Endophytic Fusarium proliferatum Reprogrammed Phytohormone Production and Antioxidant System of Oryza sativa under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Samples
2.2. Isolation and Screening of Endophytic Fungi
2.3. Isolation and Estimation of Metabolite Production
2.4. Screening of IAA- and GAs-Producing Fungal Strains
2.5. Quantification of GAs and IAA in Culture Broth
2.6. Extraction and Quantification of ABA in Culture Broth
2.7. Molecular Identification of Fungal Endophyte
2.8. Assessment of Potent Fungal Endophytes in Alleviation of Drought Stress in Host
- Group 1: Sunflower + 1/2 strength of Hoagland’s solution + 0% PEG
- Group 2: Sunflower + 1/2 strength of Hoagland’s solution + endophytes + 0% PEG
- Group 3: Sunflower + 1/2 strength of Hoagland’s solution + endophytes + 8% PEG
- Group 4: Sunflower + 1/2 strength of Hoagland’s solution + 8% PEG + no endophytes
2.9. Phenolics, Flavonoids, Proline, Antioxidants, and Lipid Peroxidation
2.10. Light and Scanning Electron Microscopy
2.11. Statistical Analysis
3. Results
3.1. Effect of Fungal CF on Waito-C Rice Growth
3.2. Screening of Potential Fungal Endophytes for Phytohormones
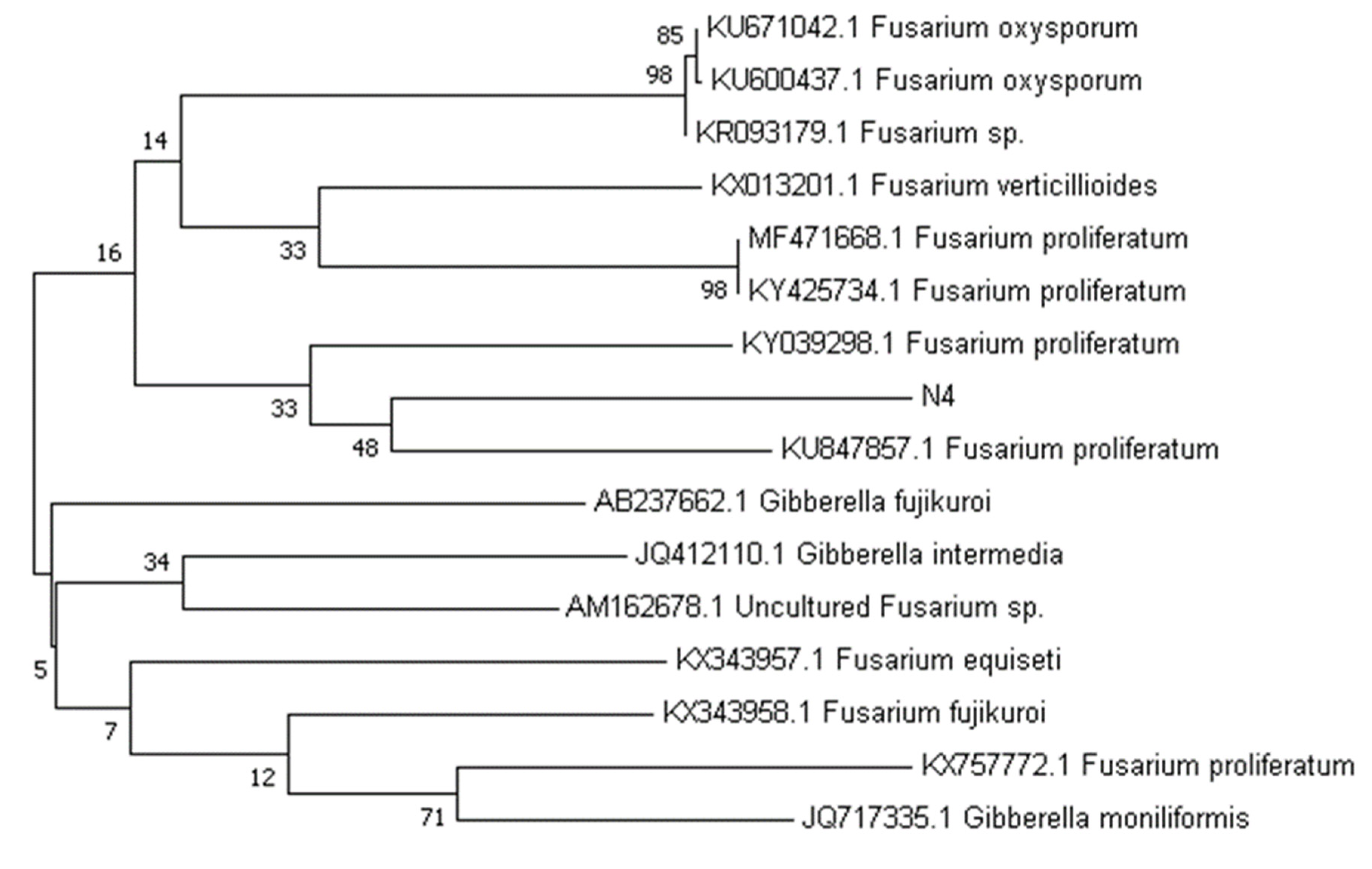
3.3. Identification and Phylogenetic Analysis of a Bioactive Endophyte
3.4. Effect of F. proliferatum Association on Sunflower Growth in Drought Stress
3.4.1. Fresh and Dry Weight
3.4.2. Chlorophyll Content (a, b)
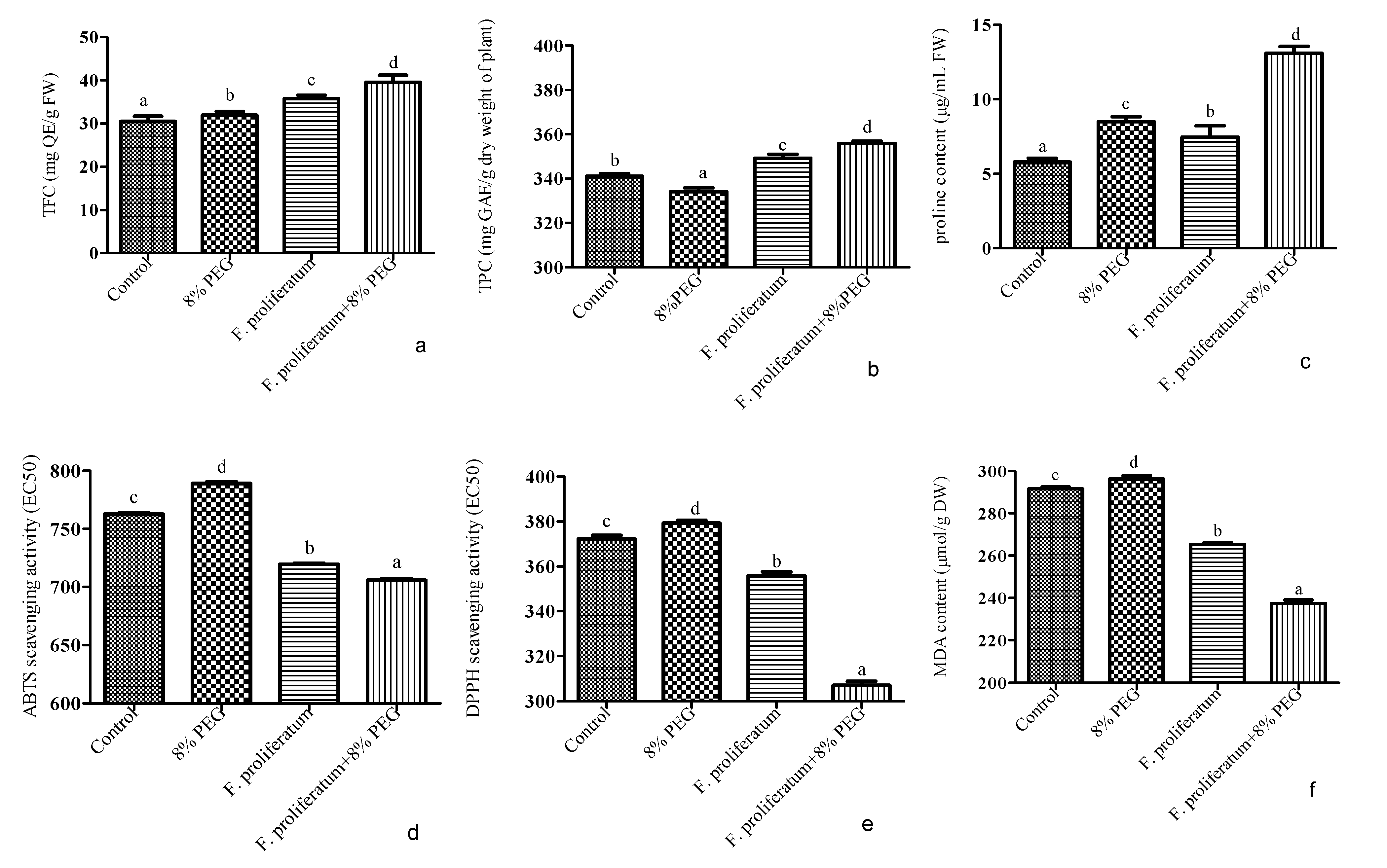
3.5. Phenolics, Flavonoids, Proline, Antioxidants, and Lipid Peroxidation
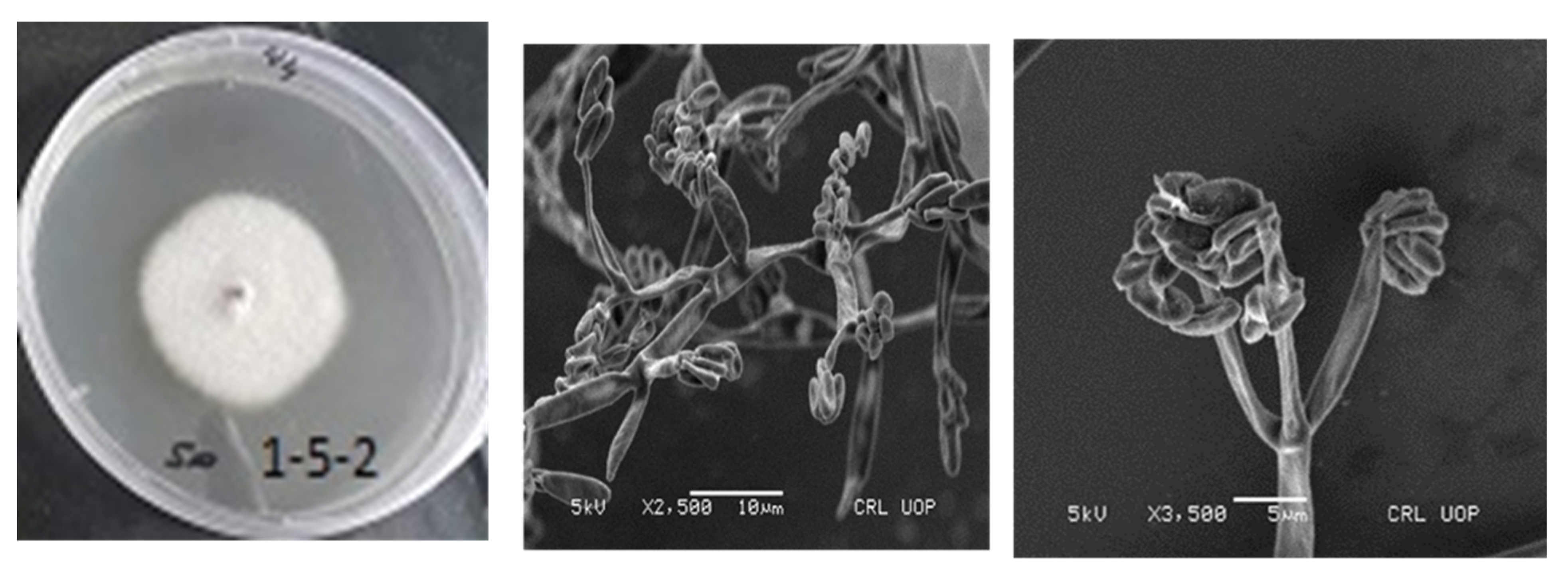
3.6. Light and Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Org.: Rome, Italy, 2018. [Google Scholar]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Muhammad, H.; Khan, S.A.; Amjad, I.; Anwar, H.; Lee, I. Cheap and rigorous method of screening soybean cultivars for drought tolerance. Fresenius Environ. Bull. 2019, 28, 8568–8575. [Google Scholar]
- Naz, F.; Hamayun, M.; Rauf, M.; Arif, M.; Khan, S.A.; Ud-Din, J.; Gul, H.; Hussain, A.; Iqbal, A.; Kim, H.-Y. Molecular mechanism of Cu metal and drought stress resistance triggered by Porostereum spadiceum AGH786 in Solanum lycopersicum L. Front. Plant Sci. 2022, 13, 1029836. [Google Scholar] [CrossRef] [PubMed]
