Simulated Nitrogen Deposition Decreases the Ratios of Soil C to P and N to P, Changes Soil Enzyme Activity, and Reduces Soil Microbial Biomass in Paddy Soil in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sampling
2.3.1. Plant Samplings
2.3.2. Soil Samplings
2.4. Measurements and Determinations
2.5. Soil Microbial Biomass
2.6. Soil Bacterial Community Structure by Determining 16S RNA Genes
2.7. Data Analysis
3. Results
3.1. Rice Yield and Biomass
3.2. Rice Plant TC, TN, and TP Contents and TC/TN, TC/TP, and TN/TP Ratios
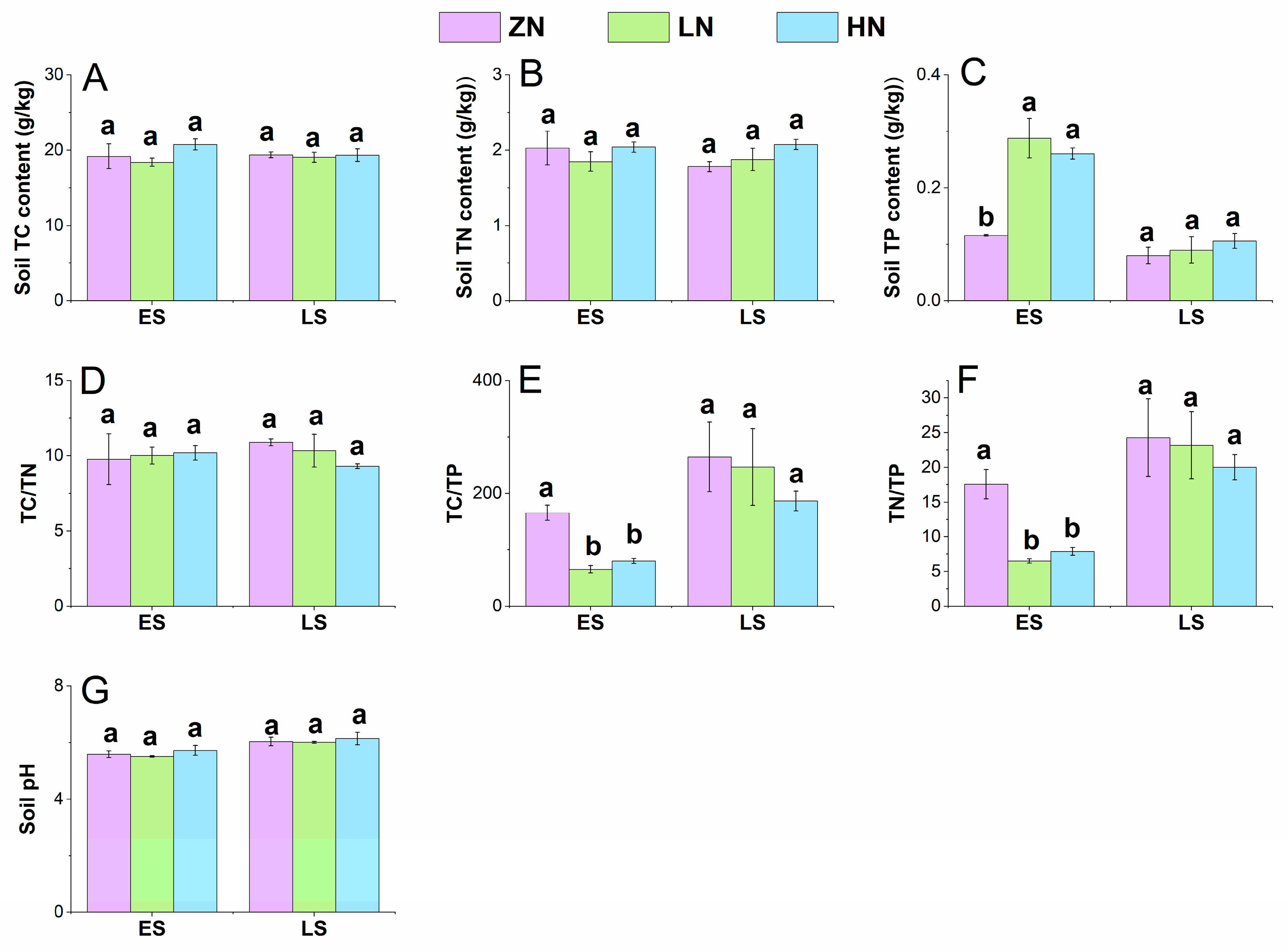
3.3. Soil Physicochemical Properties
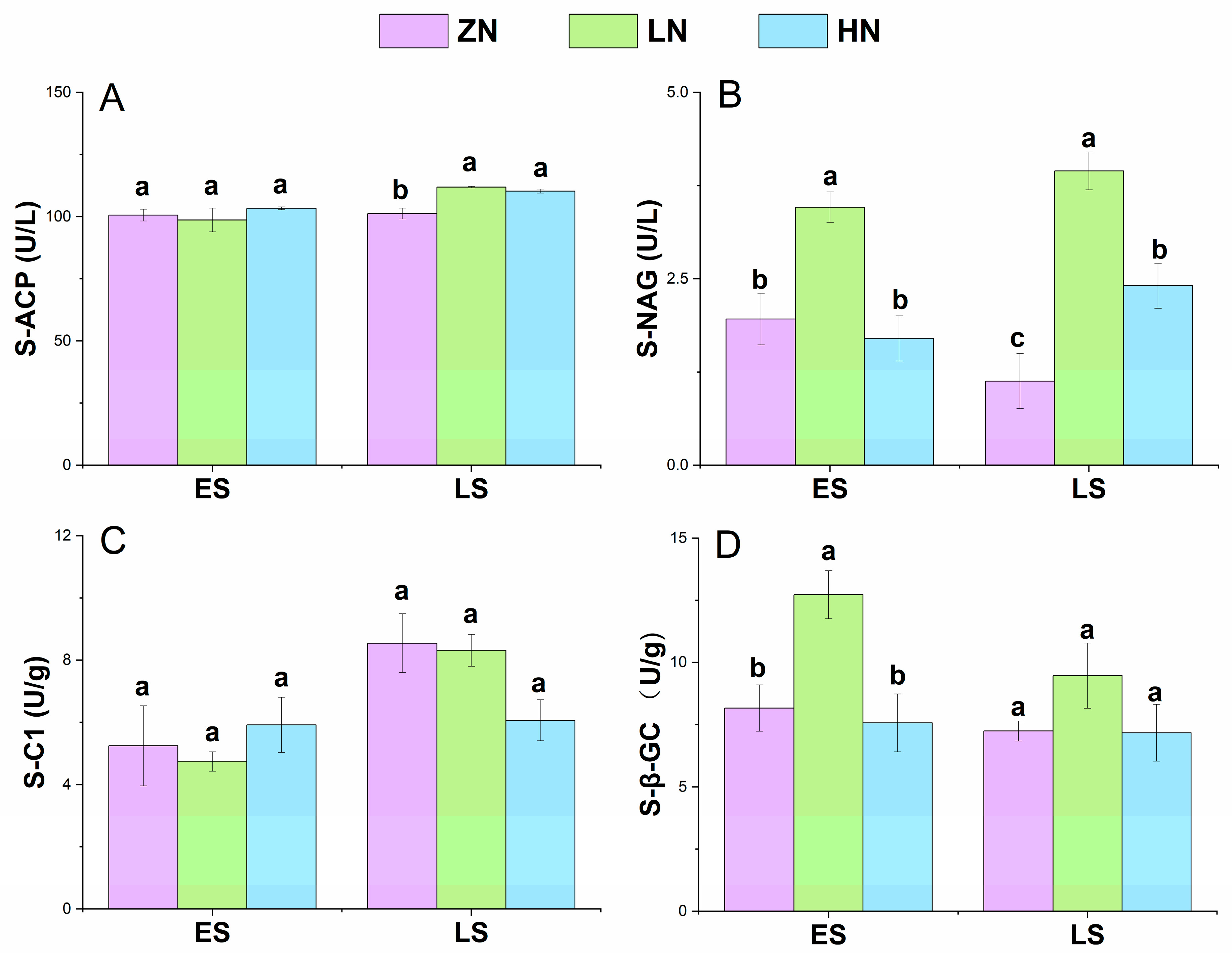
3.4. Soil Enzyme Activities
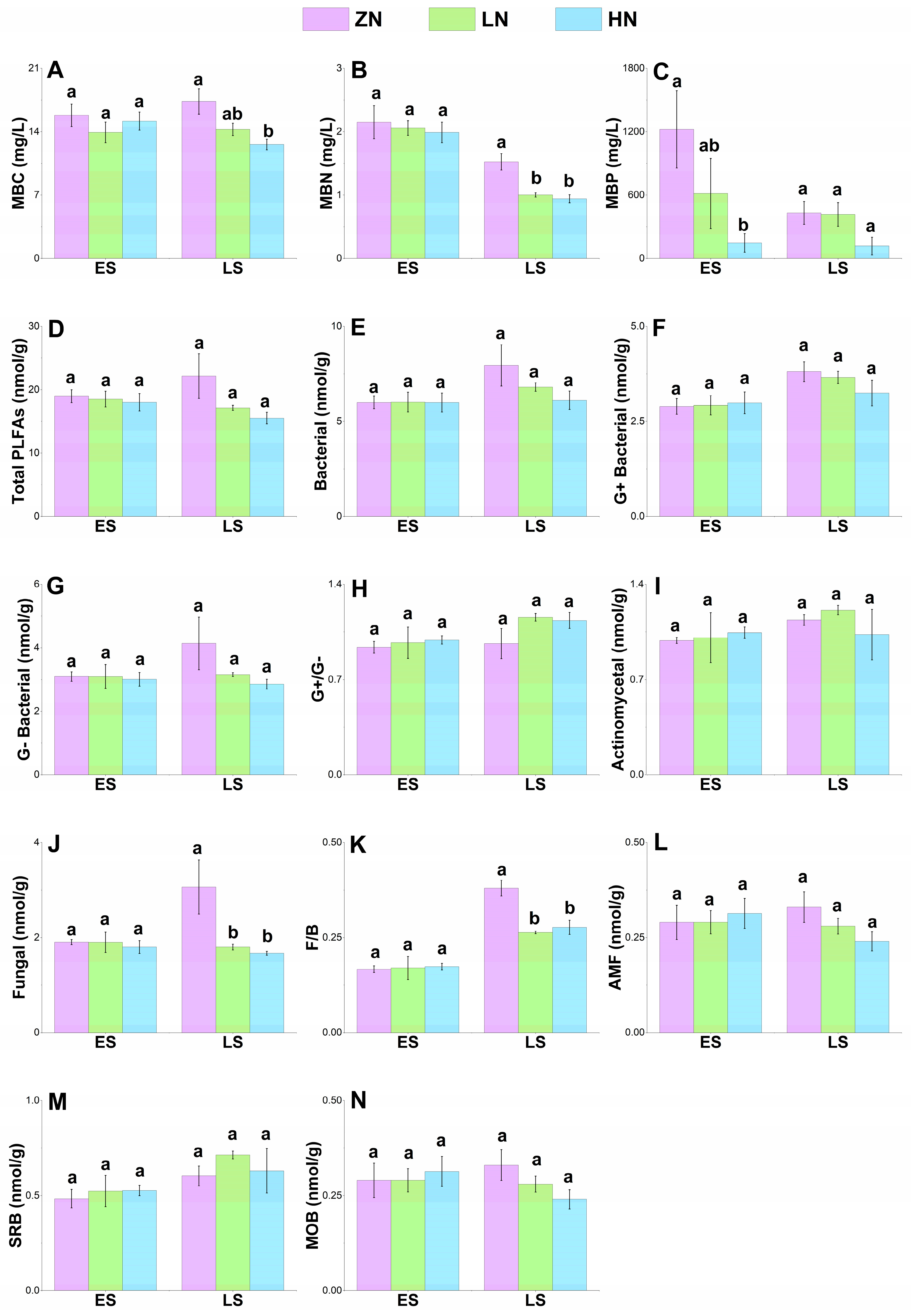
3.5. Soil Microbial Biomass
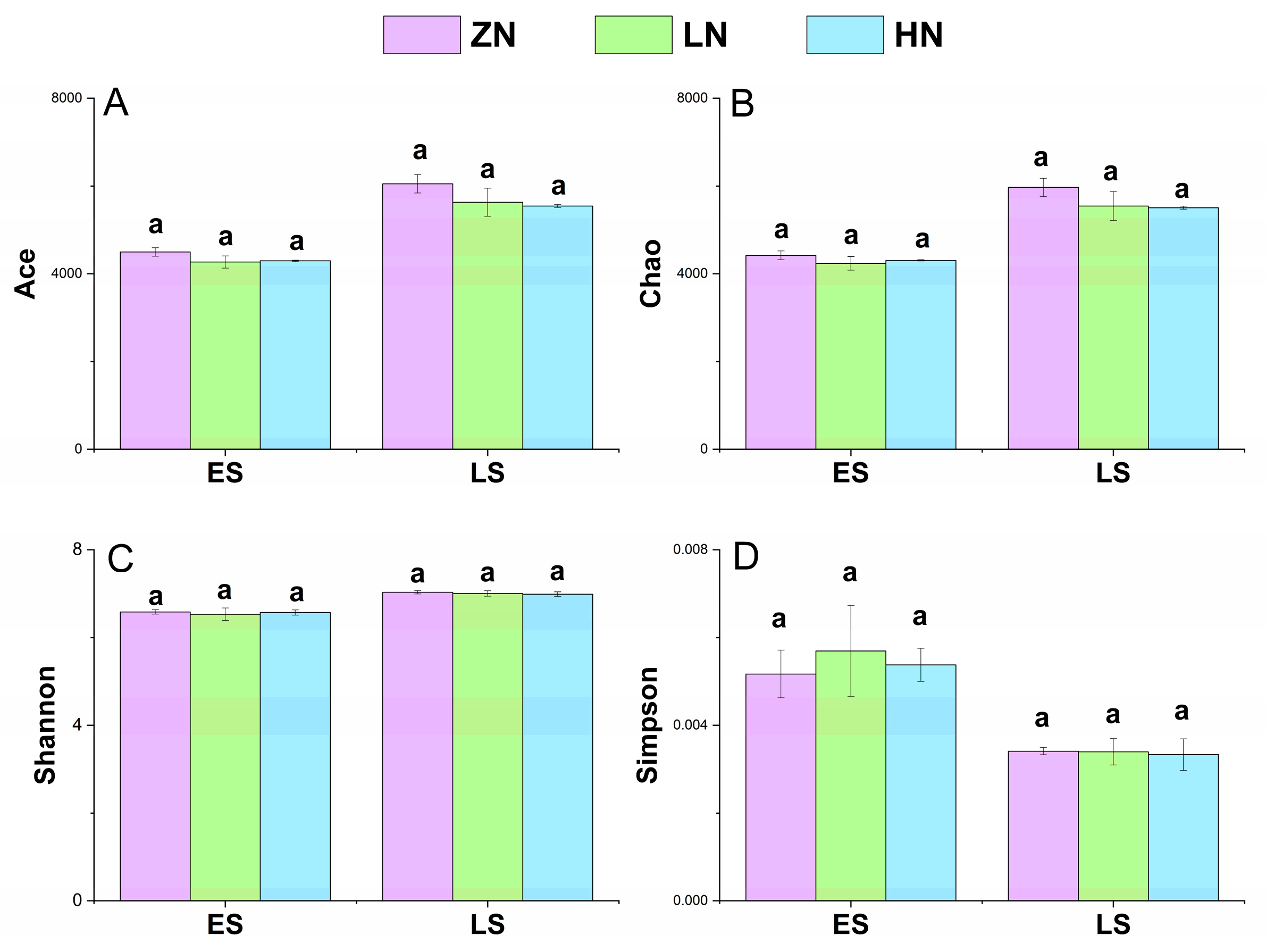
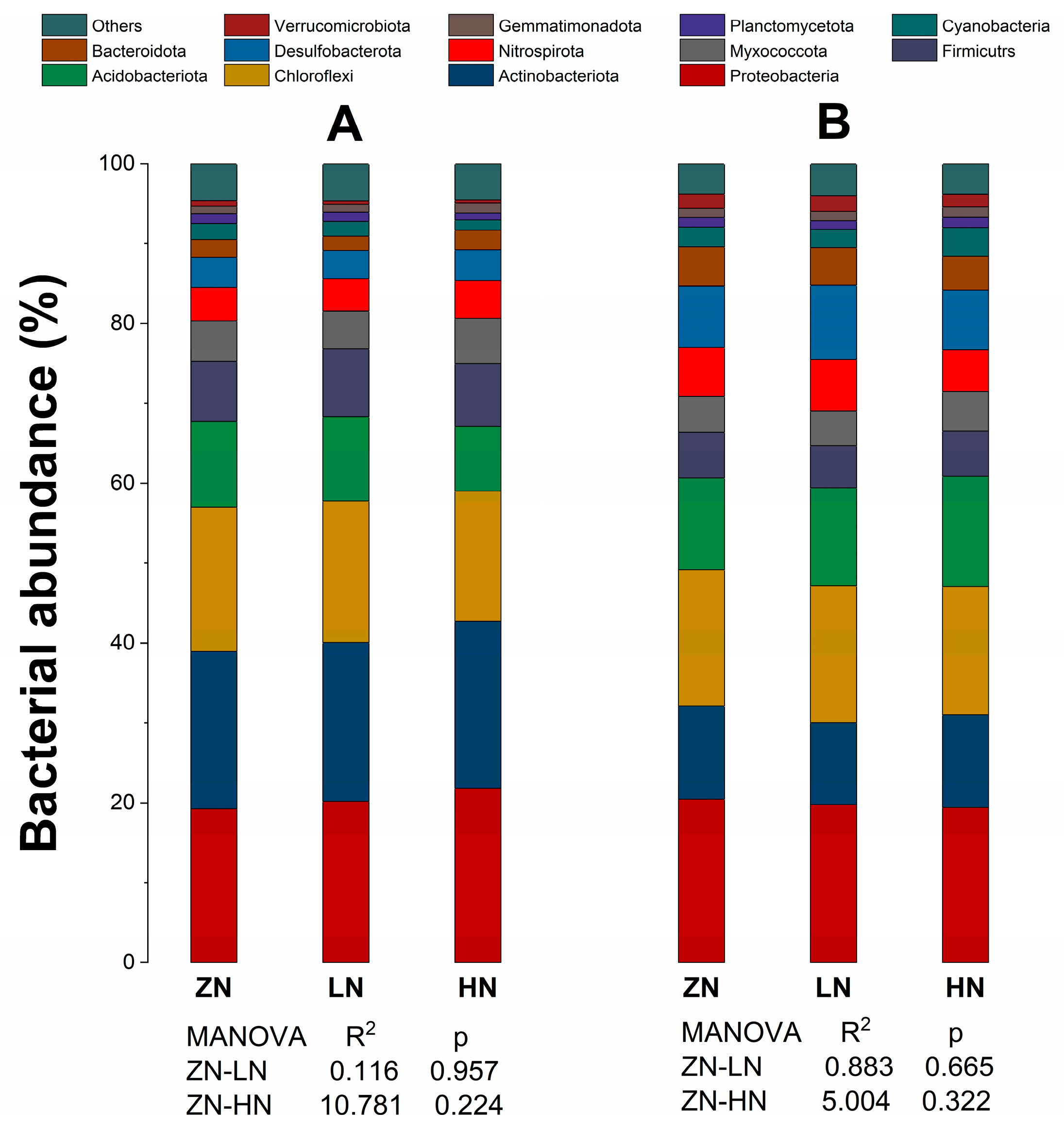
3.6. Soil Microbial Community Structure
4. Discussion
4.1. Effects of Nitrogen Deposition on Rice Yield and Biomass
4.2. Effects of Nitrogen Deposition on Rice TC, TN, and TP Contents
