Genetic Diversity of Flavescence Dorée Phytoplasmas in Vineyards of Serbia: From the Widespread Occurrence of Autochthonous Map-M51 to the Emergence of Endemic Map-FD2 (Vectotype II) and New Map-FD3 (Vectotype III) Epidemic Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapevine Samples
2.2. Clematis vitalba, Ailanthus altissima, and Alnus glutinosa Samples
2.3. DNA Extraction and Phytoplasma Identification
2.4. FDp Genotyping
3. Results
3.1. Diversity and Incidence of FDp Map Genotypes in Serbian Vineyards
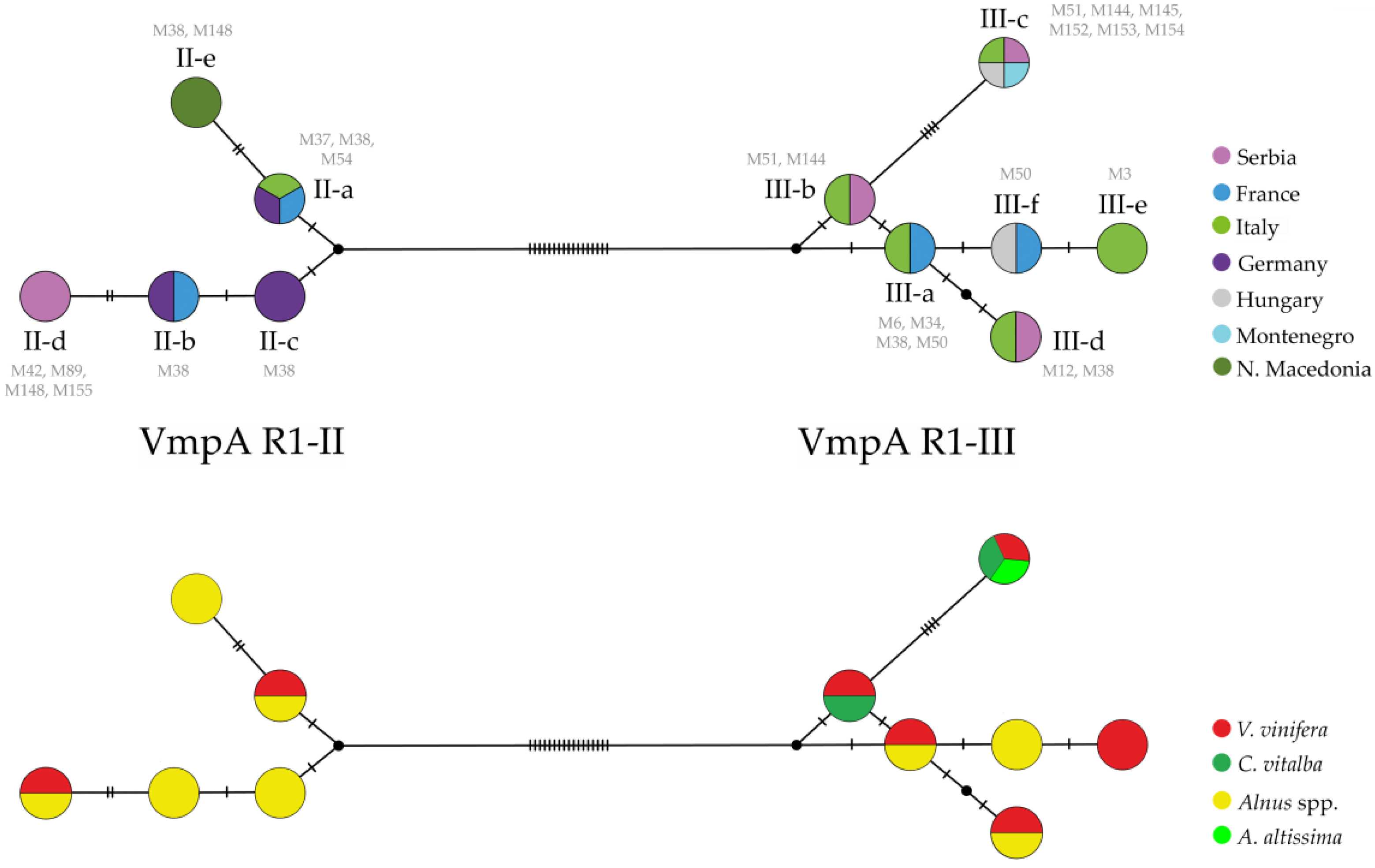
3.2. Diversity of FDp VmpA Genotypes in Serbian Vineyards
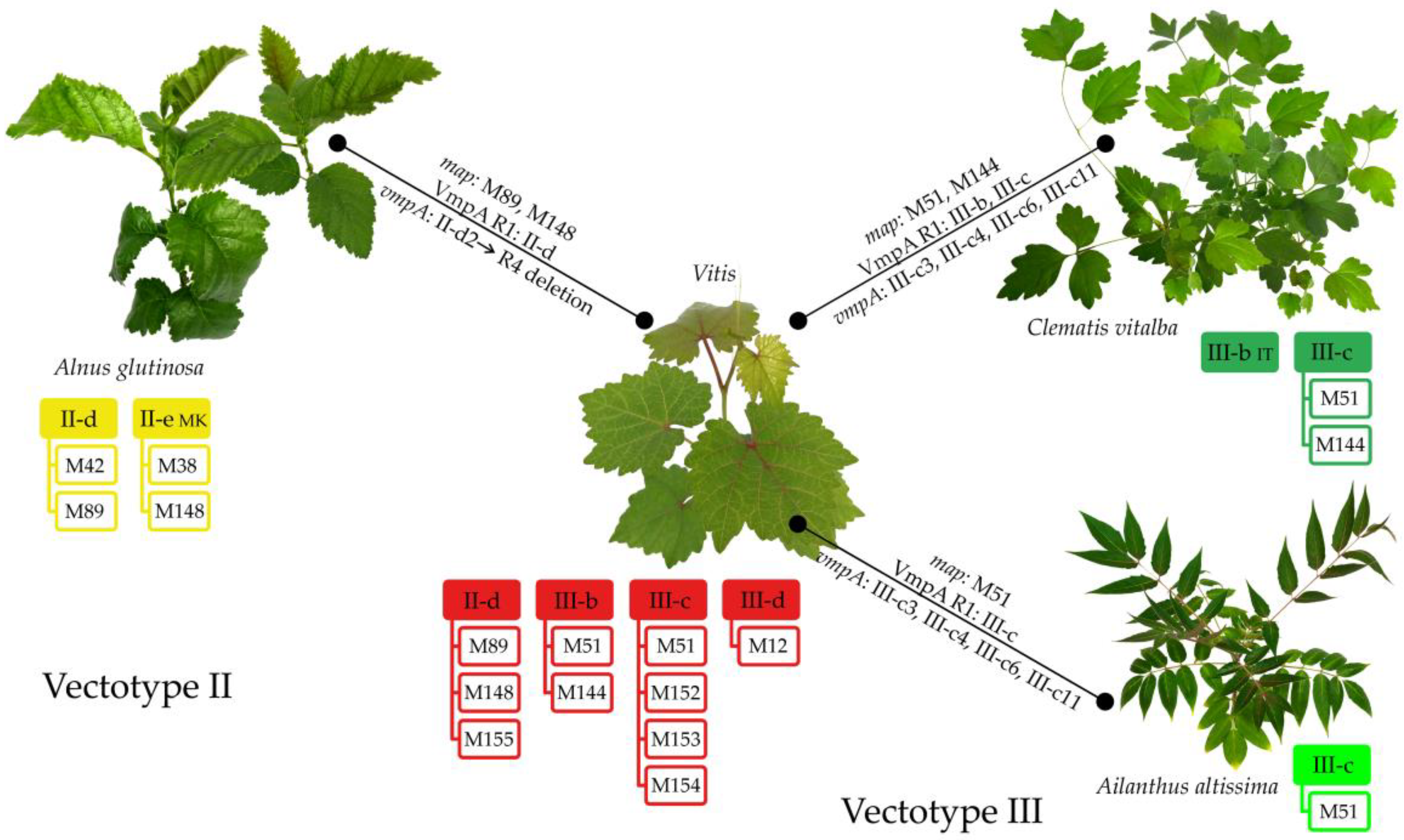
3.3. Ecological Components of FDp Epidemiological Cycle in Serbia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caudwell, A. Deux années d’étude sur la Flavescence dorée, nouvelle maladie grave de la vigne. Ann. Amélior. Plantes 1957, 4, 359–363. [Google Scholar]
- Constable, F.E. Phytoplasma epidemiology: Grapevines as a model. In Phytoplasmas: Genomes, Plant Hosts and Vectors; Weintraub, P.G., Jones, P., Eds.; CAB International: Wallingford, UK, 2010; pp. 188–212. [Google Scholar]
- Chuche, J.; Thiéry, D. Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef] [Green Version]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Jaques Miret, J.A.; MacLeod, A.; Navajas Navarro, M.; et al. Risk to plant health of Flavescence doree for the EU territory. EFSA J. 2016, 14, 4603. [Google Scholar]
- Tramontini, S.; Delbianco, A.; Vos, S. Pest survey card on Flavescence dorée phytoplasma and its vector Scaphoideus titanus. EFSA Support. Publ. 2020, 17, 1909E. [Google Scholar]
- Jarausch, B.; Biancu, S.; Kugler, S.; Wetzel, T.; Baumann, M.; Winterhagen, P.; Jarausch, W.; Kortekamp, A.; Maixner, M. First Report of Flavescence Dorée-Related Phytoplasma in a Productive Vineyard in Germany. Plant Dis. 2021, 105, 3285. [Google Scholar] [CrossRef]
- Arnaud, G.; Malembic-Maher, S.; Salar, P.; Bonnet, P.; Maixner, M.; Marcone, C.; Boudon-Padieu, E.; Foissac, X. Multilocus Sequence Typing Confirms the Close Genetic Interrelatedness of Three Distinct Flavescence dorée Phytoplasma Strain Clusters and Group 16SrV Phytoplasmas Infecting Grapevine and Alder in Europe. Appl. Environ. Microbiol. 2007, 73, 4001–4010. [Google Scholar] [CrossRef] [Green Version]