- Siraj, S.; Khan, M.A.; Hamayun, M.; Ali, S.; Khan, S.A.; Hussain, A.; Iqbal, A.; Khan, H.; Kang, S.-M.; Lee, I.-J. Microbacterium oxydans regulates physio-hormonal and molecular attributes of Solanum lycopersicum under drought stress. Agronomy 2022, 12, 3224. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–53. [Google Scholar]
- Chakma, R.; Saekong, P.; Biswas, A.; Ullah, H.; Datta, A. Growth, fruit yield, quality, and water productivity of grape tomato as affected by seed priming and soil application of silicon under drought stress. Agric. Water Manag. 2021, 256, 107055. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Su, Y.; Qin, C.; Begum, N.; Ashraf, M.; Zhang, L.J.E. Acetylcholine ameliorates the adverse effects of cadmium stress through mediating growth, photosynthetic activity and subcellular distribution of cadmium in tobacco (Nicotiana benthamiana). Ecotoxicol. Environ. Saf. 2020, 198, 110671. [Google Scholar] [CrossRef]
- Nahakpam, S. Chlorophyll Stability: A Better Trait for Grain Yield in Rice under Drought. Indian J. Ecol. 2017, 44, 77. [Google Scholar]
- Čanak, P.; Jeromela, A.M.; Vujošević, B.; Kiprovski, B.; Mitrović, B.; Alberghini, B.; Facciolla, E.; Monti, A.; Zanetti, F.J.A. Is Drought Stress Tolerance Affected by Biotypes and Seed Size in the Emerging Oilseed Crop Camelina? Agronomy 2020, 10, 1856. [Google Scholar] [CrossRef]
- Wada, S.; Miyake, C.; Makino, A.; Suzuki, Y. Photorespiration coupled with CO2 assimilation protects photosystem I from photoinhibition under moderate poly (ethylene glycol)-induced osmotic stress in rice. Front. Plant Sci. 2020, 11, 1121. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, N.; Khan, M.N.; Qadir, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Rehman, K.U.; Lee, I.-J. Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) WT Aiton. Biocell 2021, 45, 363. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Shah, M.; Lee, I.J.; Iqbal, A.; Irshad, M.; Sayyed, A.; Ahmad, A.; Hamayun, M. Comparative assessment of chromate bioremediation potential of Pantoea conspicua and Aspergillus niger. J. Hazard. Mater. 2022, 424, 127314. [Google Scholar] [CrossRef]