4.3. Effects of Nitrogen Deposition on Soil Physicochemical Properties
4.4. Effects of Nitrogen Deposition on Soil Enzyme Activities
4.5. Effects of Nitrogen Deposition on Soil Microbial Biomass
4.6. Effects of Nitrogen Deposition on Soil Microbial Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Flore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20, 1–21. [Google Scholar]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar]
- Fowler, D.; Pyle, J.A.; Raven, J.A.; Sutton, M.A. The global nitrogen cycle in the twenty-first century: Introduction. Biol. Sci. 2013, 368, 1–2. [Google Scholar]
- Xu, F.D.; Gao, Y.; Dong, W.Y.; Hao, Z.; Xu, Y.J. Impact of atmospheric nitrogen and phosphorus wet deposition on nitrogen and phosphorus export and associated water quality: A case study of forest watershed in the red soil area, southern China. Acta Ecol. Sin. 2016, 36, 6409–6419. (In Chinese) [Google Scholar]
- Mao, Q.G.; Lu, X.K.; Zhou, K.J.; Chen, H.; Zhu, X.M.; Mori, T.K.; Mo, J.M. Effects of long- term nitrogen and phosphorus depositions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar]
- Lu, X.K.; Mo, J.M.; Zhang, W.; Mao, Q.G.; Liu, R.Z.; Wang, C.; Wang, S.H.; Zheng, M.H.; Mori, T.; Mao, J.H.; et al. Effects of simulated atmospheric nitrogen deposition on forest ecosystems in China: An overview. J. Trop. Subtrop. Bot. 2019, 27, 500–522. (In Chinese) [Google Scholar]
- The State of National Grain Production in China. 2020. Available online: http://www.stats.gov.cn/xxgk/jd/sjjd2020/202012/t20201211_1808749.html (accessed on 16 November 2021). (In Chinese)
- Yan, Z.Q.; Qi, Y.C.; Li, S.J.; Dong, Y.S.; Peng, Q.; He, Y.L.; Li, Z.L. Soil microorganisms and enzyme activity of grassland ecosystem affected by changes in precipitation pattern and increase in nitrogen deposition-a review. Microbiol. China 2017, 44, 1481–1490. (In Chinese) [Google Scholar]
