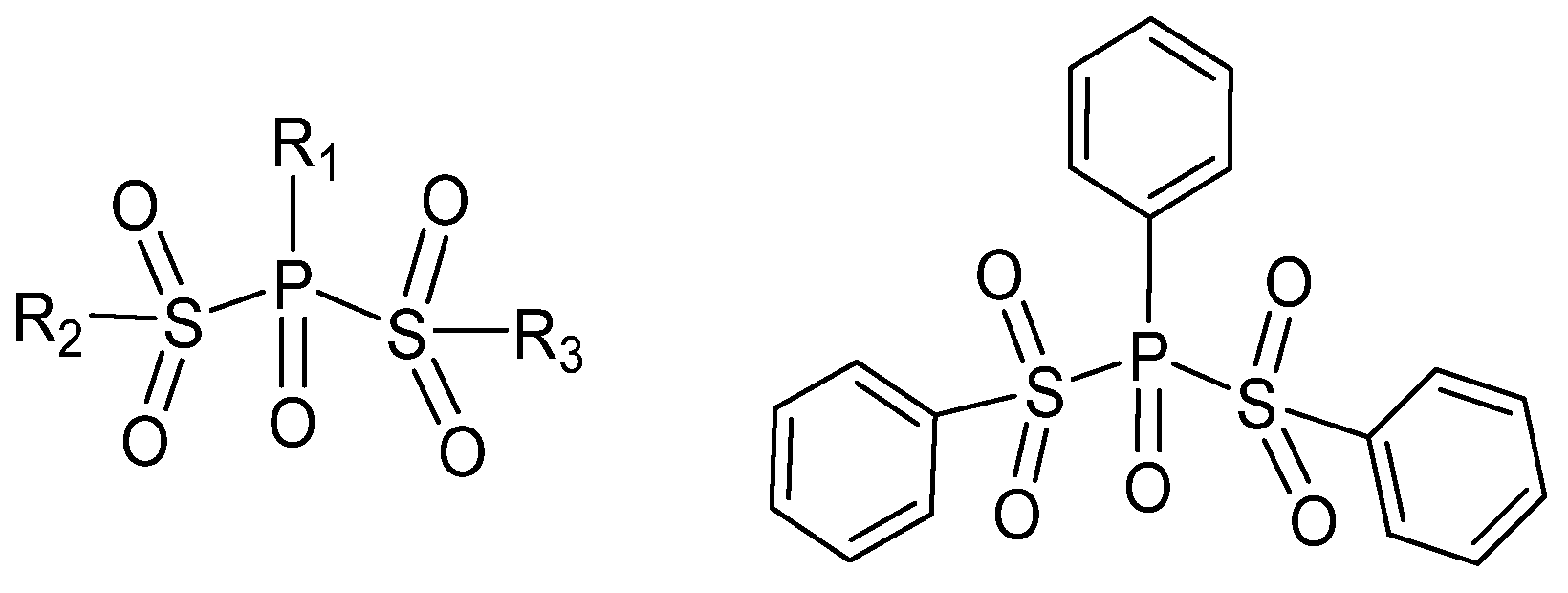
3.3.1. IEDs Containing Sulfonyl Groups
3.3.2. IEDs Containing Dibenzoyl Sulfide Groups
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.-X.; Ko, E.-B.; Park, J.-H.; Moon, Y.-K.; Zhang, X.-Q.; Yoon, K.-B. Fabrication of polyethylene/graphene nanocomposites through in situ polymerization with a spherical graphene/MgCl2-supported Ziegler-Natta catalyst. Compos. Sci. Technol. 2016, 136, 61–66. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Y.; Zhao, S.; Dong, J.-Y. In Situ Promotion of Long-Chain Branching in Polyethylene from Ziegler–Natta Catalysts. ACS Appl. Polym. Mater. 2021, 3, 6455–6467. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, Y.; Fan, Z.; Xu, J. Structure and morphology of polyethylene/polypropylene in-reactor alloys synthesized by spherical high-yield Ziegler–Natta catalyst. J. Appl. Polym. Sci. 2007, 103, 2075–2085. [Google Scholar] [CrossRef]
- Kalita, A.; Boruah, M.; Das, D.; Dolui, S.K. Ethylene polymerization on polymer supported Ziegler-Natta catalyst. J. Polym. Res. 2012, 19, 9892. [Google Scholar] [CrossRef]
- An, M.; Cui, B.; Duan, X. Preparation and applications of linear low-density polyethylene. J. Phys. Conf. Ser. 2022, 2229, 012009. [Google Scholar] [CrossRef]
- Cariño, A.C.; Ko, Y.S. Effect of preparation condition in one-pot synthesis of MgCl2-supported Ziegler-Natta catalyst on ethylene-1-octene copolymerization. J. Ind. Eng. Chem. 2023, 118, 483–487. [Google Scholar] [CrossRef]
- Chatarsa, C.; Prasassarakich, P.; Rempel, G.L.; Hinchiranan, N. The influence of Ni/Nd-based Ziegler–Natta catalyst on microstructure configurations and properties of butadiene rubber. J. Appl. Polym. Sci. 2015, 132, 41834. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; He, A. Synthesis of amine-capped Trans-1,4-polyisoprene. Polymer 2021, 235, 124231. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Niu, Q.; Wang, R.; He, A. Trans-1,4-stereospecific polymerization of isoprene with MgCl2-supported Ziegler-Natta catalyst I. Initial polymerization kinetic and polymerization mechanism. Polymer 2018, 140, 255–268. [Google Scholar] [CrossRef]
- Zheng, W.; He, A.; Liu, C.; Shao, H.; Wang, R. The influences of alkylaluminium as cocatalyst on butene-1 polymerization with MgCl2-supported TiCl4 Ziegler-Natta catalysts. Polymer 2020, 210, 122998. [Google Scholar] [CrossRef]
