Characterizing Hyperspectral Microscope Imagery for Classification of Blueberry Firmness with Deep Learning Methods
Abstract
:1. Introduction
2. Materials and Methods
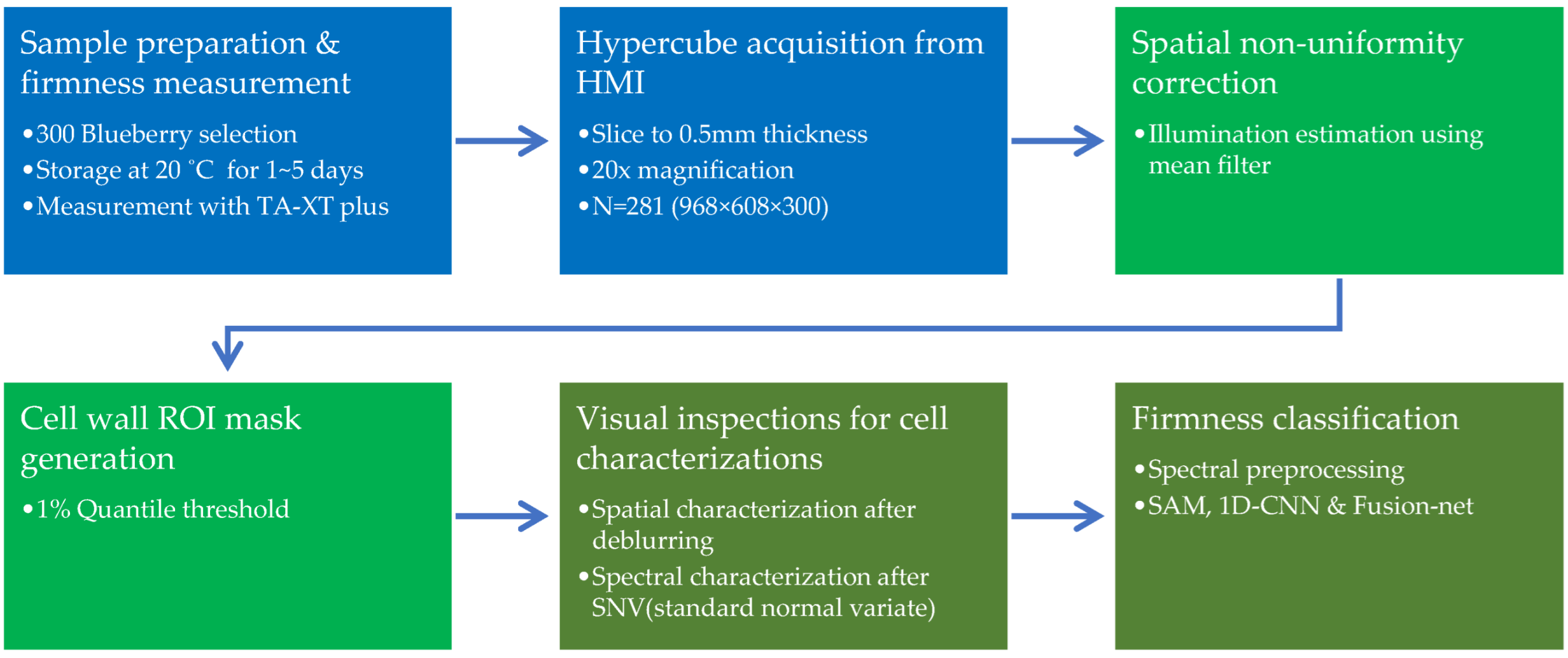
2.1. Summary Pipeline of Approaches
2.2. Sample Preparation and Firmness Measurement