- Tu, L.H.; Dai, H.Z.; Hu, T.X.; Zhang, J.; Luo, S.H. Effects of simulated nitrogen deposition on soil respiration in a Bambusa pervariabilis × Dendrocala mopsi Plantation in Rainy Area of West China. Chin. J. Appl. Ecol. 2011, 22, 829–836. (In Chinese) [Google Scholar]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benitez-Lopez, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen deposition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar]
- Rousk, J.; Philip, C.B.; Brookes, P.C.; Baath, E. Fungal and bacterial growth response to N fertilization and pH in the 150-year ‘Park Grass’ UK grassland experiment. FEMS Microbiol. Ecol. 2011, 76, 89–99. [Google Scholar]
- Lin, B.L.; Kumon, Y.; Inoue, K.; Tobari, N.; Xue, M.Q.; Tsunemi, K.; Terada, A. Increased nitrogen deposition contributes to plant biodiversity loss in Japan: Insights from long-term historical monitoring data. Environ. Pollut. 2021, 290, 118033. [Google Scholar] [CrossRef]
- Hou, X.D.; Liu, S.T.; Wang, S.J.; Jia, S.G.; Hou, Y.L.; Lu, L. Correlation analysis of fertilization parameters in rice. J. Agric. Resour. Environ. 2019, 36, 614–619. (In Chinese) [Google Scholar]