- Ismail, I.; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Ahmad, A.; Gul, S.; Kim, H.-Y.; Lee, I.-J. Stemphylium solani stabilized the physicochemical characteristics of host plant species during stress. Pol. J. Environ. Stud. 2022, 31, 1125–1136. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Husssin, A.; Khan, S.A.; Shafique, M.; Arif, M.; Ahmad, A.; Rehman, G. Aspergillus violaceofuscus alleviates cadmium and chromium stress in okra through biochemical modulation. PLoS ONE 2022, 17, e0273908. [Google Scholar] [CrossRef]
- Khan, M.I.; Ali, N.; Jan, G.; Hamayun, M.; Jan, F.G.; Iqbal, A.; Hussain, A.; Lee, I.-J. Salt stress alleviation in Triticum aestivum through primary and secondary metabolites modulation by Aspergillus terreus BTK-1. Front. Plant Sci. 2022, 13, 167. [Google Scholar] [CrossRef]
- Gul, S.L.; Moon, Y.-S.; Hamayun, M.; Khan, S.A.; Iqbal, A.; Khan, M.A.; Hussain, A.; Shafique, M.; Kim, Y.-H.; Ali, S. Porostereum spadiceum-AGH786 regulates the growth and metabolites production in Triticum aestivum L. under salt stress. Curr. Microbiol. 2022, 79, 159. [Google Scholar] [CrossRef]
- Husna, H.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Asim, S.; Lee, I.-J. Stemphylium lycopersici and Stemphylium solani improved antioxidant system of soybean under chromate stress. Front. Microbiol. 2022, 13, 4314. [Google Scholar] [CrossRef]
- Asim, S.; Hussain, A.; Murad, W.; Hamayun, M.; Iqbal, A.; Rehman, H.; Tawab, A.; Irshad, M.; Alataway, A.; Dewidar, A.Z. Endophytic Fusarium oxysporum GW controlling weed and an effective biostimulant for wheat growth. Front. Plant Sci. 2022, 13, 2714. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Varma, A. Unravelling the role of endophytes in micronutrient uptake and enhanced crop productivity. In Symbiotic Soil Microorganisms; Springer: Berlin/Heidelberg, Germany, 2021; pp. 63–85. [Google Scholar]