- Malembic-Maher, S.; Desqué, D.; Khalil, D.; Salar, P.; Bergey, B.; Danet, J.L.; Duret, S.; Dubrana-Ourabah, M.P.; Beven, L.; Ember, I.; et al. When a Palearctic bacterium meets a Nearctic insect vector: Genetic and ecological insights into the emergence of the grapevine Flavescence dorée epidemics in Europe. PLoS Pathog. 2020, 16, e1007967. [Google Scholar] [CrossRef] [Green Version]
- Casati, P.; Jermini, M.; Quaglino, F.; Corbani, G.; Schaerer, S.; Passera, A.; Bianco, P.A.; Rigamonti, I.E. New insights on Flavescence dorée phytoplasma ecology in the vineyard agro-ecosystem in southern Switzerland. Ann. Appl. Biol. 2017, 171, 37–51. [Google Scholar] [CrossRef]
- de Sousa, E.; Casati, P.; Cardoso, F.; Baltazar, C.; Durante, G.; Quaglino, F.; Bianco, P.A. Flavescence dorée phytoplasma affecting grapevine (Vitis vinifera) newly reported in Portugal. Plant Pathol. 2010, 59, 398. [Google Scholar] [CrossRef] [Green Version]
- Angelini, E.; Clair, D.; Borgo, M.; Bertaccini, A.; Boudon-Padieu, E. Flavescence dorée in France and Italy—Occurrence of closely related phytoplasma isolates and their near relationships to Palatinate grapevine yellows and an alder yellows phytoplasma. Vitis 2001, 40, 79–86. [Google Scholar]
- Martini, M.; Botti, S.; Marcone, C.; Marzachı, C.; Casati, P.; Bianco, P.A.; Benedetti, R.; Bertaccini, A. Genetic variability among Flavescence dorée phytoplasmas from different origins in Italy and France. Mol. Cell. Probes 2002, 16, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Pegoraro, M.; Ripamonti, M.; Abbà, S.; Beal, D.; Giraudo, A.; Veratti, F.; Malembic-Maher, S.; Salar, P.; Bosco, D.; et al. Genetic Diversity of Flavescence Dorée Phytoplasmas at the Vineyard Scale. Appl. Environ. Microbiol. 2019, 85, e03123-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriston, É.; Krizbai, L.; Juhász, E.; Melika, G. Status of grapevine “Flavescence dorée” in Hungary. Phytopathog. Mollicutes 2019, 9, 63–64. [Google Scholar] [CrossRef]
- Plavec, J.; Budinšćak, Ž.; Križanac, I.; Škorić, D.; Foissac, X.; Šeruga Musić, M. Multilocus sequence typing reveals the presence of three distinct Flavescence dorée phytoplasma genetic clusters in Croatian vineyards. Plant Pathol. 2019, 68, 18–30. [Google Scholar] [CrossRef]
- Mehle, N.; Rupar, M.; Seljak, G.; Ravnikar, M.; Dermastia, M. Molecular diversity of ‘Flavescence dorée’ phytoplasma strains in Slovenia. Bull. Insectology 2011, 64, S29–S30. [Google Scholar]