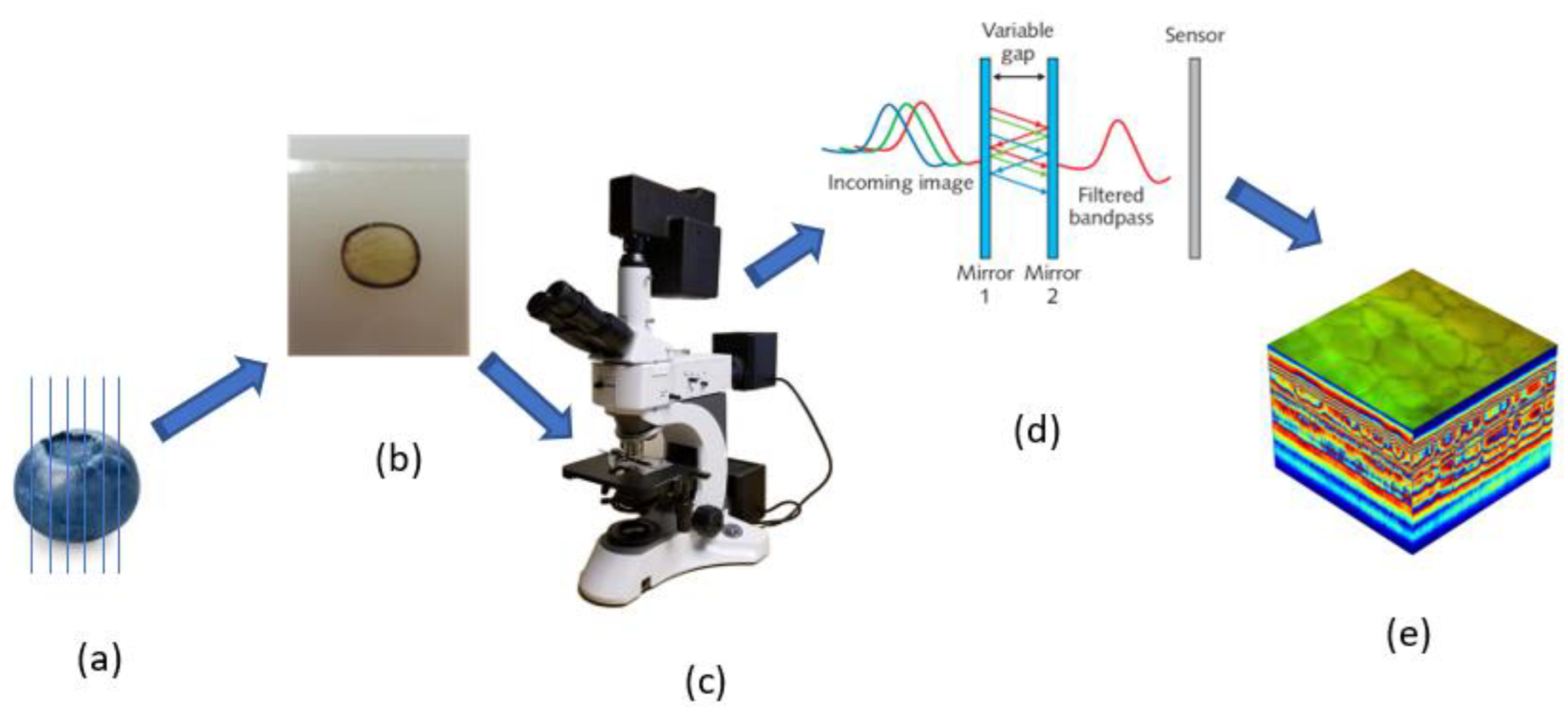
2.3. Hyperspectral Microscope Imaging
2.4. Hypercube Processing
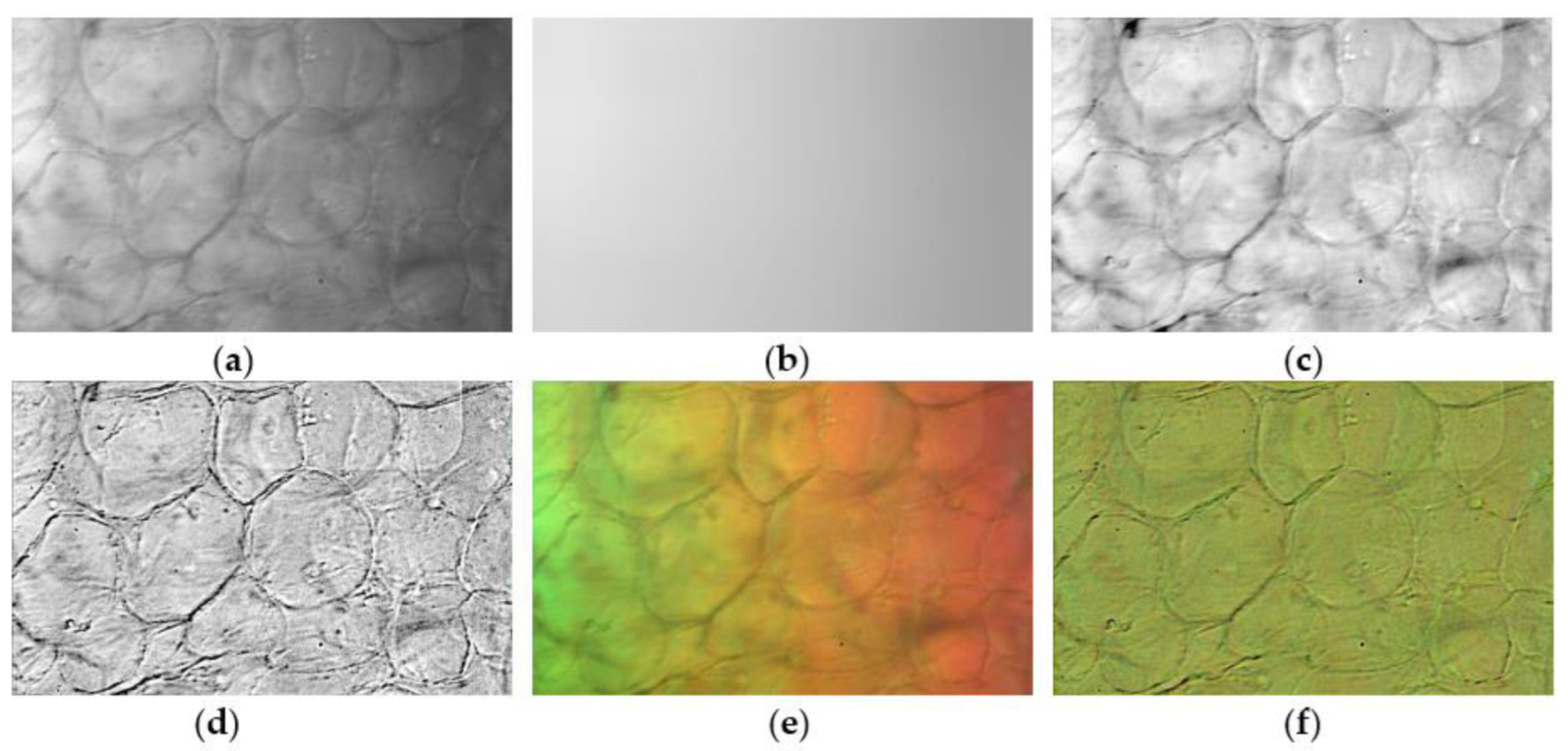
2.4.1. Spatial Non-Uniformity Correction for Every Wavelength
2.4.2. ROI Mask Generation of Cell Walls Using Quantile Threshold