- Rajput, M.; Vivekanand, V.; Pareek, N. Biofertilizers and Biopesticides: A Whole New Dimension for Ameliorating Soil Fertility and Organic Agriculture Practice. In Plant, Soil and Microbes in Tropical Ecosystems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 369–389. [Google Scholar]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Siddiqui, Z.S. Physiological performance of sunflower genotypes under combined salt and drought stress environment. Acta Bot. Croat. 2018, 77, 36–44. [Google Scholar] [CrossRef] [Green Version]
- UN. 2018 UN World Water Development Report, Nature-Based Solutions for Water; UNESCO Publishing: Paris, France, 2018. [Google Scholar]
- Howitt, R.; MacEwan, D.; Medellín-Azuara, J.; Lund, J.; Sumner, D. Economic Analysis of the 2015 Drought for California; Center for Watershed Sciences University of California, Davis: Davis, CA, USA, 2016. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Gond, S.K.; Kumar, A.; Kharwar, R.N.; Boulanger, L.-A.; Strobel, G.A. Endophytic fungal flora from roots and fruits of an Indian neem plant Azadirachta indica A. Juss., and impact of culture media on their isolation. Indian J. Microbiol. 2011, 51, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.L.; Shinwari, Z.K.; Kim, Y.-H.; Waqas, M.; Hamayun, M.; Kamran, M.; Lee, I.J. Role of endophyte Chaetomium globosum lk4 in growth of Capsicum annuum by production of gibberellins and indole acetic acid. Pak. J. Bot. 2012, 44, 1601–1607. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Ahmad, N.; Nawaz, Y.; Sher, H.; Lee, I.-J. Gibberellin producing Neosartorya sp. CC8 reprograms Chinese cabbage to higher growth. Sci. Hortic. 2011, 129, 347–352. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kang, K.; Lee, S.-H.; Lee, I.-J.; Paek, N.-C.J. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J. Exp. Bot. 2016, 67, 1677–1687. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.G. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Matić, P.; Sabljić, M.; Jakobek, L. Validation of spectrophotometric methods for the determination of total polyphenol and total flavonoid content. J. AOAC Int. 2017, 100, 1795–1803. [Google Scholar] [CrossRef]
