Changes in the Soil Phosphorus Supply with Rice Straw Return in Cold Region
Abstract
:1. Introduction
2. Materials and Methods
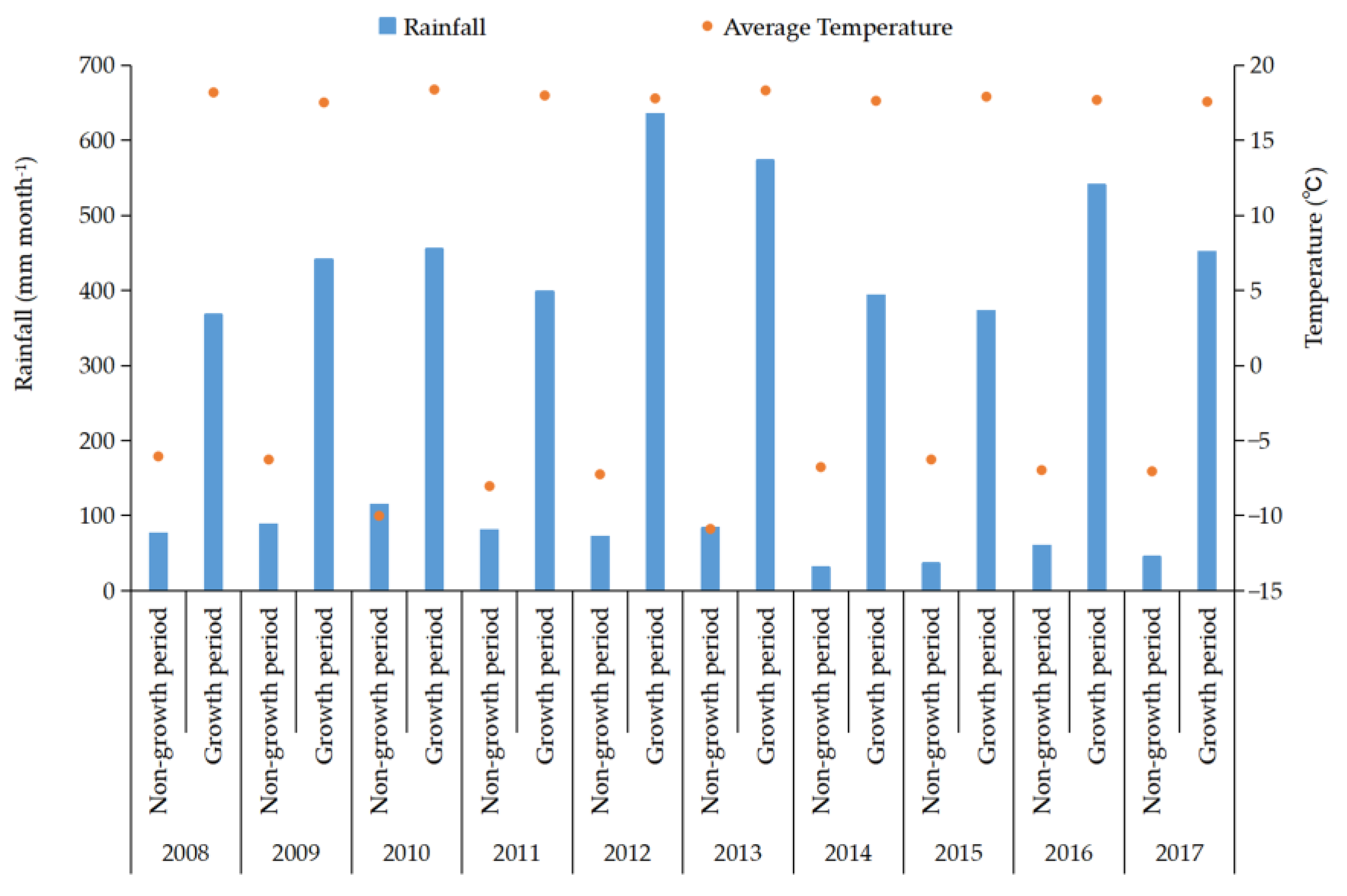
2.1. Overview of the Experimental Site
2.2. Experimental Design
2.3. Plant and Soil Sample Collection
2.4. Measurement Indicators and Methods
2.5. P Uptake and P Budget
2.6. Data Processing and Analysis
3. Results
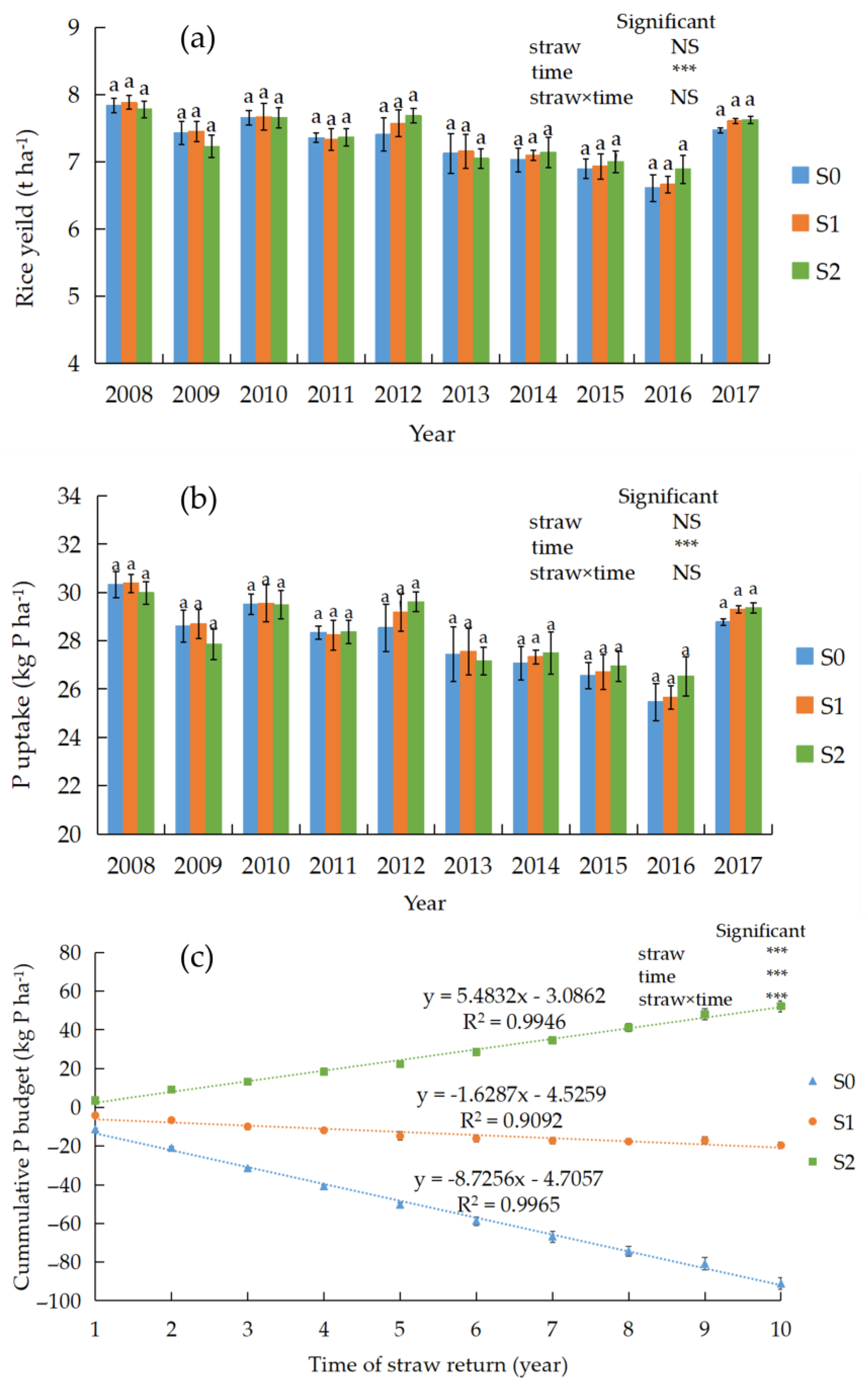
3.1. Grain Yield, P Uptake, and P Budget
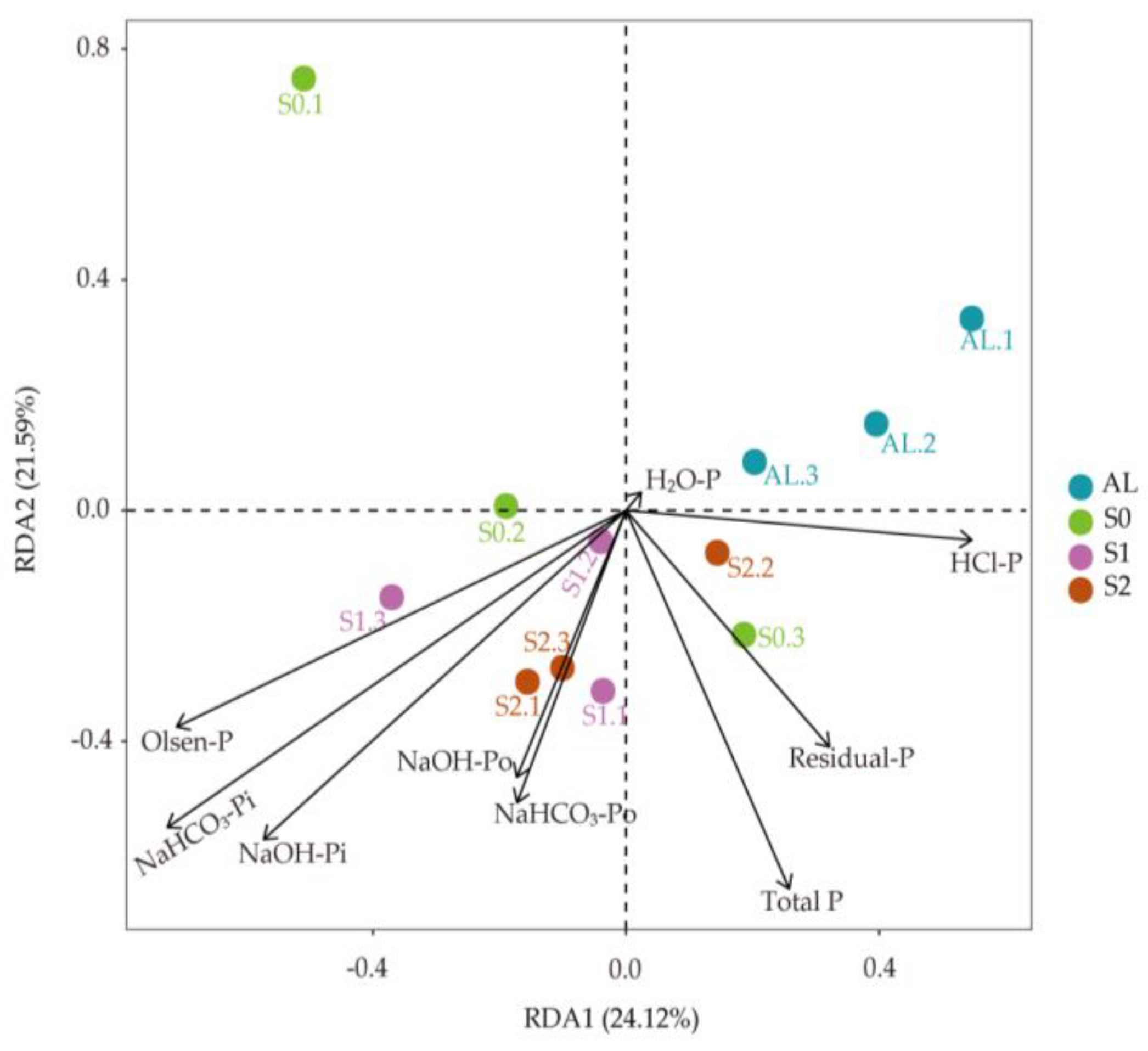
3.2. Soil P Fractions
3.3. Soil Phosphatase Activity
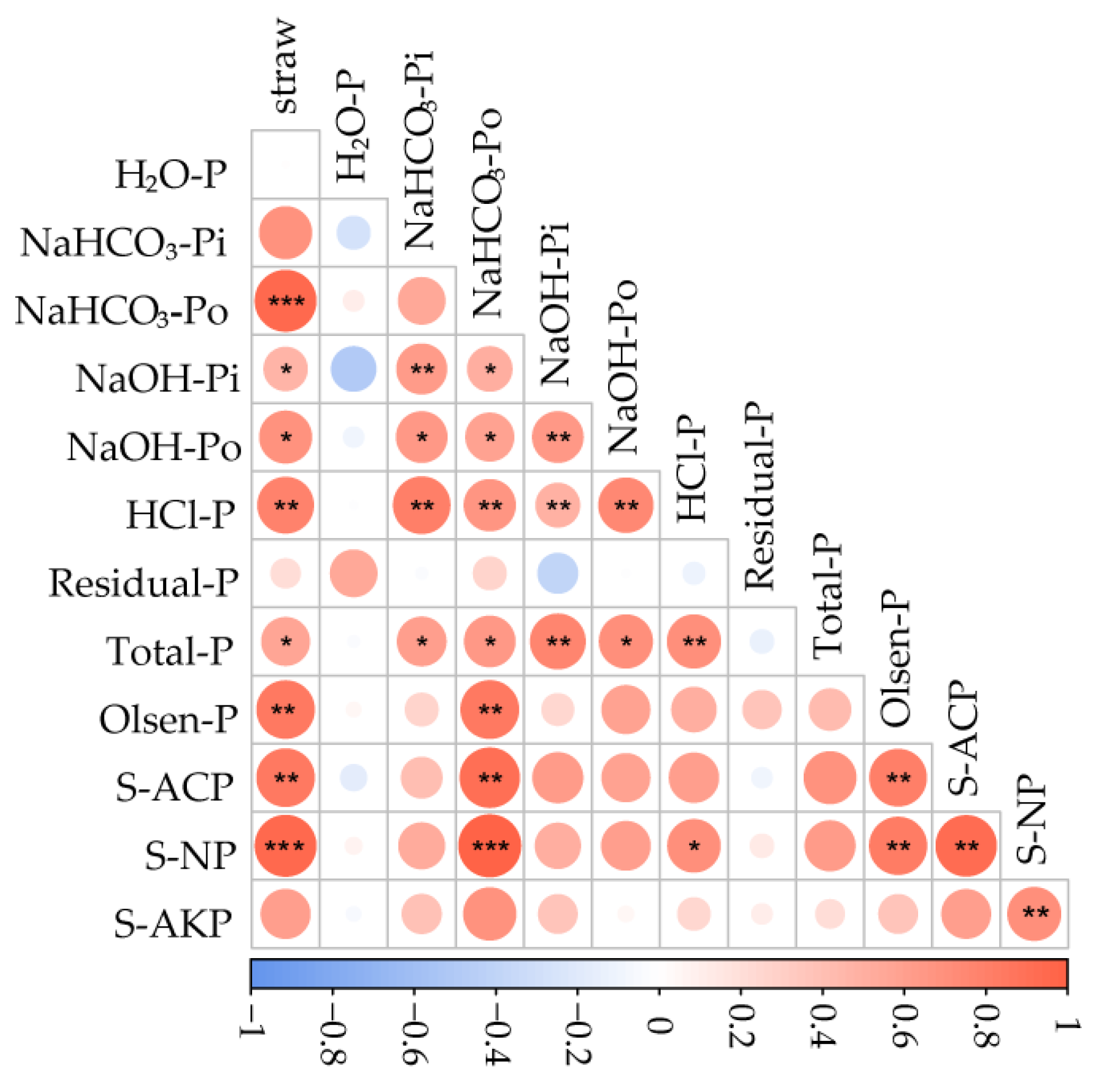
3.4. Correlation between Straw Amount, Soil P Fractions, and Phosphatase Activity
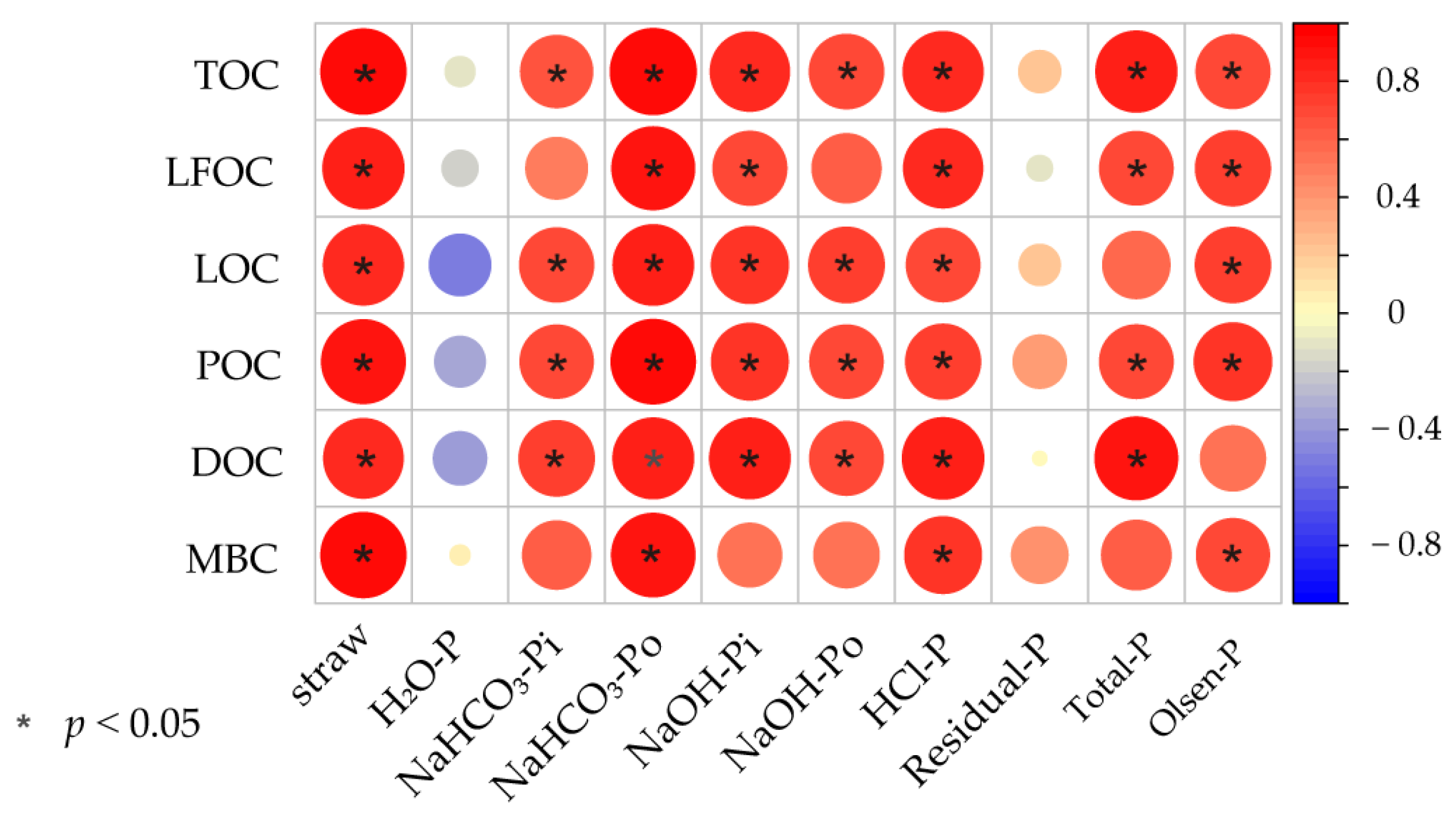
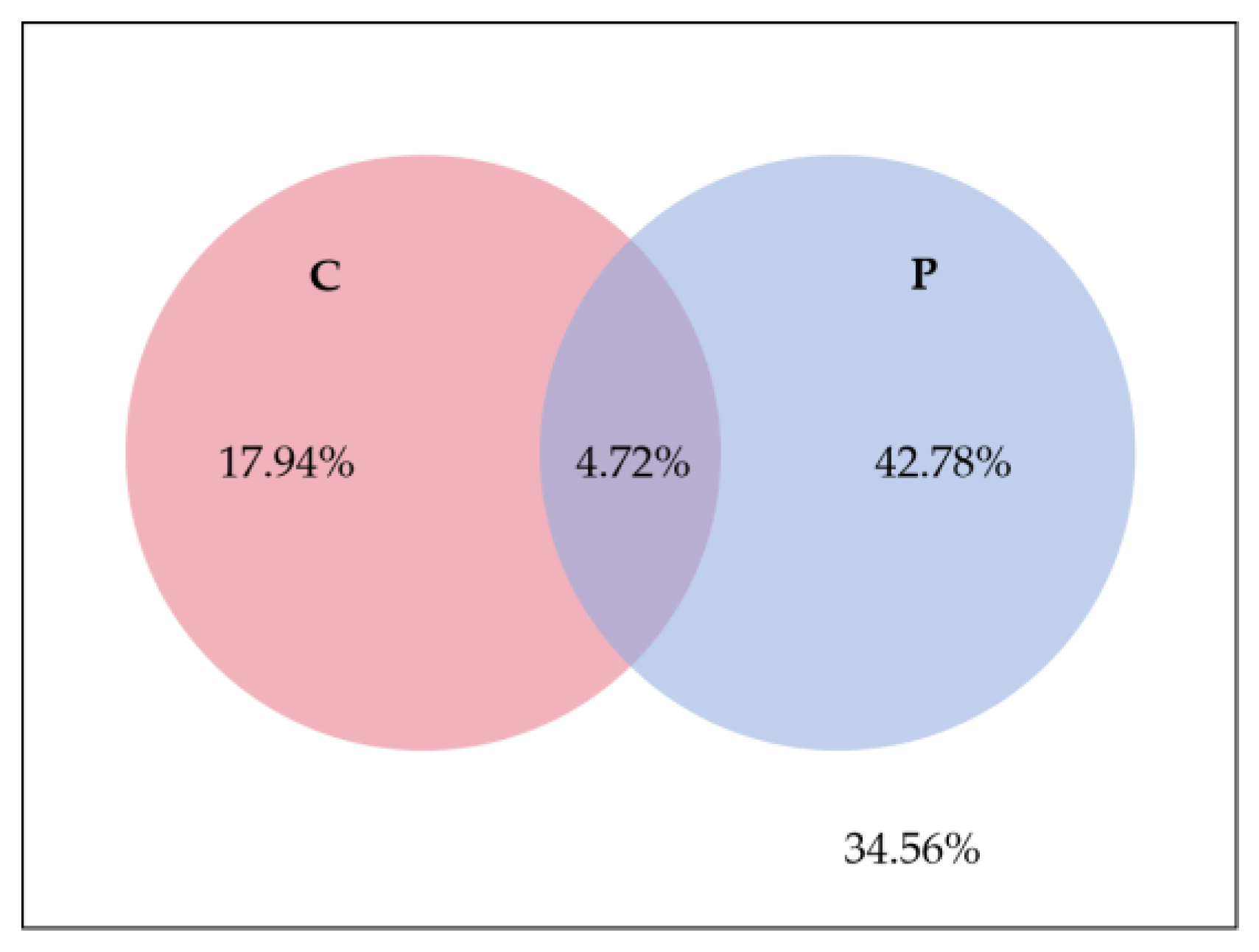
3.5. Correlation between Soil P Fractions and C Fractions
3.6. Effect of Straw Return on PSB Abundance
4. Discussion
4.1. Effect of Straw Return on Rice Yield, P Uptake, and P Budget
4.2. Effect of Straw Return on Soil P Content
4.3. Correlation between Soil P Fractions and Enzyme Activities and C Fractions
4.4. Relationship between Soil P Fractions and Microbial Communities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Sanguin, H.; Wilson, N.L.; Kertesz, M.A. Assessment of functional diversity and structure of phytate-hydrolysing bacterial community in Lolium perenne rhizosphere. Plant Soil 2016, 401, 151–167. [Google Scholar] [CrossRef]
- Sanyal, S.K.; De Datta, S.K. Chemistry of Phosphorus Transformations in Soil: Advances in Soil Science; Springer: New York, NY, USA, 1991; Volume 16. [Google Scholar]
