Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields
Abstract
:1. Introduction
2. Materials and Methods
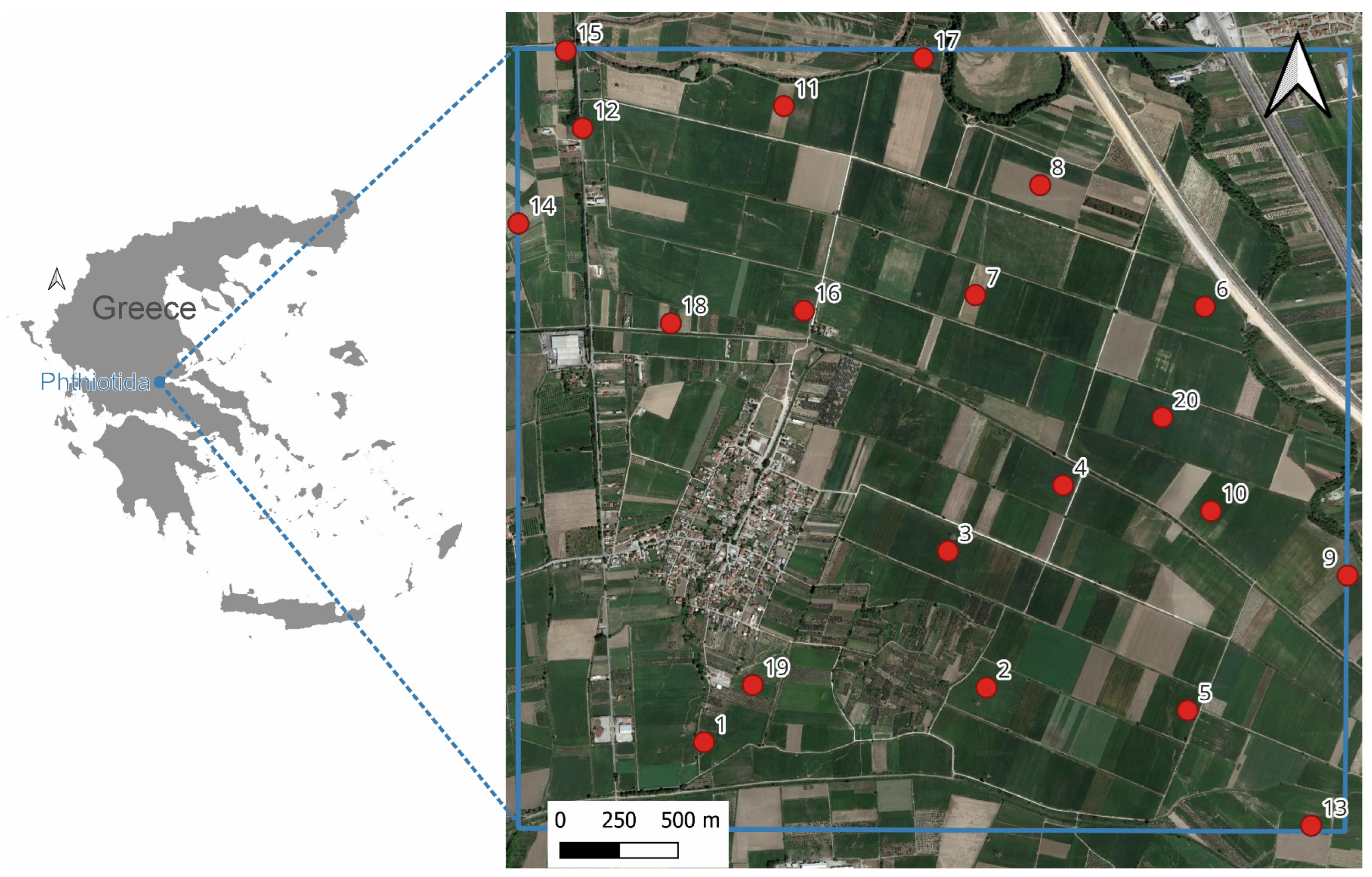
2.1. Site of Experimentation
2.2. Traps and Pheromones
2.3. Experimental Design
2.4. Statistical Analysis
3. Results
3.1. Trap Comparison
3.2. Population Fluctuations per Field
3.3. Detection Sensitivity and Correlation
3.4. Spatio-Temporal Distribution of H. armigera
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–53. [Google Scholar] [CrossRef]
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Fitt, G.P. Cotton pest management: Part 3. An Australian perspective. Annu. Rev. Entomol. 1994, 39, 543–562. [Google Scholar] [CrossRef]
- Achaleke, J.; Brévault, T. Inheritance and stability of pyrethroid resistance in the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) in Central Africa. Pest Manag. Sci. 2009, 66, 137–141. [Google Scholar]
- Aheer, G.M.; Aziz, M.A.; Hameed, A.; Ali, A. Evaluation of resistance to different insecticides in field strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in Punjab, Pakistan. Entomol. Res. 2009, 39, 159–167. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, W.; Zhao, J.; Jin, L.; Yang, Y.; Wu, S.; Tabashnik, B.E.; Wu, Y. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS ONE 2011, 6, e22874. [Google Scholar] [CrossRef]
- Abbade-Neto, D.; Amado, D.; Pereira, R.M.; Basso, M.; Spineli-Silva, S.; Gonçalves, T.M.; Corrêa, A.S.; Omoto, C. First report of Helicoverpa armigera (Lepidoptera: Noctuidae) resistance to flubendiamide in Brazil: Genetic basis and mechanisms of the resistance. Agronomy 2022, 12, 1664. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Ota, N.; Hutchison, W.D.; Beddow, J.; Walsh, T.; Tay, W.T.; Borchert, D.M.; Paula-Moreas, S.V.; Czepak, C.; Zalucki, M.P. The potential distribution of invading Helicoverpa armigera in North America: Is it just a matter of time? PLoS ONE 2015, 10, e0119618. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Salcedo, C.; Fang, Y.-L.; Zhang, R.-J.; Zhang, Z.-N. An overlooked component: (Z)-9-tetradecenal as a sex pheromone in Helicoverpa armigera. J. Insect Physiol. 2012, 58, 1209–1216. [Google Scholar] [CrossRef]
- Nyambo, B.T. Assessment of pheromone traps for monitoring and early warning of Heliothis armigera Hübner (Lepidoptera, Noctuidae) in the western cotton-growing areas of Tanzania. Crop Prot. 1989, 8, 188–192. [Google Scholar] [CrossRef]
- Srivastava, C.P.; Srivastava, R.P. Monitoring of Helicoverpa armigera (Hbn.) by pheromone trapping in chickpea (Cicer arietinum L.). J. Appl. Entomol. 1995, 119, 607–609. [Google Scholar] [CrossRef]
- Baker, G.H.; Tann, C.R.; Fitt, G.P. A tale of two trapping methods: Helicoverpa spp. (Lepidoptera, Noctuidae) in pheromone and light traps in Australian cotton production systems. Bull. Entomol. Res. 2011, 101, 9–23. [Google Scholar] [CrossRef]