- Bates, C.J. Proline and hydroxyproline excretion and vitamin C status in elderly human subjects. Clin. Sci. Mol. Med. 1977, 52, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Anwar, S.; Basir, A. DPPH free radical scavenging activity and phenotypic difference in hepatoprotective plant (Silybum marianum L.). Toxicol. Ind. Health 2013, 29, 460–467. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)/Trolox®-Equivalent Antioxidant Capacity) radical scavenging mixed-mode assay. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 117–139. [Google Scholar]
- Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T.J.M. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef] [Green Version]
- Salih, A.; Smith, D.; Price, J.; Dawson, L.J.P.S. Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult. Sci. 1987, 66, 1483–1488. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring cell death by trypan blue uptake and light microscopy. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Ketjarun, K.; Staples, G.W.; Swangpol, S.C.; Traiperm, P. Micro-morphological study of Evolvulus spp. (Convolvulaceae): The old world medicinal plants. Bot. Stud. 2016, 57, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Hamayun, M.; Khan, S.A.; Khan, A.L.; Lee, I.-J.; Shin, D.-H. Gibberellin-producing endophytic fungi isolated from Monochoria vaginalis. J. Microbiol. Biotechnol. 2010, 20, 1744–1749. [Google Scholar]
- Im Kim, J.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Yun, D.J. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant 2013, 6, 337–349. [Google Scholar] [CrossRef] [Green Version]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C.; Lee, H.S.; Li, H.; Kwak, S.S. Transgenic poplar expressing Arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol. Biochem. 2015, 94, 19–27. [Google Scholar] [CrossRef]