- Kashiwa, N.; Yoshitake, J.; Mizuno, A.; Tsutsui, T. Polymerization of butene-1 with highly active MgCl2-supported TiCl4 catalyst system. Polymer 1987, 28, 1227–1231. [Google Scholar] [CrossRef]
- Niu, Q.; Zhang, J.; Peng, W.; Fan, Z.; He, A. Effect of alkylaluminium on the regio- and stereoselectivity in copolymerization of isoprene and butadiene using TiCl4/MgCl2 type Ziegler-Natta catalyst. Mol. Catal. 2019, 471, 1–8. [Google Scholar] [CrossRef]
- Kaminsky, W. (Ed.) Polyolefins: 50 Years after Ziegler and Natta II; Springer: Berlin/Heidelberg, Germany, 2013; pp. 37–57. [Google Scholar]
- Andoni, A.; Chadwick, J.C.; Milani, S.; Niemantsverdriet, H.; Thüne, P.C. Introducing a new surface science model for Ziegler–Natta catalysts: Preparation, basic characterization and testing. J. Catal. 2007, 247, 129–136. [Google Scholar] [CrossRef]
- Capone, F.; Rongo, L.; D’Amore, M.; Budzelaar, P.H.M.; Busico, V. Periodic Hybrid DFT Approach (Including Dispersion) to MgCl2-Supported Ziegler–Natta Catalysts. 2. Model Electron Donor Adsorption on MgCl2 Crystal Surfaces. J. Phys. Chem. C 2013, 117, 24345–24353. [Google Scholar] [CrossRef]
- Pirinen, S.; Pakkanen, T.T. Polyethers as potential electron donors for Ziegler–Natta ethylene polymerization catalysts. J. Mol. Catal. A Chem. 2015, 398, 177–183. [Google Scholar] [CrossRef]
- Wondimagegn, T.; Ziegler, T. The Role of External Alkoxysilane Donors on Stereoselectivity and Molecular Weight in MgCl2-Supported Ziegler–Natta Propylene Polymerization: A Density Functional Theory Study. J. Phys. Chem. C 2012, 116, 1027–1033. [Google Scholar] [CrossRef]
- Pasquon, I.; Giannini, U. Catalytic Olefin Polymerization. In Catalysis: Science and Technology Volume 6; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 65–159. [Google Scholar]
- Sacchi, M.C.; Tritto, I.; Shan, C.; Mendichi, R.; Noristi, L. Role of the pair of internal and external donors in magnesium chloride-supported Ziegler-Natta catalysts. Macromolecules 1991, 24, 6823–6826. [Google Scholar] [CrossRef]
- Shen, X.-r.; Fu, Z.-s.; Hu, J.; Wang, Q.; Fan, Z.-q. Mechanism of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts Based on Counting of Active Centers: The Role of External Electron Donor. J. Phys. Chem. C 2013, 117, 15174–15182. [Google Scholar] [CrossRef]