- Fite, T.; Damte, T.; Tefera, T.; Negeri, M. Evaluation of commercial trap types and lures on the population dynamics of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) and its effects on non-targets insects. Cogent Food Agric. 2020, 6, 1771116. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Gravanis, F.T.; Koukounitsas, N.A.; Roussou, D.E. Influence of trap type, pheromone quantity and trapping location on capture of the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae). Appl. Entomol. Zool. 2002, 37, 385–391. [Google Scholar] [CrossRef]
- Karakasis, A.; Lampiri, E.; Rumbos, C.I.; Athanassiou, C.G. Factors affecting adult captures of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in pheromone-baited traps. Agronomy 2021, 11, 2539. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Mazomenos, B.E. Effect of trap type, trap color, trapping location, and pheromone dispenser on captures of male Palpita unionalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2004, 97, 321–329. [Google Scholar] [CrossRef]
- Parlak, S.; Özçankaya, İ.M.; Batur, M.; Akkaş, M.E.; Boza, Z.; Toprak, Ö. Efficiency of funnel traps in controlling pine processionary moth. J. Plant Dis. Prot. 2018, 125, 539–548. [Google Scholar] [CrossRef]
- Trematerra, P.; Colacci, M.; Athanassiou, C.G.; Kavallieratos, N.G.; Rumbos, C.I.; Boukouvala, M.C.; Nikolaidou, A.J.; Kontodimas, D.C.; Benavent-Fernández, E.; Gálvez-Settier, S. Evaluation of mating disruption for the control of Thaumetopoea pityocampa (Lepidoptera: Thaumetopoeidae) in suburban recreational areas in Italy and Greece. J. Econ. Entomol. 2019, 112, 2229–2235. [Google Scholar] [CrossRef]
- Cardim Ferreira Lima, M.; Damascena de Almeida Leandro, M.E.; Valero, C.; Pereira Coronel, L.C.; Gonçalves Bazzo, C.O. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
- Moral García, F.J. Analysis of the spatio–temporal distribution of Helicoverpa armigera Hb. in a tomato field using a stochastic approach. Biosyst. Eng. 2006, 93, 253–259. [Google Scholar] [CrossRef]
- Milonas, P.; Gogou, C.; Papadopoulou, A.; Fountas, S.; Liakos, V.; Papadopoulos, N.T. Spatio-temporal distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) and Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in a cotton production area. Neotrop. Entomol. 2016, 45, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Damte, T.; Ojiewo, C.O. Incidence and within field dispersion pattern of pod borer, Helicoverpa armigera (Lepidoptera: Noctuidae) in chickpea in Ethiopia. Arch. Phytopathol. Plant Prot. 2017, 50, 868–884. [Google Scholar] [CrossRef]