2.5. Spatial and Spectral Cell Characterization on Blueberry Firmness
2.6. Firmness Classification
Algorithm Implementations for Classification Models
3. Results
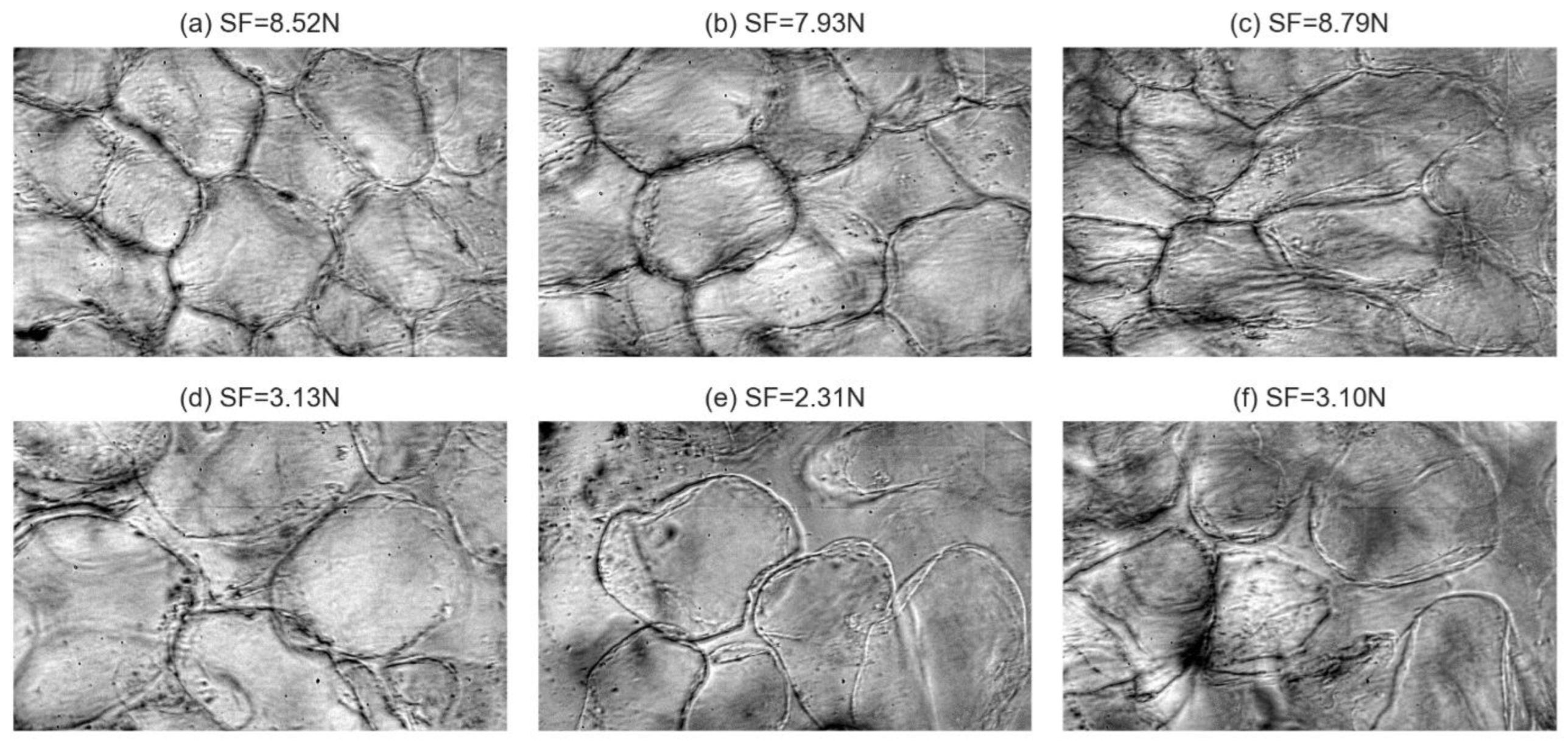
3.1. Spatial Cell Characteristics Based on Firmness