- Guo, X.; Cui, L.; Wang, Y.; Yi, J.; Sun, J.; Liu, Z.; Liu, B. Mechanistic Study on Effect of Electron Donors in Propylene Polymerization Using the Ziegler–Natta Catalyst. J. Phys. Chem. C 2021, 125, 8533–8542. [Google Scholar] [CrossRef]
- Hermans, J.P.; Henrioulle, P. Catalytic Complexes. U.S. Patent 4,210,738, 1 July 1980. [Google Scholar]
- Natta, G.; Pasquon, I.; Zambelli, A.; Gatti, G. Highly stereospecific catalytic systems for the polymerization of α-olefins to isotactic polymers. J. Polym. Sci. 1961, 51, 387–398. [Google Scholar] [CrossRef]
- Karian, H. (Ed.) Handbook of Polypropylene and Polypropylene Composites, Revised and Expanded; CRC Press: New York, NY, USA, 2003; pp. 10–28. [Google Scholar]
- Erik, T.; Seelbach, C.W.; Langer, J.A.; Exxon Research and Engineering Co. Preparation of Partially Reduced Transition Metal Halide Catalyst Compositions. U.S. Patent 3,128,252, 7 April 1964. [Google Scholar]
- Norio, K.; Yoshihisa, U. Process for Polymerizing or Copolymerizing Olefins. U.S. Patent 4,442,276, 10 April 1984. [Google Scholar]
- Galli, P. The reactor granule technology: The ultimate expansion of polypropylene properties? J. Macromol. Sci. Part A 1999, 36, 1561–1586. [Google Scholar] [CrossRef]
- Rotzinger, B.; Brunner, M. In-reactor stabilization of polypropene. Polym. Degrad. Stab. 2008, 93, 316–320. [Google Scholar] [CrossRef]
- Morini, G.; Albizzati, E.; Balbontin, G.; Baruzzi, G.; Cristofori, A. 1,3-Diethers and Components and Catalysts for the Polymerization of Olefins, Containing Said Diethers. U.S. Patent 7,022,640, 4 April 2006. Available online: https://ppubs.uspto.gov/dirsearch-public/print/downloadBasicPdf/7022640?requestToken=eyJzdWIiOiJhZTNiMWY0ZS1lOWQ4LTQzNjEtOGEyMy02YzE3MzJhMTdjY2YiLCJ2ZXIiOiI3YTAwMTAwMS0yMzVkLTRlZDAtODg3NC0zOTkyMzNlOTViOWEiLCJleHAiOjB9 (accessed on 14 August 2024).
- Morini, G.; Albizzati, E.; Balbontin, G.; Baruzzi, G.; Cristofori, A. 1,3-Diethers and Components and Catalysts for the Polymerization of Olefins, Containing Said Diethers. U.S. Patent 7,049,377, 23 May 2006. Available online: https://ppubs.uspto.gov/dirsearch-public/print/downloadBasicPdf/7049377?requestToken=eyJzdWIiOiJhZTNiMWY0ZS1lOWQ4LTQzNjEtOGEyMy02YzE3MzJhMTdjY2YiLCJ2ZXIiOiI3YTAwMTAwMS0yMzVkLTRlZDAtODg3NC0zOTkyMzNlOTViOWEiLCJleHAiOjB9 (accessed on 14 August 2024).