- Hou, Y.P.; Yang, J.; Li, Q.; Qin, Y.B.; Kong, L.L.; Yin, C.X.; Wang, L.C.; Xie, J.G. Effects of nitrogen application on yield, nitrogen utilization and accumulation of soil inorganic nitrogen of rice. Chin. J. Soil Sci. 2016, 47, 118–124. (In Chinese) [Google Scholar]
- Zhang, Q.; Chang, M.; Wang, X.M. Advances in nitrogen deposition observation in China and its application in the Pearl River Delta. China Environ. Sci. 2017, 37, 4401–4416. (In Chinese) [Google Scholar]
- Hei, Z.W.; Xiang, H.M.; Zhang, J.E.; Liang, K.M.; Ren, X.Q.; Sun, Y.R.; Wu, R.Q. Water mimosa (Neptunia oleracea Lour.) can fix and transfer nitrogen to rice in their intercropping system. J. Sci. Food Agric. 2021, 102, 156–166. [Google Scholar] [PubMed]
- Lu, R.K. Soil Argro-Chemical Analysis; Agricultural Technical Press of China: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Mecino, C.; Godoy, R.; Matus, F. Soil enzymes and biological activity at different levels of organic matter stability. J. Soil Sci. Plant Nutr. 2016, 16, 14–30. [Google Scholar]