- Seethalam, M.; Bapatla, K.G.; Kumar, M.; Nisa, S.; Chandra, P.; Mathyam, P.; Sengottaiyan, V. Characterization of Helicoverpa armigera spatial distribution in pigeonpea crop using geostatistical methods. Pest Manag. Sci. 2021, 77, 4942–4950. [Google Scholar] [CrossRef]
- Zar, H.J. Biostatistical Analysis; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Zalucki, M.P.; Daglish, G.; Firempong, S.; Twine, P. The biology and ecology of Helicoverpa armigera (Hübner) and H. punctigera Wallengren (Lepidoptera: Noctuidae) in Australia: What do we know? Aust. J. Zool. 1986, 34, 779–814. [Google Scholar] [CrossRef]
- Guo, Y.Y. Progress in the researches on migration regularity of Helicoverpa armigera and relationships between the pest and its host plants. Acta Entomol. Sin. 1997, 40, 1–6. [Google Scholar]
- Czepak, C.; Albernaz, K.C. First reported occurrence of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil. Agric. Res. Trop. 2013, 43, 110–113. [Google Scholar]
- Murúa, M.G.; Scalora, F.S.; Navarro, F.R.; Cazado, L.E.; Casmuz, A.; Villagrán, M.E.; Lobos, E.; Gastaminza, G. First record of Helicoverpa armigera (Lepidoptera: Noctuidae) in Argentina. Fla Entomol. 2014, 97, 854–856. [Google Scholar] [CrossRef]
- Gilligan, T.M.; Goldstein, P.Z.; Timm, A.E.; Farris, R.; Ledezma, L.; Cunningham, A.P. Identification of Heliothine (Lepidoptera: Noctuidae) larvae intercepted at U.S. ports of entry from the new world. J. Econ. Entomol. 2019, 112, 603–615. [Google Scholar] [CrossRef]
- Jones, C.M.; Parry, H.; Tay, W.T.; Reynolds, D.R.; Chapman, J.W. Movement ecology of pest Helicoverpa: Implications for ongoing spread. Annu. Rev. Entomol. 2019, 64, 277–295. [Google Scholar] [CrossRef]
- Buchelos, C.T.; Papadopoulou, S.C. Evaluation of the effectiveness of a new pheromonic trap for monitoring Lasioderma serricorne (F.) in tobacco stores. Anz. Schädlingskd. J. Pest Sci. 1999, 72, 92–94. [Google Scholar] [CrossRef]
- Röth, F.; Galli, Z.; Tóth, M.; Fail, J.; Jenser, G. The hypothesized visual system of Thrips tabaci Lindeman and Frankliniella occidentalis (Pergande) based on different coloured traps’ catches. North West. J. Zool. 2016, 12, 40–49. [Google Scholar]
- Campbell, J.F.; Dowdy, A.K.; Mullen, M.A. Monitoring stored-product pests in food processing plants with pheromone trapping, contour mapping, and mark-recapture. J. Econ. Entomol. 2002, 95, 1089–1101. [Google Scholar] [CrossRef]
- Chu, C.C.; Henneberry, T.J. Pink bollworm seasonal distribution, yearly variation, and male moth trap catch relationships to population increases in cotton. Southwest. Entomol. 1990, 15, 273–280. [Google Scholar]
- Lu, Z.Z.; Baker, G. Spatial and temporal dynamics of Helicoverpa armigera (Lepidoptera, Noctuidae) in contrasting agricultural landscapes in northwestern China. Int. J. Pest Manag. 2013, 59, 25–34. [Google Scholar] [CrossRef]





| Degrees of Freedom | F | p | |
|---|---|---|---|
| Whole model | 269 | 2.4 | <0.001 |