- Wolters, H.; Jürgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Akbar, F.; Shah, A. Role of potassium, zinc and gibberellic acid in increasing drought stress tolerance in sunflower (Helianthus annuus L.). Pak. J. Bot. 2019, 51, 809–815. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef] [PubMed]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Qadir, M.; Iqbal, A. Heavy metal tolerant endophytic fungi Aspergillus welwitschiae improves growth, ceasing metal uptake and strengthening antioxidant system in Glycine max L. Environ. Sci. Pollut. Res. 2021, 29, 15501–15515. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Husna; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.; Hamayun, M.; Sayyed, A.; Din, I.; Gul, H.; Hussain, A. Gibberellin and indole acetic acid production capacity of endophytic fungi isolated from Zea mays L. Int. J. Biosci. 2016, 8, 35–43. [Google Scholar]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.-D.; Mahgoub, H.A.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.J.C. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.-J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Khan, A.L.; Lee, I.-J. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J. Plant Interact. 2014, 9, 478–487. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef]
- Samaga, P.V. Screening of Endophytes from Nothapodytes Foetida and Hypericum Species for Bioactive Compounds. Ph.D. Thesis, University of Mysore, Mysore, India, 2014. [Google Scholar]
- Qadir, M.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Nadia. Enhancement of chromate phytoremediation and soil reclamation potential of Brassica campestris L. by Aspergillus niger. Environ. Sci. Pollut. Res. 2022, 30, 9471–9482. [Google Scholar] [CrossRef]
- Uzma, F.; Mohan, C.D.; Siddaiah, C.N.; Chowdappa, S. Endophytic fungi: Promising source of novel bioactive compounds. In Advances in Endophytic Fungal Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 243–265. [Google Scholar]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.; Škrbić, B.D.; Jigjiddorj, E.-A.; Vágvölgyi, C.J.M. Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial bioformulation-based plant biostimulants: A plausible approach toward next generation of sustainable agriculture. In Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–225. [Google Scholar]