- Zhu, H.; Wu, J.; Huang, D.; Zhu, Q.; Liu, S.; Su, Y.; Wei, W.; Syers, J.K.; Li, Y. Improving fertility and productivity of a highly-weathered upland soil in subtropical China by incorporating rice straw. Plant Soil 2010, 331, 427–437. [Google Scholar] [CrossRef]
- Suriyagoda, L.; De Costa, W.A.J.M.; Lambers, H. Growth and phosphorus nutrition of rice when inorganic fertiliser application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 2014, 384, 53–68. [Google Scholar] [CrossRef]
- Yadvinder, S.; Bijay, S.; Timsina, J. Crop residue management for nutrient cycling and improving soil productivity in rice-based cropping systems in the tropics. Adv. Agron. 2005, 85, 269–407. [Google Scholar]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 2014, 74, 127–137. [Google Scholar] [CrossRef]
- Lan, Z.M.; Lin, X.J.; Wang, F.; Zhang, H.; Chen, C.R. Phosphorus availability and rice grain yield in a paddy soil in response to long-term fertilization. Biol. Fertil. Soils 2012, 48, 579–588. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Zhang, H.; Tian, G. The influence of no-till coupled with straw return on soil phosphorus speciation in a two-year rice-fallow practice. Soil Tillage Res. 2019, 195, 104389. [Google Scholar] [CrossRef]
- Gupta, R.K.; Yadvinder, S.; Ladha, J.K.; Bijay, S.; Singh, J.; Singh, G.; Pathak, H. Yield and phosphorus transformations in a rice–wheat system with crop residue and phosphorus management. Soil Sci. Soc. Am. J. 2007, 71, 1500–1507. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Q.; Wu, Q.; Zhang, S.; Zhu, P.; Peng, C.; Huang, S.; Wang, B.; Zhang, H. The response of soil Olsen-P to the P budgets of three typical cropland soil types under long-term fertilization. PLoS ONE 2020, 15, e0230178. [Google Scholar] [CrossRef]