- Reisenzein, H.; Steffek, R. First outbreaks of grapevine ‘Flavescence dorée’ in Austrian viticulture. Bull. Insectology 2011, 64, S223–S224. [Google Scholar]
- Krnjajić, S.; Mitrović, M.; Cvrković, T.; Jović, J.; Petrović, A.; Forte, V.; Angelini, E.; Toševski, I. Occurrence and distribution of Scaphoideus titanus in multiple outbreaks of “flavescence dorée” in Serbia. Bull. Insectology 2007, 60, 197–198. [Google Scholar]
- Holz, S.; Duduk, B.; Büttner, C.; Kube, M. Genetic variability of Alder yellows phytoplasma in Alnus glutinosa in its natural Spreewald habitat. For. Pathol. 2016, 46, 11–21. [Google Scholar] [CrossRef]
- Krstić, O.; Cvrković, T.; Mitrović, M.; Radonjić, S.; Hrnčić, S.; Toševski, I.; Jović, J. Wolbachia infection in natural populations of Dictyophara europaea, an alternative vector of grapevine Flavescence dorée phytoplasma: Effects and interactions. Ann. Appl. Biol. 2018, 172, 47–64. [Google Scholar] [CrossRef]
- Angelini, E.; Squizzato, F.; Lucchetta, G.; Borgo, M. Detection of a Phytoplasma Associated with Grapevine Flavescence dorée in Clematis vitalba. Eur. J. Plant Pathol. 2004, 110, 193–201. [Google Scholar] [CrossRef]
- Filippin, L.; Jović, J.; Cvrković, T.; Forte, V.; Clair, D.; Toševski, I.; Boudon-Padieu, E.; Borgo, M.; Angelini, E. Molecular characteristics of phytoplasmas associated with Flavescence dorée in clematis and grapevine and preliminary results on the role of Dictyophara europaea as a vector. Plant Pathol. 2009, 58, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Valiunas, D.; Alminaite, A.; Staniulis, J.; Jomantiene, R.; Davis, R.E. First Report of Alder Yellows Phytoplasma in the Eastern Baltic Region. Plant Dis. 2001, 85, 1120. [Google Scholar] [CrossRef] [PubMed]
- Cvrković, T.; Jović, J.; Mitrović, M.; Petrović, A.; Krnjajić, S.; Malembic-Maher, S.; Toševski, I. First report of alder yellows phytoplasma on common alder (Alnus glutinosa) in Serbia. Plant Pathol. 2008, 57, 773. [Google Scholar] [CrossRef]
- Ember, I.; Acs, Z.; Salar, P.; Danet, J.L.; Foissac, X.; Koelber, M.; Malembic-Maher, S. Survey and genetic diversity of phytoplasmas from the 16SrV-C and -D subgroups in Hungary. Bull. Insectology 2011, 64, S33–S34. [Google Scholar]