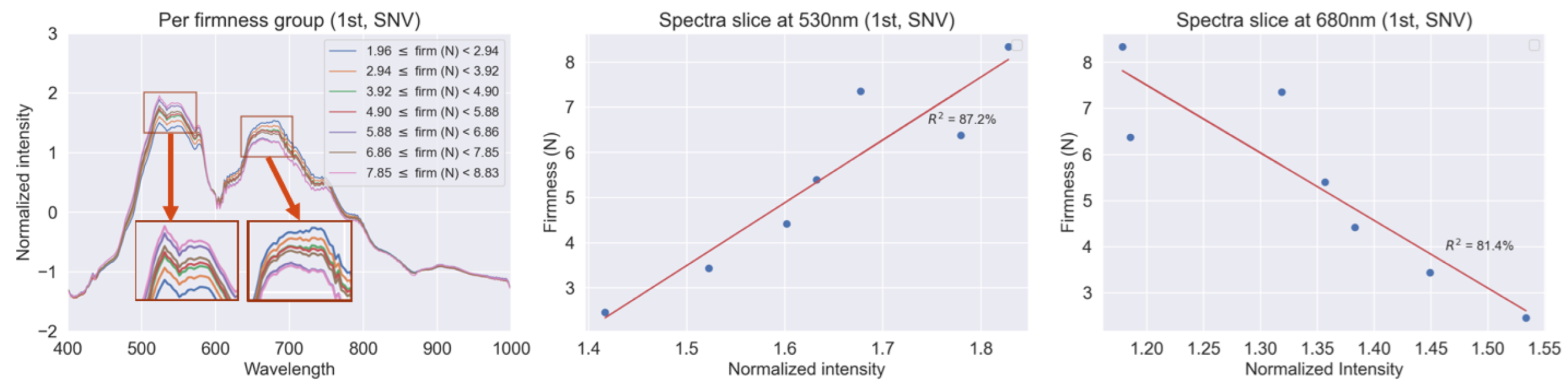
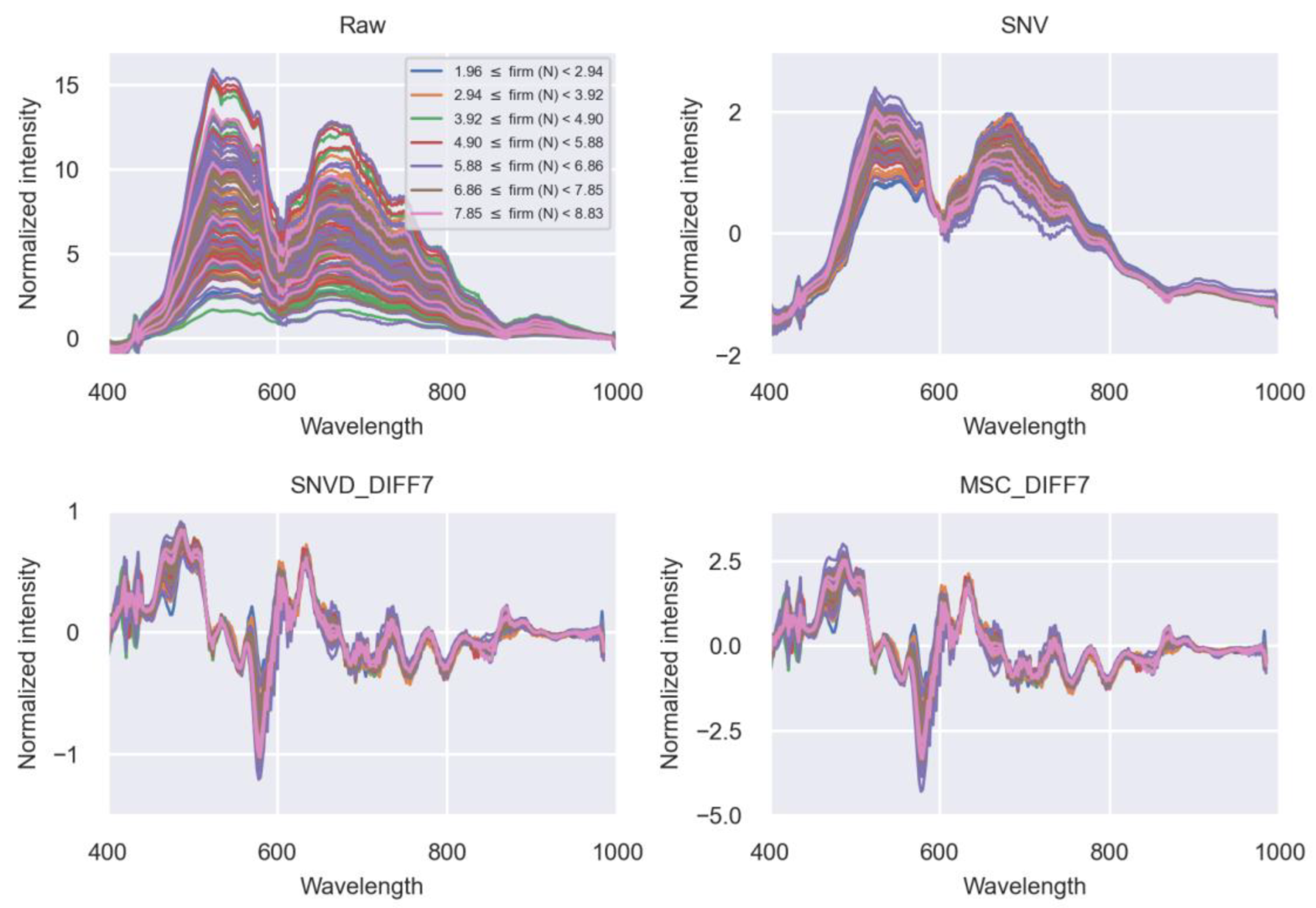
3.2. Spectral Cell Characteristics Based on Firmness
3.3. Firmness Classification
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allan-Wojtas, P.M.; Forney, C.F.; Carbyn, S.E.; Nicholas, K.U. Microstructural indicators of quality-related characteristics of blueberries—An integrated approach. LWT-Food Sci. Technol. 2001, 34, 23–32. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- FAOUN. FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 18 November 2020).