- Blake, L.; Johnston, A.; Poulton, P.; Goulding, K.J.P. Changes in soil phosphorus fractions following positive and negative phosphorus balances for long periods. Plant Soil 2003, 254, 245–261. [Google Scholar] [CrossRef]
- Shen, J.; Li, R.; Zhang, F.; Fan, J.; Tang, C.; Rengel, Z. Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil. Field Crops Res. 2004, 86, 225–238. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Lu, C. Crop yield and soil organic matter after long-term straw return to soil in China. Nutr. Cycl. Agroecosys. 2015, 102, 371–381. [Google Scholar] [CrossRef]
- Wei, K.; Bao, H.; Huang, S.; Chen, L. Effects of long-term fertilization on available P, P composition and phosphatase activities in soil from the Huang-Huai-Hai Plain of China. Agric. Ecosyst. Environ. 2017, 237, 134–142. [Google Scholar] [CrossRef]
- Malik, M.A.; Marschner, P.; Khan, K.S. Addition of organic and inorganic P sources to soil–Effects on P pools and microorganisms. Soil Biol. Biochem. 2012, 49, 106–113. [Google Scholar] [CrossRef]
- Yang, C.; Yang, L.; Jianhua, L. Organic phosphorus fractions in organically amended paddy soils in continuously and intermittently flooded conditions. J. Environ. Qual. 2006, 35, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Kwabiah, A.B.; Palm, C.A.; Stoskopf, N.C.; Voroney, R.P. Response of soil microbial biomass dynamics to quality of plant materials with emphasis on P availability. Soil Biol. Biochem. 