- Radonjić, S.; Hrnčić, S.; Krstić, O.; Cvrković, T.; Mitrović, M.; Jović, J.; Toševski, I. First Report of Alder Yellows Phytoplasma Infecting Common and Grey Alder (Alnus glutinosa and A. incana) in Montenegro. Plant Dis. 2013, 97, 686. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, B.; Spasov, D.; Jakovljević, M.; Jović, J.; Krstić, O.; Mitrović, M.; Cvrković, T. First Report of Alder Yellows Phytoplasma Associated with Common Alder (Alnus glutinosa) in the Republic of Macedonia. Plant Dis. 2014, 98, 1268. [Google Scholar] [CrossRef] [PubMed]
- Jurga, M.; Zwolińska, A. Artemisia vulgaris, a new host of 16SrV-C phytoplasma related strains infecting black alder in Poland. J. Phytopathol. 2020, 168, 659–667. [Google Scholar] [CrossRef]
- Reisenzein, H.; Strauss, G. Sporadic outbreaks of “Flavescence dorée” in Austrian vineyards and the role of Phlogottetix cyclops as a potential vector. Phytopathog. Mollicutes 2019, 9, 61–62. [Google Scholar] [CrossRef]
- Seljak, G. Contribution to the knowledge of planthoppers and leafhoppers of Slovenia (Hemiptera: Auchenorrhyncha). Acta Entomol. Slov. 2004, 12, 189–216. [Google Scholar]
- Mifsud, D.; Cocquempot, C.; Mühlethaler, R.; Wilson, M.; Streito, J.-C. Other Hemiptera Sternorrhyncha (Aleyrodidae, Phylloxeroidea, and Psylloidea) and Hemiptera Auchenorrhyncha Chapter 9.4. BioRisk 2020, 4, 511–552. [Google Scholar] [CrossRef]
- EPPO. Orientus ishidae: A potential phytoplasma vector spreading in the EPPO region. EPPO Report. Serv. 2015, 5, 8–10. [Google Scholar]
- Klejdysz, T.; Zwolińska, A.; Walczak, M.; Kobiałka, M. The first record of a potential pest Orientus ishidae (Matsumura, 1902) (Hemiptera: Cicadellidae) in Poland. J. Plant Prot. Res. 2017, 57, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Chireceanu, C.; Teodoru, A.; Gutue, M.; Dumitru, M.; Anastasiu, P. Two new invasive hemipteran species first recorded in Romania: Orientus ishidae (Matsumura 1902) (Cicadellidae) and Acanalonia conica (Say 1830) (Acanaloniidae). J. Entomol. Zool. Stud. 2017, 5, 824–830. [Google Scholar]
- Gaffuri, F.; Sacchi, S.; Cavagna, B. First detection of the mosaic leafhopper, Orientus ishidae, in northern Italian vineyards infected by the flavescence dorée phytoplasma. New Dis. Rep. 2011, 24, 22. [Google Scholar] [CrossRef] [Green Version]
- Lessio, F.; Picciau, L.; Gonella, E.; Tota, F.; Mandrioli, M.; Alma, A. The mosaic leafhopper Orientus ishidae: Host plants, spatial distribution, infectivity, and transmission of 16SrV phytoplasmas to vines. Bull. Insectology 2016, 69, 277–289. [Google Scholar]