- Seema, N.; Hamayun, M.; Ara, H.; Khan, R.S. Inoculation of Sunflower with Endophytic Fungi Alters Soil Physio-Chemical Properties to Nullify Drought Stress. Sarhad J. Agric. 2019, 35. [Google Scholar] [CrossRef]


| Fungal Isolates | Shoot Lengths (cm) | Root Lengths (cm) | Fresh Weights (g/Seedling) | Dry Weights (g/Seedlings) | CC (SPAD) | Growth Status |
|---|---|---|---|---|---|---|
| Control | 5.92 ± 2.27 | 6.67 ± 2.98 | 0.07 ± 0.02 | 0.004 ± 0.003 | 35.60 ± 13.15 | |
| CZPK | 6.50 ± 0.45 | 6.58 ± 1.68 | 0.06 ± 0.01 | 0.006 ± 0.003 | 34.70 ± 9.09 | |
| 1-1-1 | 7.50 ± 0.71 | 6.00 ± 1.05 | 0.08 ± 0.03 | 0.010 ± 0.003 | 35.10 ± 9.23 | Promoted |
| 1-1-2 | 7.42 ± 1.53 | 7.00 ± 2.60 | 0.08 ± 0.02 | 0.012 ± 0.003 | 32.36 ± 4.20 | Promoted |
| 1-1-3 | 8.25 ± 1.60 | 8.25 ± 3.19 | 0.11 ± 0.02 | 0.011 ± 0.003 | 32.23 ± 7.54 | Promoted |
| 1-1-4 | 7.92 ± 0.86 | 5.83 ± 2.40 | 0.12 ± 0.02 | 0.010 ± 0.003 | 24.83 ± 1.09 | Promoted |
| 1-2-2 | 7.50 ± 0.84 | 7.67 ± 2.25 | 0.05 ± 0.04 | 0.011 ± 0.003 | 27.13 ± 4.17 | Promoted |
| 1-2-3 | 7.50 ± 1.22 | 8.17 ± 2.46 | 0.08 ± 0.03 | 0.010 ± 0.003 | 26.30 ± 3.33 | Promoted |
| 1-2-4 | 7.58 ± 0.80 | 5.75 ± 1.92 | 0.11 ± 0.03 | 0.010 ± 0.003 | 30.33 ± 10.88 | Promoted |
| 1-2-5 | 7.92 ± 1.32 | 7.33 ± 1.13 | 0.12 ± 0.02 | 0.011 ± 0.003 | 28.23 ± 3.76 | Promoted |
| 1-3-1 | 7.90 ± 1.14 | 7.67 ± 1.66 | 0.11 ± 0.03 | 0.011 ± 0.003 | 43.73 ± 2.11 | Promoted |
| 1-3-2 | 7.67 ± 0.41 | 6.42 ± 2.54 | 0.08 ± 0.02 | 0.011 ± 0.003 | 43.90 ± 2.22 | Promoted |
| 1-3-3 | 8.50 ± 1.00 | 7.25 ± 1.04 | 0.07 ± 0.01 | 0.011 ± 0.003 | 42.07 ± 1.19 | Promoted |
| 1-4-2 | 7.58 ± 1.07 | 7.75 ± 3.25 | 0.06 ± 0.01 | 0.011 ± 0.003 | 42.70 ± 3.48 | Promoted |
| 1-4-6 | 7.50 ± 0.84 | 7.67 ± 2.25 | 0.05 ± 0.04 | 0.011 ± 0.003 | 53.26 ± 5.49 | Promoted |
| 1-5-2 | 8.33 ± 0.75 | 8.00 ± 3.03 | 0.05 ± 0.01 | 0.012 ± 0.003 | 57.20 ± 5.05 | Promoted |
| 1-5-3 | 7.83 ± 0.52 | 6.42 ± 1.36 | 0.08 ± 0.02 | 0.011 ± 0.003 | 31.40 ± 9.28 | Promoted |