- Busico, V.; Chadwick, J.C.; Cipullo, R.; Ronca, S.; Talarico, G. Propene/Ethene-[1-13C] Copolymerization as a Tool for Investigating Catalyst Regioselectivity. MgCl2/Internal Donor/TiCl4−External Donor/AlR3 Systems. Macromolecules 2004, 37, 7437–7443. [Google Scholar] [CrossRef]
- D’Amore, M.; Credendino, R.; Budzelaar, P.H.M.; Causá, M.; Busico, V. A periodic hybrid DFT approach (including dispersion) to MgCl2-supported Ziegler–Natta catalysts—1: TiCl4 adsorption on MgCl2 crystal surfaces. J. Catal. 2012, 286, 103–110. [Google Scholar] [CrossRef]
- Takasao, G.; Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. Insight into structural distribution of heterogeneous Ziegler–Natta catalyst from non-empirical structure determination. J. Catal. 2021, 394, 299–306. [Google Scholar] [CrossRef]
- Kuklin, M.S.; Bazhenov, A.S.; Denifl, P.; Leinonen, T.; Linnolahti, M.; Pakkanen, T.A. Stabilization of magnesium dichloride surface defects by mono- and bidentate donors. Surf. Sci. 2015, 635, 5–10. [Google Scholar] [CrossRef]
- Thushara, K.S.; Gnanakumar, E.S.; Mathew, R.; Ajithkumar, T.G.; Rajamohanan, P.R.; Bhaduri, S.; Gopinath, C.S. MgCl2·4((CH3)2CHCH2OH): A new molecular adduct for the preparation of TiClx/MgCl2 catalyst for olefin polymerization. Dalton Trans. 2012, 41, 11311–11318. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Morini, G.; Balbontin, G.; Camurati, I.; Heere, J.J.R.; Mingozzi, I.; Testoni, F. Effects of Internal and External Donors on the Regio- and Stereoselectivity of Active Species in MgCl2-Supported Catalysts for Propene Polymerization. Macromol. Chem. Phys. 2001, 202, 1995–2002. [Google Scholar] [CrossRef]
- Wen, X.; Ji, M.; Yi, Q.; Niu, H.; Dong, J.-Y. Magnesium chloride supported Ziegler-Natta catalysts containing succinate internal electron donors for the polymerization of propylene. J. Appl. Polym. Sci. 2010, 118, 1853–1858. [Google Scholar] [CrossRef]
- Song, B.G.; Choi, Y.H.; Ihm, S.-K. Characteristics of diether- and phthalate-based Ziegler-Natta catalysts for copolymerization of propylene and ethylene and terpolymerization of propylene, ethylene, and 1-butene. J. Appl. Polym. Sci. 2013, 130, 851–859. [Google Scholar] [CrossRef]
- Soga, K.; Shiono, T.; Doi, Y. Influence of internal and external donors on activity and stereospecificity of ziegler-natta catalysts. Die Makromol. Chem. 1988, 189, 1531–1541. [Google Scholar] [CrossRef]
- Potapov, A.G.; Bukatov, G.D.; Zakharov, V.A. DRIFT study of internal donors in supported Ziegler–Natta catalysts. J. Mol. Catal. A Chem. 2006, 246, 248–254. [Google Scholar] [CrossRef]
- Mori, H.; Hasebe, K.; Terano, M. XPS study of the interaction of titanium species with internal electron donors on MgCl2-supported Ziegler catalysts. J. Mol. Catal. A Chem. 1999, 140, 165–172. [Google Scholar] [CrossRef]
- Terano, M.; Kataoka, T.; Keii, T. A Study on the States of Ethylbenzoate and TiCl4 in MgCl2-Supported Catalysts by Thermal Analysis. In Catalytic Polymerization of Olefins; Keii, T., Soga, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 25, pp. 407–418. [Google Scholar]
- Andoni, A.; Chadwick, J.C.; Niemantsverdriet, H.J.W.; Thüne, P.C. The role of electron donors on lateral surfaces of MgCl2-supported Ziegler–Natta catalysts: Observation by AFM and SEM. J. Catal. 2008, 257, 81–86. [Google Scholar] [CrossRef]
- Weng, Y.; Jiang, B.; Fu, Z.; Fan, Z. Mechanism of internal and external electron donor effects on propylene polymerization with MgCl2-supported Ziegler–Natta catalyst: New evidences based on active center counting. J. Appl. Polym. Sci. 2018, 135, 46605. [Google Scholar] [CrossRef]
- Zheng, W.-P.; Ma, Y.-P.; Du, D.-L.; He, A.-H.; Shao, H.-F.; Liu, C.-G. Polymerization Kinetics of Propylene with the MgCl2-Supported Ziegler-Natta Catalysts—Active Centers with Different Tacticity and Fragmentation of the Catalyst. Chin. J. Polym. Sci. 2021, 39, 70–80. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, Y.; Ren, S.; Dang, X.; Zhang, L.; Li, H.; Hu, Y. Study on the sequence length distribution of polypropylene by the successive self-nucleation and annealing (SSA) calorimetric technique. Polym. Chem. 2012, 3, 2909–2919. [Google Scholar] [CrossRef]