- Cvrković, T.; Jović, J.; Jakovljević, M.; Krstić, O.; Marinković, S.; Mitrović, M.; Toševski, I. The “code red” for Balkan vineyards: Occurrence of Orientus ishidae (Matsumura, 1902) (Hemiptera: Cicadellidae) in Serbia. BioInvasions Rec. 2021, 10, 544–554. [Google Scholar] [CrossRef]
- Filippin, L.; De Pra, V.; Zottini, M.; Borgo, M.; Angelini, E. Nucleotide sequencing of imp gene in phytoplasmas associated to ‘Flavescence dorée’ from Ailanthus altissima. Bull. Insectology 2011, 64, S49–S50. [Google Scholar]
- Miladinović, Z.; Radulović, M.; Hrnčić, S.; Delić, D. Genetic variability of Scaphoideus titanus populations and a new host plant of 16SrV group phytoplasmas in Bosnia and Herzegovina. Phytopathog. Mollicutes 2019, 9, 111–112. [Google Scholar] [CrossRef]
- Mehle, N.; Jakoš, N.; Mešl, M.; Miklavc, J.; Matko, B.; Rot, M.; Rus, A.F.; Brus, R.; Dermastia, M. Phytoplasmas associated with declining of hazelnut (Corylus avellana) in Slovenia. Eur. J. Plant Pathol. 2019, 155, 1117–1132. [Google Scholar] [CrossRef] [Green Version]
- Duduk, B.; Ivanović, M.; Dukić, N.; Botti, S.; Bertaccini, A. First Report of an Elm Yellows Subgroup 16SrV-C Phytoplasma Infecting Grapevine in Serbia. Plant Dis. 2003, 87, 599. [Google Scholar] [CrossRef]
- Magud, B.; Toševski, I. Scaphoideus titanus Ball. (Homoptera, Cicadellidae): A new pest in Serbia. Plant Dr. 2004, 32, 348–352. [Google Scholar]
- Filippin, L.; Jović, J.; Forte, V.; Cvrković, T.; Toševski, I.; Borgo, M.; Angelini, E. Occurrence and diversity of phytoplasmas detected in clematis and their relationships with grapevine” Flavescence dorée” phytoplasmas. Bull. Insectology 2007, 60, 327–328. [Google Scholar]
- Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Dictyophara europaea (Hemiptera: Fulgoromorpha: Dictyopharidae): Description of immatures, biology and host plant associations. Bull. Entomol. Res. 2016, 106, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ivanišević, D.; Jakšić, D.; Korać, N. Vineyard Atlas: Census of Agriculture 2012 in the Republic of Serbia; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2015; pp. 1–413.
- Statistical Office of the Republic of Serbia. Statistical Yearbook of the Republic of Serbia 2020; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2020; pp. 1–445. ISSN 0354-4206.