| 2-1-1 | 8.42 ± 0.80 | 8.42 ± 2.28 | 0.07 ± 0.03 | 0.012 ± 0.003 | 34.63 ± 6.40 | Promoted |
| 2-1-2 | 8.42 ± 0.97 | 9.33 ± 2.44 | 0.04 ± 0.02 | 0.011 ± 0.003 | 44.10 ± 3.19 | Promoted |
| 2-1-3 | 8.42 ± 0.58 | 8.33 ± 0.82 | 0.07 ± 0.02 | 0.012 ± 0.003 | 45.76 ± 4.94 | Promoted |
| 2-2-1 | 7.50 ± 1.22 | 8.17 ± 2.46 | 0.08 ± 0.03 | 0.010 ± 0.003 | 26.07 ± 3.09 | Promoted |
| 2-2-2 | 7.58 ± 1.07 | 7.75 ± 3.25 | 0.06 ± 0.01 | 0.011 ± 0.003 | 30.50 ± 2.18 | Promoted |
| 3-1-1 | 7.67 ± 0.75 | 7.67 ± 2.80 | 0.06 ± 0.01 | 0.011 ± 0.003 | 29.06 ± 6.38 | Promoted |
| 3-1-2 | 8.00 ± 0.84 | 8.08 ± 2.41 | 0.13 ± 0.03 | 0.011 ± 0.003 | 30.50 ± 6.87 | Promoted |
| 3-1-3 | 8.50 ± 1.14 | 6.83 ± 1.91 | 0.12 ± 0.02 | 0.011 ± 0.003 | 33.90 ± 10.79 | Promoted |
| 3-2-2 | 8.08 ± 1.39 | 6.67 ± 2.66 | 0.09 ± 0.04 | 0.011 ± 0.003 | 31.10 ± 6.09 | Promoted |
| 3-3-2 | 7.67 ± 1.03 | 6.08 ± 2.13 | 0.14 ± 0.02 | 0.011 ± 0.003 | 27.93 ± 4.08 | Promoted |
| 3-4-1 | 7.92 ± 0.58 | 5.50 ± 1.30 | 0.14 ± 0.03 | 0.011 ± 0.003 | 46.93 ± 0.98 | Promoted |
| 3-5-2 | 8.00 ± 0.63 | 7.33 ± 2.42 | 0.04 ± 0.01 | 0.011 ± 0.003 | 46.03 ± 2.02 | Promoted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seema, N.; Hamayun, M.; Hussain, A.; Shah, M.; Irshad, M.; Qadir, M.; Iqbal, A.; Alrefaei, A.F.; Ali, S. Endophytic Fusarium proliferatum Reprogrammed Phytohormone Production and Antioxidant System of Oryza sativa under Drought Stress. Agronomy 2023, 13, 873. https://doi.org/10.3390/agronomy13030873
Seema N, Hamayun M, Hussain A, Shah M, Irshad M, Qadir M, Iqbal A, Alrefaei AF, Ali S. Endophytic Fusarium proliferatum Reprogrammed Phytohormone Production and Antioxidant System of Oryza sativa under Drought Stress. Agronomy. 2023; 13(3):873. https://doi.org/10.3390/agronomy13030873
Chicago/Turabian StyleSeema, Nighat, Muhammad Hamayun, Anwar Hussain, Mohib Shah, Muhammad Irshad, Muhammad Qadir, Amjad Iqbal, Abdulwahed Fahad Alrefaei, and Sajid Ali. 2023. "Endophytic Fusarium proliferatum Reprogrammed Phytohormone Production and Antioxidant System of Oryza sativa under Drought Stress" Agronomy 13, no. 3: 873. https://doi.org/10.3390/agronomy13030873