2003, 35, 207–216. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Chu, H.; Yin, R.; Zhang, J. Population size and specific potential of P-mineralizing and -solubilizing bacteria under long-term P-deficiency fertilization in a sandy loam soil. Pedobiologia 2009, 53, 49–58. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, K.K.; Singh, S.; Shivay, Y.S.; Meena, M.C.; Nain, L. Effect of paddy straw burning on soil microbial dynamics in sandy loam soil of Indo-Gangetic plains. Environ. Technol. Innov. 2019, 16, 100469. [Google Scholar] [CrossRef]
- Yan, S.; Song, J.; Fan, J.; Yan, C.; Dong, S.; Ma, C.; Gong, Z. Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Glob. Ecol. Conser. 2020, 22, e00962. [Google Scholar] [CrossRef]
- Lu, R. Methods of Agricultural Chemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available P in Soil by Extraction with Sodium Bicarbonate; Government Printing Office: Washington, DC, USA, 1953; p. 19. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Messiga, A.J.; Ziadi, N.; Plénet, D.; Parent, L.E.; Morel, C. Long-term changes in soil phosphorus status related to P budgets under maize monoculture and mineral P fertilization. Soil Use Manag. 2010, 26, 354–364. [Google Scholar] [CrossRef]
- Yuan, G.; Huan, W.; Song, H.; Lu, D.; Chen, X.; Wang, H.; Zhou, J. Effects of straw incorporation and potassium fertilizer on crop yields, soil organic carbon, and active carbon in the rice–wheat system. Soil Tillage Res. 2021, 209, 104958. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.; Jiang, N.; Zhang, Y.; Feng, J.; Tian, J.; Chen, X.; Lou, C.; Chen, L. Effects of mineral phosphorus fertilizer reduction and maize straw incorporation on soil phosphorus availability, acid phosphatase activity, and maize grain yield in northeast China. Arch. Agron. Soil Sci. 2021, 67, 66–78. [Google Scholar] [CrossRef]
- Vandermoere, S.; Van De Sande, T.; Tavernier, G.; Lauwers, L.; Goovaerts, E.; Sleutel, S.; De Neve, S. Soil phosphorus (P) mining in agriculture–Impacts on P availability, crop yields and soil organic carbon stocks. Agric. Ecosyst. Environ. 2021, 322, 107660. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Taranu, Z.E. The influence of time, soil characteristics, and land-use history on soil phosphorus legacies: A global meta-analysis. Glob. Chang. Biol. 2012, 18, 1904–1917. [Google Scholar] [CrossRef]
- Mao, X.; Xu, X.; Lu, K.; Gielen, G.; Luo, J.; He, L.; Donnison, A.; Xu, Z.; Xu, J.; Yang, W.; et al. Effect of 17 years of organic and inorganic fertilizer applications on soil phosphorus dynamics in a rice–wheat rotation cropping system in eastern China. J. Soils Sediments 2015, 15, 1889–1899. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Gao, R.; Wei, H.; Chen, A.; Li, Y. Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil. J. Integr. Agric. 2015, 14, 365–373. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Li, Z.; Han, F. Phosphorus Adsorption and Bioavailability in a Paddy Soil Amended with Pig Manure Compost and Decaying Rice Straw. Commun. Soil Sci. Plant Anal. 2009, 40, 2185–2199. [Google Scholar] [CrossRef]