- Bukatov, G.D.; Zakharov, V.A.; Barabanov, A.A. Mechanism of olefin polymerization on supported Ziegler-Natta catalysts based on data on the number of active centers and propagation rate constants. Kinet. Catal. 2005, 46, 166–176. [Google Scholar] [CrossRef]
- Shetty, S. Synergistic, reconstruction and bonding effects during the adsorption of internal electron donors and TiCl4 on MgCl2 surface: A periodic-DFT investigation. Surf. Sci. 2016, 653, 55–65. [Google Scholar] [CrossRef]
- Busico, V.; Corradini, P.; De Martino, L.; Proto, A.; Savino, V.; Albizzati, E. Polymerization of propene in the presence of MgCl2-supported Ziegler-Natta catalysts, 1. The role of ethyl benzoate as “internal” and “external” base. Die Makromol. Chem. 1985, 186, 1279–1288. [Google Scholar] [CrossRef]
- Stukalov, D.V.; Zakharov, V.A.; Potapov, A.G.; Bukatov, G.D. Supported Ziegler–Natta catalysts for propylene polymerization. Study of surface species formed at interaction of electron donors and TiCl4 with activated MgCl2. J. Catal. 2009, 266, 39–49. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, S.; Makwana, U.; Patankar, R.B.; Gupta, V.K. Influence of Internal Donors on the Performance and Structure of MgCl2 Supported Titanium Catalysts for Propylene Polymerization. Macromol. Chem. Phys. 2009, 210, 69–76. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Hu, Y.; Vizzini, J.C. Superactive and stereospecific catalysts. IV. Influence of structure of esters on MgCl2 supported olefin polymerization catalysts. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 273–284. [Google Scholar] [CrossRef]
- Sobota, P.; Ejfler, J.; Utko, J.; Lis, T. Di-μ-chloro-tetrachlorobis(diethyl o-phthalate)dititanium(III), a new intermediate for the synthesis of titanium(III) compounds: Crystal structure and properties. J. Organomet. Chem. 1991, 410, 149–157. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Hu, Y. Superactive and stereospecific catalysts. III. Definitive identification of active sites by electron paramagnetic resonance. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 897–913. [Google Scholar] [CrossRef]
- Sobota, P.; Utko, J.; Lis, T. Interaction between TiCl4 and o-, m- and p-diesters. The crystal structures of [o-C6H4(COO-i-Bu)2TiCl4]·CH2Cl2 and [p-C6H4(COOMe)2TiCl4]. J. Organomet. Chem. 1990, 393, 349–358. [Google Scholar] [CrossRef]
- Kakkonen, H.J.; Pursiainen, J.; Pakkanen, T.A.; Ahlgrén, M.; Iiskola, E. TiCl4 diester complexes: Relationships between the crystal structures and properties of Ziegler-Natta catalysts. J. Organomet. Chem. 1993, 453, 175–184. [Google Scholar] [CrossRef]
- Gao, M.Z.; Cai, X.X.; Liu, H.T.; Ma, J.X.; Chen, J.H.; Wang, J.; Hu, J.J.; Li, X.Z.; Ma, J.; Li, C.X. The Invention Discloses A Method For Preparing A Catalyst For Olefin Polymerization. C.N. Patent 104,558,292, 29 April 2015. [Google Scholar]
- Zhang, R.; Tan, Z.; Zhou, Q.L.; Xu, X.D.; Li, F.K.; Song, W.W.; Yu, J.H.; Yin, S.S. Solid Catalyst Components for Preparing Polyolefin, Catalyst System and Application. C.N. Patent 112,661,883, 16 April 2021. [Google Scholar]
- Li, H.Y.; Li, Q.; Hu, Y.L. Solid Components of Olefin Polymerization Catalyst and Preparation Method. C.N. Patent 102,212,154, 12 October 2011. [Google Scholar]
- Guo, J.; Hu, G.; Chen, Z. Synthesis of novel electron donors and their application to propylene polymerization. Trans. Tianjin Univ. 2012, 18, 8–14. [Google Scholar] [CrossRef]
- Tsukahara, T.; Nobuyuki, T.; Sugano, Y.; Yamamoto, K.; Nakajima, T. Polypropylene Compositions and Molded Products. C.N. Patent 111,712,541, 25 September 2020. Available online: https://cpquery.cponline.cnipa.gov.cn/detail/index?zhuanlisqh=Qd7zPZ2WzNTRIUP7J1qIyg%253D%253D&anjianbh (accessed on 13 August 2024).