- Hren, M.; Boben, J.; Rotter, A.; Kralj, P.; Gruden, K.; Ravnikar, M. Real-time PCR detection systems for Flavescence dorée and Bois noir phytoplasmas in grapevine: Comparison with conventional PCR detection and application in diagnostics. Plant Pathol. 2007, 56, 785–796. [Google Scholar] [CrossRef]
- Pelletier, C.; Salar, P.; Gillet, J.; Cloquemin, G.; Very, P.; Foissac, X.; Malembic-Maher, S. Triplex real-time PCR assay for sensitive and simultaneous detection of grapevine phytoplasmas of the 16SrV and 16SrXII-A groups with an endogenous analytical control. Vitis 2009, 48, 87–95. [Google Scholar]
- Deng, S.; Hiruki, C. Amplification of 16S rRNA genes from culturable and nonculturable Mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Smart, C.D.; Schneider, B.; Blomquist, C.L.; Guerra, L.J.; Harrison, N.A.; Ahrens, U.; Lorenz, K.H.; Seemüller, E.; Kirkpatrick, B.C. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl. Environ. Microbiol. 1996, 62, 2988–2993. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Gundersen, D.E.; Hammond, R.W.; Davis, R.E. Use of Mycoplasmalike Organism (MLO) Group-Specific Oligonucleotide Primers for Nested-PCR Assays to Detect Mixed-MLO Infections in a Single Host Plant. Phytopathology 1994, 84, 559–566. [Google Scholar] [CrossRef]
- Gibb, K.S.; Padovan, A.C.; Mogen, B.D. Studies on Sweet Potato Little-Leaf Phytoplasma Detected in Sweet Potato and Other Plant Species Growing in Northern Australia. Phytopathology 1995, 85, 169–174. [Google Scholar] [CrossRef]
- Padovan, A.C.; Gibb, K.S.; Bertaccini, A.; Vibio, M.; Bonfiglioli, R.E.; Magarey, P.A.; Sears, B.B. Molecular detection of the Australian grapevine yellows phytoplasma and comparison with grapevine yellows phytoplasmas from Italy. Aust. J. Grape Wine Res. 1995, 1, 25–31. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jović, J.; Krstić, O.; Mitrović, M.; Marinković, S.; Toševski, I.; Cvrković, T. Diversity of phytoplasmas identified in the polyphagous leafhopper Euscelis incisus (Cicadellidae, Deltocephalinae) in Serbia: Pathogen inventory, epidemiological significance and vectoring potential. Eur. J. Plant Pathol. 2020, 156, 201–221. [Google Scholar] [CrossRef]
- Mittelberger, C.; Obkircher, L.; Oberkofler, V.; Ianeselli, A.; Kerschbamer, C.; Gallmetzer, A.; Reyes-Dominguez, Y.; Letschka, T.; Janik, K. Development of a universal endogenous qPCR control for eukaryotic DNA samples. Plant Methods 2020, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jović, J.; Cvrković, T.; Mitrović, M.; Petrović, A.; Krstić, O.; Krnjajić, S.; Toševski, I. Multigene sequence data and genetic diversity among ‘Candidatus Phytoplasma ulmi’ strains infecting Ulmus spp. in Serbia. Plant Pathol. 2011, 60, 356–368. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoli, A.; Belgeri, E.; Jermini, M.; Conedera, M.; Filippin, L.; Angelini, E. Alnus glutinosa and Orientus ishidae (Matsumura, 1902) share phytoplasma genotypes linked to the ‘Flavescence dorée’ epidemics. J. Appl. Entomol. 2021, 145, 1015–1028. [Google Scholar] [CrossRef]
- Posada, D.; Crandall, K.A. Selecting the Best-Fit Model of Nucleotide Substitution. Syst. Biol. 2001, 50, 580–601. [Google Scholar] [CrossRef]
- Cassens, I.; Mardulyn, P.; Milinkovitch, M.C. Evaluating Intraspecific “Network” Construction Methods Using Simulated Sequence Data: Do Existing Algorithms Outperform the Global Maximum Parsimony Approach? Syst. Biol. 2005, 54, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Kuzmanović, S.; Martini, M.; Ermacora, P.; Ferrini, F.; Starović, M.; Tošić, M.; Carraro, L.; Osler, R. Incidence and molecular characterization of Flavescence dorée and stolbur phytoplasmas in grapevine cultivars from different viticultural areas of Serbia. Vitis 2008, 47, 105–111. [Google Scholar]