- USDA. National Agricultural Statistics Service. 2020. Available online: https://www.nass.usda.gov/Statistics_by_Subject/result.php?CBDCF536-13A5-3D67-94CA-D068D478FBD4§or=CROPS&group=FRUIT%20%26%20TREE%20NUTS&comm=BLUEBERRIES (accessed on 21 August 2020).
- Retamales, J.B.; Palma, M.J.; Morales, Y.A.; Lobos, G.A.; Moggia, C.E.; Mena, C.A. Blueberry production in Chile: Current status and future developments. Rev. Bras. Frutic. 2014, 36, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Moggia, C.; Graell, J.; Lara, I.; Schmeda-Hirschmann, G.; Thomas-Valdés, S.; Lobos, G.A. Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Sci. Hortic. 2016, 211, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Takeda, F.; Krewer, G.; Li, C. Measure of mechanical impacts in commercial blueberry packing lines and potential damage to blueberry fruit. Postharvest Biol. Technol. 2015, 110, 103–113. [Google Scholar] [CrossRef]
- USDA; Agricultural Marketing Service. Fruit and Vegetable Programs: Blueberries—Shipping Point and Market Inspection Instructions. 2002. Available online: https://www.ams.usda.gov/sites/default/files/media/Blueberry_Inspection_Instructions%5B1%5D.pdf (accessed on 18 November 2020).
- Sanford, K.A.; Lidster, P.D.; McRae, K.B.; Jackson, E.D.; Lawrence, R.A.; Stark, R.; Prange, R.K. Lowbush blueberry quality changes in response to mechanical damage and storage temperature. J. Am. Soc. Hortic. Sci. 1991, 116, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.L.; Marroquin, E.; Matta, F.B.; Garner, J.O., Jr.; Stojanovic, J. Physicochemical, carbohydrate and sensory characteristics of highbush and rabbiteye blueberry cultivars. J. Sci. Food Agric. 2005, 85, 1815–1821. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; MacLean, D. A novel instrument to delineate varietal and harvest effects on blueberry fruit texture during storage. J. Sci. Food Agric. 2011, 91, 1653–1658. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Prussia, S.E.; Astleford, J.J.; Hewlett, B.; Hung, Y.C. University of Georgia Research Foundation Inc. UGARF. Non-Destructive Firmness Measuring Device. U.S. Patent 5,372,030, 13 December 1994. [Google Scholar]
- VSCNews. Methods for Measuring Fruit Firmness. 2019. Available online: https://vscnews.com/methods-for-measuring-fruit-firmness/ (accessed on 21 August 2020).