- Tsukahara, T.; Nobuyuki, T.; Sugano, Y.; Yamamoto, K.; Nakajima, T. Polypropylene Compositions and Molded Products. C.N. Patent 111,741,987, 2 October 2020. Available online: https://cpquery.cponline.cnipa.gov.cn/detail/index?zhuanlisqh=2sh8eLQK48jnLeHjDuY%252BEQ%253D%253D&anjianbh (accessed on 13 August 2024).
- Gaddy, B.; Bockino, M.; Collina, G.; Fusco, O.; Guidotti, S.; Mazucco, A.; Pantaglioni, R.; Piermertes, F. Preparation Method of Porous Propylene Polymer. C.N. Patent 105,636,994, 16 March 2021. [Google Scholar]
- Pater, J.T.M.; Liguori, D.; Vitale, G.; Collina, G.; Brita, D.; Dall’Occo, T.; Morini, G. Catalyst Components for the Polymerization of Olefins. U.S. Patent 10,155,825, 18 December 2018. [Google Scholar]
- Zhang, R.; Tan, Z.; Zhou, Q.; Xu, X. Research of the effctive structure of succinate as internal donor. Spec. Petrochem. 2018, 35, 19–23. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhao, X.T.; Wei, S.Y.; Zhu, B.C.; Hu, Y.L.; Gao, L.; Wang, X.; Hao, P.; Yao, P.H.; Liao, Z.F.; et al. Olefin Polymerization Catalytic Components and Catalysts. C.N. Patent 101,423,571, 6 May 2009. [Google Scholar]
- Liu, Y.X.; Xia, X.Z.; Peng, R.Q.; Zhang, T.Y.; Tan, Y.; Zhao, J.; Zhang, J.G.; Ling, Y.T.; Gao, F.T.; Li, W.L.; et al. The Invention Discloses an Propylene Polymer and Preparation Method. C.N. Patent 105,504,111, 20 April 2016. [Google Scholar]
- Zhao, J.; Xia, X.; Liu, Y.; Yang, T.; Gao, F.; Ling, Y.; Gao, P.; Peng, R.; Li, W.; Zhang, J. Olefin Polymerization Catalyst Components and Olefin Polymerization Catalyst and Olefin Polymerization Method. C.N. Patent 104,250,316, 13 December 2014. [Google Scholar]
- Gao, M.; Liu, H.; Wang, J.; Li, C.; Ma, J.; Wei, G. Novel MgCl2-supported catalyst containing diol dibenzoate donor for propylene polymerization. Polymer 2004, 45, 2175–2180. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liu, Y.; Zhou, Q.; Li, C.; Gao, M. Development of catalysts with dioldiesters as internal donor for propylene polymerization. Chem. Ind. Eng. Prog. 2015, 34, 1809–1816. [Google Scholar] [CrossRef]
- Zhang, J.; Nan, F.; Yu, H.; Zhang, S.; Xia, X.; Huang, Q.; Yi, J.; Li, H.; Zhao, Z. Direct Preparation of Transparent Isotactic Polypropylene with Supported Ziegler–Natta Catalysts Containing Novel Eco-friendly Internal Electron Donors. Ind. Eng. Chem. Res. 2020, 59, 8995–9003. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Xia, X.; Zhang, S.; Huang, Q.; Li, C.; Miao, Q.; Zhu, F.; Yi, J.; Zhao, Z. Sustainable rosin acid ester as internal electron donors in Ziegler-Natta catalysts for synthesis of isotactic polypropylene with high melt flow rate. J. Macromol. Sci. Part A 2021, 58, 686–693. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, A.; Li, H.; Luo, Z.; Zheng, T.; Zhang, L.; Hu, Y. Microstructure of polypropylene and active center in Ziegler–Natta catalyst: Effect of novel salicylate internal donor. RSC Adv. 2016, 6, 75023–75031. [Google Scholar] [CrossRef]
- Ratanasak, M.; Hasegawa, J.-y.; Parasuk, V. Roles of Salicylate Donors in Enhancement of Productivity and Isotacticity of Ziegler–Natta Catalyzed Propylene Polymerization. Polymers 2020, 12, 883. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, C.; Liu, H. Research in imino ester compounds as internal electron donors. Petrochem. Ind. Technol. 2022, 29, 98–100. [Google Scholar] [CrossRef]