- Tanasijević, N. Constant and temporary members of leafhopper fauna in alfalfa in Yugoslavia. Plant Prot. 1964, 80, 379–388. [Google Scholar]
- Tanasijević, N. A new contribution to the study of leafhopper fauna (Homoptera; Auchenorrhyncha). Plant Prot. 1966, 91–92, 205–212. [Google Scholar]
- Tanasijević, N. The significance of leafhoppers in agriculture and forestry. Agrohemija 1967, 1–2, 73–78. [Google Scholar]
- Cvrković, T.; Jović, J.; Mitrović, M.; Krstić, O.; Krnjajić, S.; Toševski, I. Potential new hemipteran vectors of stolbur phytoplasma in Serbian vineyards. Bull. Insectology 2011, 64, S129–S130. [Google Scholar]
- Malembic-Maher, S.; Salar, P.; Filippin, L.; Carle, P.; Angelini, E.; Foissac, X. Genetic diversity of European phytoplasmas of the 16SrV taxonomic group and proposal of ‘Candidatus Phytoplasma rubi’. Int. J. Syst. Evol. Microbiol. 2011, 61, 2129–2134. [Google Scholar] [CrossRef]
- Arricau-Bouvery, N.; Duret, S.; Dubrana, M.P.; Batailler, B.; Desqué, D.; Béven, L.; Danet, J.L.; Monticone, M.; Bosco, D.; Malembic-Maher, S.; et al. Variable Membrane Protein A of Flavescence Dorée Phytoplasma Binds the Midgut Perimicrovillar Membrane of Euscelidius variegatus and Promotes Adhesion to Its Epithelial Cells. Appl. Environ. Microbiol. 2018, 84, e02487-17. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Vallino, M.; Galetto, L.; Marzachì, C. Competitive exclusion of Flavescence dorée phytoplasma strains in Catharanthus roseus plants. Plants 2020, 9, 1594. [Google Scholar] [CrossRef]
- Belgeri, E.; Rizzoli, A.; Jermini, M.; Angelini, E.; Filippin, L.; Rigamonti, I.E. First report of Flavescence dorée phytoplasma identification and characterization in three species of leafhoppers. J. Plant Pathol. 2021. [Google Scholar] [CrossRef]




| Region | District | Collection Date | Number of Characterized FDp Isolates a | Number of Map Genotypes b | ||
|---|---|---|---|---|---|---|
| M12 | M51 | Single c | ||||
| North Serbia | 1. North Banat | August-2017, July-2018 | 20 | 19 | 1 | - |
| 2. North Bačka | July-2017, July-2018 | 20 | 16 | 4 | - | |
| 3. West Bačka | July-2018, July-2019 | 20 | 15 | 5 | - | |
| 4. South Bačka | August-2017, July-2018 | 10 | 1 | 9 | - | |
| 5. Central Banat | July-2018 | 5 | 3 | 2 | - | |
| 6. South Banat | July-2019 | 10 | - | 9 | M155 | |
| 7. Srem | August-2017, July-2018 | 25 | - | 24 | M153 | |
| Central Serbia | 8. Belgrade | August-2017, July-2018 | 15 | - | 14 | M154 |
| 9. Mačva | July-2018 | 3 | - | 2 | M148 | |
| 10. Kolubara | July-2018, July-2019 | 2 | - | 1 | M148 | |
| 11. Podunavlje | July-2018, July-2019 | 15 | - | 13 | M89, M152 | |
| 12. Šumadija | August-2017, July-2019 | 10 | - | 9 | M151 | |
| 13. Pomoravlje | August-2017, July-2019 | 10 | - | 9 | M150 | |
| East Serbia | 14. Braničevo | August-2017, July-2018 | 5 | - | 5 | - |
| 15. Bor | July-2019 | 3 | - | 3 | - | |
| 16. Zaječar | July-2018, July-2019 | 10 | - | 5 | M144 (5) | |
| 17. Pirot | July-2018, July-2019 | 25 | - | 25 | - | |
| South Serbia | 18. Rasina | August-2017, July-2018 | 25 | - | 25 | - |
| 19. Nišava | July-2017, July-2018 | 25 | - | 25 | - | |
| 20. Toplica | July-2019 | 5 | - | 5 | - | |
| 21. Jablanica | July-2018 | 5 | - | 5 | - | |
| 22. Pčinja | July-2018 | 2 | - | 2 | - | |
| Total | 270 | 54 | 202 | 14 | ||
| VmpA | Associated Map | Representative Isolate or Identity (Sequence Type) a | Host (No) b | |||