- Xavier, F.A.D.S.; De Oliveira, T.S.; Andrade, F.V.; De Sá Mendonça, E. Phosphorus fractionation in a sandy soil under organic agriculture in Northeastern Brazil. Geoderma 2009, 151, 417–423. [Google Scholar] [CrossRef]
- Neufeldt, H.; da Silva, J.E.; Ayarza, M.A.; Zech, W. Land-use effects on phosphorus fractions in Cerrado oxisols. Biol. Fertil. Soils 2000, 31, 30–37. [Google Scholar] [CrossRef]
- Rocha Junior, P.R.D.; Ribeiro, P.H.; Mesquita, L.F.; Andrade, F.V.; Mendonça, E.D.S. Distribution of C and inorganic phosphorus fractions in different aggregate sizes under forestry, agroforestry system and pasture. J. Soil Sci. Plant Nut. 2018, 18, 361–375. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding how long-term organic amendments increase soil phosphatase activities: Insight into phoD- and phoC-harboring functional microbial populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Yan, C.; Diao, X.; Ge, H.; Wang, X.; Ma, C.; Gong, Z. Effects of rice straw return-to-field on soil solution nutrients and enzyme activities. Chin. J. Soil Sci. 2012, 43, 1232–1236. (In Chinese) [Google Scholar]
- Zhan, H.; Yan, S.; Wang, J.; Ma, C.; Gong, Z.; Dong, S.; Zhang, Q. Effects of rice straw return-to-field on soil phosphatase activity and available phosphorus content. Crops 2015, 2, 78–83. (In Chinese) [Google Scholar]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co-incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Tillage Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Dai, X.; Fu, X.; Yang, F.; Liu, X.; Sun, X.; Wen, X.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Kucey, R.M.N. Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]