- Piovano, A.; Groppo, E. Flexible ligands in heterogeneous catalysts for olefin polymerization: Insights from spectroscopy. Coord. Chem. Rev. 2022, 451, 214258. [Google Scholar] [CrossRef]
- Song, B.G.; Ihm, S.-K. The role of two different internal donors (phthalate and 1,3-diether) on the formation of surface structure in MgCl2-supported Ziegler–Natta catalysts and their catalytic performance of propylene polymerization. J. Appl. Polym. Sci. 2014, 131, 40356. [Google Scholar] [CrossRef]
- Potapov, A.G.; Politanskaya, L.V. The study of the adsorption of 1,3-diethers on the MgCl2 surface. J. Mol. Catal. A Chem. 2013, 368–369, 159–162. [Google Scholar] [CrossRef]
- Cui, N.; Ke, Y.; Li, H.; Zhang, Z.; Guo, C.; Lv, Z.; Hu, Y. Effect of diether as internal donor on MgCl2-supported Ziegler–Natta catalyst for propylene polymerization. J. Appl. Polym. Sci. 2006, 99, 1399–1404. [Google Scholar] [CrossRef]
- Ghasemi Hamedani, N.; Arabi, H.; Poorsank, F. Towards the design of a mixture of diether and succinate as an internal donor in a MgCl2-supported Ziegler–Natta catalyst. New J. Chem. 2020, 44, 15758–15768. [Google Scholar] [CrossRef]
- Blaakmeer, E.S.M.; Antinucci, G.; van Eck, E.R.H.; Kentgens, A.P.M. Probing Interactions between Electron Donors and the Support in MgCl2-Supported Ziegler–Natta Catalysts. J. Phys. Chem. C 2018, 122, 17865–17881. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, L.; Zerbi, G.; Piemontesi, F.; Nascetti, S.; Morini, G. Structure of Donor Molecule 9,9-Bis(Methoxymethyl)-Fluorene in Ziegler-Natta Catalyst by Infrared Spectroscopy and Quantum Chemical Calculation. J. Phys. Chem. C 2010, 114, 11475–11484. [Google Scholar] [CrossRef]
- Nissinen, V.; Pirinen, S.; Pakkanen, T.T. Unexpected cleavage of ether bonds of 1,3-dimethoxypropane in Grignard–Wurtz synthesis of a MgCl2–donor adduct. J. Mol. Catal. A Chem. 2016, 413, 94–99. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P.; Morini, G.; Noristi, L.; Albizzati, E. Polymerization Stereochemistry with Ziegler−Natta Catalysts Containing Dialkylpropane Diethers: A Tool for Understanding Internal/External Donor Relationships. Macromolecules 1996, 29, 3341–3345. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, M.; Han, S.; Hu, Y. Study on ziegler-natta catalyst for propene polymerization without external electron donor. Acta Polym. Sin. 2001, 2, 191–194. [Google Scholar]
- Zahedi, R.; Afshar Taromi, F.; Mirjahanmardi, S.H.; Nekoomanesh Haghighi, M.; Jadidi, K.; Jamjah, R. Propylene Polymerization over MgCl2-Supported Ziegler–Natta Catalysts Containing Tri-Ether as the Internal Donor. Adv. Polym. Technol. 2018, 37, 144–153. [Google Scholar] [CrossRef]
- Morini, G.; Albizzati, E.; Balbontin, G.; Mingozzi, I.; Sacchi, M.C.; Forlini, F.; Tritto, I. Microstructure Distribution of Polypropylenes Obtained in the Presence of Traditional Phthalate/Silane and Novel Diether Donors: A Tool for Understanding the Role of Electron Donors in MgCl2-Supported Ziegler−Natta Catalysts. Macromolecules 1996, 29, 5770–5776. [Google Scholar] [CrossRef]
- Albizzati, E.; Giannini, U.; Morini, G.; Galimberti, M.; Barino, L.; Scordamaglia, R. Recent advances in propylene polymerization with MgCl2 supported catalysts. Macromol. Symp. 1995, 89, 73–89. [Google Scholar] [CrossRef]
- Iiskola, E.; Pelkonen, A.; Kakkonen, H.J.; Pursiainen, J.; Pakkanen, T.A. A novel MgCl2-supported Ziegler-Natta catalyst composition: Stereospecific polymerization of propene without external donor. Die Makromol. Chem. Rapid Commun. 1993, 14, 133–137. [Google Scholar] [CrossRef]