|---|---|---|---|---|---|---|
| Cluster—R1 Type | No of Repeated Domains | Identical Domains | Cluster | Genotype | ||
| Malembic-Maher et al. 2020 Plos Pathogens [8] | ||||||
| II-a | 4 | no | FD2 | M54 | FD92 | Vv |
| 4 | no | FD2 | M37 | AF-06-30-25 | Ald | |
| 4 | no | FD2 | M38 | FG-15-124 | Ald | |
| II-b | 4 | no | FD2 | M38 | AG-15-115 | Ald |
| II-c | 4 | no | FD2 | M38 | AG-09-22-7 | Ald |
| II-d | 5 | R2, R3 | FD2 | M42 | AS-AL21 | Ald |
| III-a | 5 | R3, R4 | FD1 | M50 | FD70 | Vv |
| 5 | R2, R4 | FD1 | M34 | VF-04-11-19 | Vv | |
| III-c | 5 | R2, R3 | FD3 | M51 | VS-Loza232 | Vv |
| III-c | 5 | R2, R3, R4 | FD3 | M51 | CH-B37 | Cl |
| III-d | 5 | no | FD3 | M12 | Vv-AO262 | Vv |
| III-e | 5 | R2, R3 | FD3 | M3 | VI04-C28 | Vv |
| III-f | 5 | R2, R3, R4 | FD1 | M50 | AH-B38 | Ald |
| This study | ||||||
| II-d | 4 | R2, R3 | FD2 | M89 | =AS-AL21, R4 deletion (II-d2) | Vv (1) |
| 4 | R2, R3 | FD2 | M155 | =AS-AL21, R4 deletion (II-d2) | Vv (1) | |
| 5 | R2, R4 | FD2 | M148 | 99.7% AS-AL21 (II-d3) | Vv (2) | |
| II-e | 5 | R2, R3, R4 | FD2 | M148 c | 99% AS-AL21 (II-e) | Ald (1) |
| 5 | R2, R3, R4 | FD2 | M38 c | 99% AS-AL21 (II-e) | Ald (1) | |
| III-b | 5 | R3, R4 | FD3 | M144 | 99.7% FD70 (III-b1) | Vv (5) |
| 5 | no | FD3 | M51 | 99.5% FD70 (III-b2) | Vv (1) | |
| III-c | 5 | no | FD3 | M51 | 99.3% VS-Loza232 (III-c1) | Vv (3) |
| 5 | no | FD3 | M51 | 99.3% VS-Loza232 (III-c2) | Vv (1) | |
| 5 | R3, R4 | FD3 | M51 | 99.5% VS-Loza232 (III-c3) | Vv (2), Cl (1), Aa (2) | |
| 5 | no | FD3 | M51 | 99.8% VS-Loza232 (III-c4) | Vv (1), Cl (1), Aa (2) | |
| 5 | no | FD3 | M51 | 99.8% VS-Loza232 (III-c5) | Vv (1) | |
| 5 | R2, R3, R4 | FD3 | M51 | =CH-B37 (III-c6) | Vv (2), Aa (1) | |
| 5 | R2, R4 | FD3 | M51 | 99.4% VS-Loza232 (III-c8) | Vv (1) | |
| 5 | no | FD3 | M51 | 99.7% VS-Loza232 (III-c9) | Vv (1) | |
| 5 | R2, R3 | FD3 | M51 | =VS-Loza232 (III-c11) | Vv (9), Cl (3), Aa (2) | |
| 5 | R2, R3, R4 | FD3 | M152 | =CH-B37 (III-c6) | Vv (1) | |
| 5 | R2, R3 | FD3 | M153 | =VS-Loza232 (III-c11) | Vv (1) | |
| 5 | no | FD3 | M154 | 99.3% VS-Loza232 (III-c1) | Vv (1) | |
| 5 | no | FD3 | M144 d | 99.6% VS-Loza232 (III-c12) | Cl (1) | |
| 5 | R2, R4 | FD3 | M145 d | 99.4% VS-Loza232 (III-c8) | Cl (1) | |
| III-d | 5 | no | FD3 | M12 | = v-AO262 (III-d1) | Vv (8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić, O.; Cvrković, T.; Marinković, S.; Jakovljević, M.; Mitrović, M.; Toševski, I.; Jović, J. Genetic Diversity of Flavescence Dorée Phytoplasmas in Vineyards of Serbia: From the Widespread Occurrence of Autochthonous Map-M51 to the Emergence of Endemic Map-FD2 (Vectotype II) and New Map-FD3 (Vectotype III) Epidemic Genotypes. Agronomy 2022, 12, 448. https://doi.org/10.3390/agronomy12020448
Krstić O, Cvrković T, Marinković S, Jakovljević M, Mitrović M, Toševski I, Jović J. Genetic Diversity of Flavescence Dorée Phytoplasmas in Vineyards of Serbia: From the Widespread Occurrence of Autochthonous Map-M51 to the Emergence of Endemic Map-FD2 (Vectotype II) and New Map-FD3 (Vectotype III) Epidemic Genotypes. Agronomy. 2022; 12(2):448. https://doi.org/10.3390/agronomy12020448
Chicago/Turabian StyleKrstić, Oliver, Tatjana Cvrković, Slavica Marinković, Miljana Jakovljević, Milana Mitrović, Ivo Toševski, and Jelena Jović. 2022. "Genetic Diversity of Flavescence Dorée Phytoplasmas in Vineyards of Serbia: From the Widespread Occurrence of Autochthonous Map-M51 to the Emergence of Endemic Map-FD2 (Vectotype II) and New Map-FD3 (Vectotype III) Epidemic Genotypes" Agronomy 12, no. 2: 448. https://doi.org/10.3390/agronomy12020448