- Park, B.; Yoon, S.C.; Lee, S.; Sundaram, J.; Windham, W.R.; Hinton, A., Jr.; Lawrence, K.C. Acousto-optic tunable filter hyperspectral microscope imaging method for characterizing spectra from foodborne pathogens. Trans. ASABE 2012, 55, 1997–2006. [Google Scholar] [CrossRef]
- Kim, M.; Yun, J.; Cho, Y.; Shin, K.; Jang, R.; Bae, H.J.; Kim, N. Deep learning in medical imaging. Neurospine 2019, 16, 657. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A.; Bengio, Y. Deep Learning; MIT Press: Cambridge, UK, 2016. [Google Scholar]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Hu, M.H.; Dong, Q.L.; Liu, B.L.; Opara, U.L.; Chen, L. Estimating blueberry mechanical properties based on random frog selected hyperspectral data. Postharvest Biol. Technol. 2015, 106, 1–10. [Google Scholar] [CrossRef]
- Eady, M.B.; Park, B.; Yoon, S.C.; Haidekker, M.A.; Lawrence, K.C. Methods for hyperspectral microscope calibration and spectra normalization from images of bacteria cells. Trans. ASABE 2018, 61, 438–448. [Google Scholar] [CrossRef]
- Shih, F.Y. Image Processing and Pattern Recognition: Fundamentals and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 120. [Google Scholar]
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing, 4th ed.; Pearson: New York, NY, USA, 2018; pp. 352–358. [Google Scholar]
- Park, B.; Windham, W.R.; Lawrence, K.C.; Smith, D.P. Contaminant classification of poultry hyperspectral imagery using a spectral angle mapper algorithm. Biosyst. Eng. 2007, 96, 323–333. [Google Scholar] [CrossRef]
- Kang, R.; Park, B.; Eady, M.; Ouyang, Q.; Chen, K. Classification of foodborne bacteria using hyperspectral microscope imaging technology coupled with convolutional neural networks. Appl. Microbiol. Biotechnol. 2020, 104, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.C.; Windham, W.R.; Ladely, S.R.; Heitschmidt, J.W.; Lawrence, K.C.; Park, B.; Narang, N.; Cray, W.C. Hyperspectral imaging for differentiating colonies of non-0157 Shiga-toxin producing Escherichia coli (STEC) serogroups on spread plates of pure cultures. J. Near Infrared Spectrosc. 2013, 21, 81–95. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.; Park, B.; Eady, M.; Ouyang, Q.; Chen, K. Single-cell classification of foodborne pathogens using hyperspectral microscope imaging coupled with deep learning frameworks. Sens. Actuators B Chem. 2020, 309, 127789. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Ji, Q.; Huang, J.; He, W.; Sun, Y. Optimized Deep Convolutional Neural Networks for Identification of Macular Diseases from Optical Coherence Tomography Images. Algorithms 2019, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Schotsmans, W.; Molan, A.; MacKay, B. Controlled atmosphere storage of rabbiteye blueberries enhances postharvest quality aspects. Postharvest Biol. Technol. 2007, 44, 277–285. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]


| Preprocessing Methods | SAM | 1D-CNN | Fusion-Net | |||
|---|---|---|---|---|---|---|
| ACC (%) | MCC (%) | ACC (%) | MCC (%) | ACC (%) | MCC (%) | |
| SNV | 55 | −2.3 | 80 | 54.5 | 80 | 56 |
| SNVD-MA7 | 70 | 30.2 | 70 | 34.1 | 70 | 27.9 |
| SNVD-DIFF7 | 70 | 31.3 | 65 | 0 | 80 | 54.5 |
| SNVD-DIFF7-MA7 | 60 | 12.1 | 75 | 45.4 | 60 | 6.1 |
| MSC-MA7 | 60 | 12.1 | 60 | −1.5 | 70 | 39 |
| MSC-DIFF7 | 70 | 30.3 | 85 | 66.3 | 85 | 73.4 |
| MSC-DIFF7-MA7 | 60 | −1.5 | 60 | 6.1 | 75 | 45.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.; Shin, T.-S.; Cho, J.-S.; Lim, J.-H.; Park, K.-J. Characterizing Hyperspectral Microscope Imagery for Classification of Blueberry Firmness with Deep Learning Methods. Agronomy 2022, 12, 85. https://doi.org/10.3390/agronomy12010085
Park B, Shin T-S, Cho J-S, Lim J-H, Park K-J. Characterizing Hyperspectral Microscope Imagery for Classification of Blueberry Firmness with Deep Learning Methods. Agronomy. 2022; 12(1):85. https://doi.org/10.3390/agronomy12010085
Chicago/Turabian StylePark, Bosoon, Tae-Sung Shin, Jeong-Seok Cho, Jeong-Ho Lim, and Ki-Jae Park. 2022. "Characterizing Hyperspectral Microscope Imagery for Classification of Blueberry Firmness with Deep Learning Methods" Agronomy 12, no. 1: 85. https://doi.org/10.3390/agronomy12010085