- Blaakmeer, E.S.M.; Antinucci, G.; Correa, A.; Busico, V.; van Eck, E.R.H.; Kentgens, A.P.M. Structural Characterization of Electron Donors in Ziegler–Natta Catalysts. J. Phys. Chem. C 2018, 122, 5525–5536. [Google Scholar] [CrossRef]
- Vanka, K.; Singh, G.; Iyer, D.; Gupta, V.K. DFT Study of Lewis Base Interactions with the MgCl2 Surface in the Ziegler−Natta Catalytic System: Expanding the Role of the Donors. J. Phys. Chem. C 2010, 114, 15771–15781. [Google Scholar] [CrossRef]
- Lee, J.W.; Jo, W.H. Chemical structure–stereospecificity relationship of internal donor in heterogeneous Ziegler–Natta catalyst for propylene polymerization by DFT and MM calculations. J. Organomet. Chem. 2009, 694, 3076–3083. [Google Scholar] [CrossRef]
- Li, H.; Yi, J.; Cui, C. Bis(trifluoromethylsulfonyl)phenylamines as Internal Donors for Ziegler-Natta Polymerization Catalysts. China Pet. Process. Petrochem. Technol. 2008, 3, 51–54. [Google Scholar]
- Wang, F.; Cui, L.; Yi, J.; Yin, L.; Wang, L.; Cui, C.; Han, B. The Property of Sulfonylimine as Internal Donor for Polymerization Catalyst. Polym. Bull. 2010, 12, 43–47. [Google Scholar] [CrossRef]
- Cui, C.M.; Wang, L.; Li, H.S.; Zhang, Y.J. The Invention Telates to a Phosphorus-Substituted Sulfonyl Compound as an Internal Electron Donor Polymerization Catalyst and Its Preparation and Application. C.N. Patent 101,787,088, 27 March 2013. [Google Scholar]
- Kim, G.-H.; Um, B.-H.; Son, K.-C.; Oh, K.; Koh, H.-L. MgCl2-supported Ziegler–Natta catalyst containing dibenzoyl sulfide donor for propylene polymerization. J. Appl. Polym. Sci. 2014, 131, 40734. [Google Scholar] [CrossRef]


| Gen | Catalyst Composition | Activity a | II b | Morphological Characteristics | Remakes |
|---|---|---|---|---|---|
| 1st | 3TiCl3·AlCl3-AlEt2Cl | 0.8–1.2 | 90–94 | Irregular powder | Need reprocess |
| 2nd | TiCl3-AlEt2Cl | 3–5 | 96–98 | Particle | Deashing catalyst powder |
| 3rd | TiCl4/MgCl2/Benzoate-AlEt3 | 5–10 | 95–97 | Regular particle | Deashing ataic polymer |
| 4th | TiCl4/MgCl2/Phthalate-AlEt3/Silane | 15–25 | 95–99 | Similar Sphere | Nothing operations |
| 5th | TiCl4/MgCl2/Diether-AlEt3/Silane | 25–35 | 95–99 | Sphere | Nothing operations |
| 5th | TiCl4/MgCl2/Succinate-AlEt3/Silane | 15–20 | 95–99 | Sphere | Nothing operations |
| Catalyst | Internal Donor | Activity a | Ti wt% | II b | MWD | MFR c |
|---|---|---|---|---|---|---|
| Ref | DNBP | 42.5 | 2.3 | 98.0 | 5.5 | 4.5 |
| Cat-1 | PDB | 56.7 | 2.4 | 96.0 | 7.8 | 0.8 |
| Cat-2 | PDB+DNBP | 57.9 | 2.5 | 98.3 | 6.5 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, H.; Hu, H.; Zhou, Y.; Mao, G.; Xin, S. The Effects of Internal Electron Donors on MgCl2-Supported Ziegler–Natta Catalysts for Isotactic PP. Polymers 2024, 16, 2687. https://doi.org/10.3390/polym16192687
Li B, Li H, Hu H, Zhou Y, Mao G, Xin S. The Effects of Internal Electron Donors on MgCl2-Supported Ziegler–Natta Catalysts for Isotactic PP. Polymers. 2024; 16(19):2687. https://doi.org/10.3390/polym16192687
Chicago/Turabian StyleLi, Bin, Huashu Li, Hongfan Hu, Yi Zhou, Guoliang Mao, and Shixuan Xin. 2024. "The Effects of Internal Electron Donors on MgCl2-Supported Ziegler–Natta Catalysts for Isotactic PP" Polymers 16, no. 19: 2687. https://doi.org/10.3390/polym16192687