| Treatment | Olsen-P | Total P | Labile P | Moderately Labile P | Nonlabile P | ||||
|---|---|---|---|---|---|---|---|---|---|
| H2O-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl-P | Residual P | |||
| S0 | 80.52 b | 991.25 c | 13.05 a | 155.75 b | 25.40 d | 122.86 c | 61.21 b | 377.75 c | 38.68 a |
| S1 | 81.27 ab | 1156.77 ab | 12.80 a | 162.63 a | 41.41 b | 144.69 b | 68.07 a | 396.00 b | 40.22 a |
| S2 | 83.79 a | 1187.28 a | 11.88 a | 164.31 a | 54.99 a | 152.54 a | 69.09 a | 394.21 b | 37.34 a |
| AL | 61.38 c | 1101.05 b | 12.03 a | 131.14 c | 32.55 c | 98.32 d | 64.73 ab | 405.57 a | 39.19 a |
| Treatment | S-ACP | S-NP | S-AKP |
|---|---|---|---|
| S0 | 21.31 b | 4.11 c | 4.82 a |
| S1 | 22.78 b | 5.09 b | 4.92 a |
| S2 | 26.52 a | 6.57 a | 5.26 a |
| AL | 17.16 c | 3.32 c | 3.36 b |
| Taxonomy | Relative Abundance | ||||
|---|---|---|---|---|---|
| S0 | S1 | S2 | AL | ||
| Phylum | Proteobacteria | 0.2618 | 0.2770 | 0.2935 | 0.3188 |
| Bacteroidetes | 0.2525 | 0.2493 | 0.2513 | 0.1460 | |
| Actinobacteria | 0.0497 | 0.0215 | 0.0304 | 0.0272 | |
| Class | Betaproteobacteria | 0.0694 | 0.0916 | 0.0977 | 0.1621 |
| Alphaproteobacteria | 0.0543 | 0.0325 | 0.0642 | 0.0576 | |
| Gammaproteobacteria | 0.0504 | 0.0370 | 0.0416 | 0.0314 | |
| Bacilli | 0.0283 | 0.0222 | 0.0123 | 0.0026 | |
| Family | Sphingomonadaceae | 0.0086 | 0.0060 | 0.0250 | 0.0072 |
| Comamonadaceae | 0.0142 | 0.0198 | 0.0230 | 0.0180 | |
| Enterobacteriaceae | 0.0087 | 0.0018 | 0.0020 | 0.0015 | |