| Intercept | 1 | 263.5 | <0.001 |
| Trap type | 2 | 34.0 | <0.001 |
| Trap check-date | 17 | 7.9 | <0.001 |
| Cotton hybrid | 4 | 3.5 | 0.008 |
| Trap type × Trap check-date | 34 | 1.6 | 0.015 |
| Trap type × Cotton hybrid | 8 | 0.2 | 0.993 |
| Trap check-date × Cotton hybrid | 68 | 1.4 | 0.020 |
| Trap type × Trap check-date × Cotton hybrid | 136 | 0.3 | 1.0 |
| Date | Trap Type | ||
|---|---|---|---|
| Striped | Green | Colored | |
| 17 July 2015 | 1.3 ± 0.3 Acd | 0.4 ± 0.2 Bc | 0.8 ± 0.2 ABc |
| 20 July 2015 | 1.7 ± 0.6 cd | 0.6 ± 0.2 c | 0.6 ± 0.4 c |
| 23 July 2015 | 1.5 ± 0.5 cd | 0.4 ± 0.2 c | 0.7 ± 0.4 c |
| 27 July 2015 | 1.8 ± 0.5 cd | 0.7 ± 0.2 bc | 0.7 ± 0.2 c |
| 1 August 2015 | 4.1 ± 0.5 Acd | 1.7 ± 0.5 Babc | 1.8 ± 0.3 Bbc |
| 4 August 2015 | 5.7 ± 1.5 Abcd | 1.6 ± 0.4 Babc | 1.8 ± 0.4 Bbc |
| 9 August 2015 | 4.0 ± 1.0 cd | 1.9 ± 0.4 abc | 1.8 ± 0.4 bc |
| 12 August 2015 | 13.6 ± 2.6 Aa | 2.4 ± 0.7 Bab | 5.4 ± 1.9 Bab |
| 17 August 2015 | 6.5 ± 0.8 Abc | 1.5 ± 0.3 Babc | 3.5 ± 0.7 Babc |
| 20 August 2015 | 3.1 ± 0.7 Acd | 0.8 ± 0.3 Bbc | 1.6 ± 0.4 ABbc |
| 24 August 2015 | 1.7 ± 0.3 Acd | 0.8 ± 0.2 Bbc | 1.0 ± 0.2 ABc |
| 28 August 2015 | 3.5 ± 0.7 Acd | 1.4 ± 0.3 Babc | 1.2 ± 0.3 Bc |
| 1 September 2015 | 2.9 ± 0.5 Acd | 1.0 ± 0.3 Bbc | 2.0 ± 0.6 ABbc |
| 4 September 2015 | 3.8 ± 0.7 Acd | 1.5 ± 0.2 Babc | 1.7 ± 0.3 Bbc |
| 8 September 2015 | 1.0 ± 0.2 d | 0.8 ± 0.2 bc | 1.2 ± 0.3 c |
| 12 September 2015 | 9.5 ± 2.3 Aab | 3.0 ± 0.5 Ba | 7.3 ± 2.1 ABa |
| 17 September 2015 | 5.8 ± 0.9 Abcd | 1.5 ± 0.3 Babc | 3.3 ± 0.6 Bbc |
| 20 September 2015 | 4.1 ± 0.5 Acd | 1.3 ± 0.3 Babc | 2.3 ± 0.5 Bbc |
| Trap Type | Trap Type | |||
|---|---|---|---|---|
| Striped | Green | Colored | Total | |
| 0 | 66 (18.3%) * | 153 (42.5%) | 129 (35.8%) | 348 (32.2%) |
| 1 | 56 (15.5%) | 86 (23.9%) | 75 (20.8%) | 217 (20.1%) |
| 2–9 | 194 (53.9%) | 119 (33.1%) | 146 (40.6%) | 459 (42.5%) |
| 10–49 | 44 (12.2%) | 2 (0.01%) | 10 (0.03%) | 56 (5.2%) |
| Trap Type | Striped | Green | Colored |
|---|---|---|---|
| Striped | - | 0.385 * | 0.643 * |
| Green | 0.385 * | - | 0.406 * |
| Colored | 0.643 * | 0.406 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakantza, E.; Rumbos, C.I.; Cavalaris, C.; Athanassiou, C.G. Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields. Agronomy 2023, 13, 1256. https://doi.org/10.3390/agronomy13051256
Karakantza E, Rumbos CI, Cavalaris C, Athanassiou CG. Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields. Agronomy. 2023; 13(5):1256. https://doi.org/10.3390/agronomy13051256
Chicago/Turabian StyleKarakantza, Elina, Christos I. Rumbos, Chris Cavalaris, and Christos G. Athanassiou. 2023. "Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields" Agronomy 13, no. 5: 1256. https://doi.org/10.3390/agronomy13051256