| Flavobacteriaceae | 0.0096 | 0.0037 | 0.0048 | 0.0014 | |
| Micrococcaceae | 0.0074 | 0.0029 | 0.0069 | 0.0031 | |
| Nocardiaceae | 0.0048 | 0.0002 | 0.0003 | 0.0001 | |
| Microbacteriaceae | 0.0061 | 0.0035 | 0.0046 | 0.0020 | |
| Burkholderiaceae | 0.0043 | 0.0006 | 0.0007 | 0.0006 | |
| Xanthobacteraceae | 0.0035 | 0.0026 | 0.0045 | 0.0080 | |
| Pseudomonadaceae | 0.0054 | 0.0013 | 0.0036 | 0.0014 | |
| Bacillaceae | 0.0031 | 0.0007 | 0.0005 | 0.0002 | |
| Moraxellaceae | 0.0033 | 0.0005 | 0.0012 | 0.0003 | |
| Oxalobacteraceae | 0.0024 | 0.0044 | 0.0054 | 0.0027 | |
| Caulobacteraceae | 0.0032 | 0.0019 | 0.0037 | 0.0016 | |
| Nocardioidaceae | 0.0012 | 0.0014 | 0.0014 | 0.0009 | |
| Paenibacillaceae | 0.0005 | 0.0005 | 0.0004 | 0.0001 | |
| Sphingobacteriaceae | 0.0035 | 0.0034 | 0.0034 | 0.0012 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Liu, C.; Li, J.; Li, J.; Cui, C.; Fan, J.; Gong, Z.; Zhang, Z.; Yan, C. Changes in the Soil Phosphorus Supply with Rice Straw Return in Cold Region. Agronomy 2023, 13, 2214. https://doi.org/10.3390/agronomy13092214
Yan S, Liu C, Li J, Li J, Cui C, Fan J, Gong Z, Zhang Z, Yan C. Changes in the Soil Phosphorus Supply with Rice Straw Return in Cold Region. Agronomy. 2023; 13(9):2214. https://doi.org/10.3390/agronomy13092214
Chicago/Turabian StyleYan, Shuangshuang, Chunxue Liu, Jianan Li, Jinwang Li, Can Cui, Jinsheng Fan, Zhenping Gong, Zhongxue Zhang, and Chao Yan. 2023. "Changes in the Soil Phosphorus Supply with Rice Straw Return in Cold Region" Agronomy 13, no. 9: 2214. https://doi.org/10.3390/agronomy13092214