- Li, Y.; Zhao, X.R.; Li, G.T.; Lin, Q.M. Effect of grazing on soil microbial biomass phosphorus and phosphatase activities on different topographic unit of a typical steppe. Acta Ecol. Sin. 2022, 42, 4137–4149. (In Chinese) [Google Scholar]
- Yang, X.Y.; Zhou, S.S.; Sun, B.H.; Wang, B.Q.; Zhang, S.L.; Gu, Q.Z. Microbial properties of a loess soil as affected by various nutrient management practices in the loess plaseau of China. Commun. Soil Sci. Plant 2010, 41, 956–967. [Google Scholar]
- Liu, Z.Q.; Liu, Z.X.; Wu, L.Z.; Li, Y.Z.; Wang, J.; Wei, H.; Zhang, J.E. Effect of polyethylene mircroplastics and acid rain on the agricultural soil ecosystem in Southern China. Environ. Pollut. 2022, 303, 119094. [Google Scholar]
- Zhang, J.F.; Feng, Y.H.; Xu, G.L.; Guan, Z.C.; Su, W.; Ou, D.; Wang, L.L.; Chen, Y. Effects of different nitrogen rates on growth and yield of Nei 5 You 5399 of hybrid rice. Hybrid Rice 2019, 34, 61–66. (In Chinese) [Google Scholar]
- Feng, S.Z.; Ding, X.D.; Wang, S.W.; Li, C.Y.; Cheng, D.D.; Zhang, S.R.; Zhou, W. Effects of different nitrogen application rates on photosynthetic rate, nitrogen fertilizer conversion efficiency and yield of rice in Linyi region. Acta Agric. Shanghai 2021, 37, 88–91. (In Chinese) [Google Scholar]
- Yan, K.; Jiang, Y.L.; Xiao, T.J.; Dai, Q.G. Effects of nitrogen amount on grain yield and quality of rice on saline-alkaline land. China Rice 2017, 23, 35–39. (In Chinese) [Google Scholar]
- Lewandowski, I.; Kauter, D. The influence of nitrogen fertilizer on the yield and combustion quality of whole grain crops for solid fuel use. Ind. Crops Prod. 2003, 17, 103–117. [Google Scholar]
- Vicensi, M.; Lopes, C.; Koszalka, V.; Umburanas, R.C.; Vidigal, J.C.B.; Avila, F.W.; Muller, M.M.L. Soil fertility, root and aboveground growth of black oat under gypsum and urea rates in no till. J. Soil Sci. Plant Nutr. 2020, 20, 1271–1286. [Google Scholar]
- Finnan, J.; Burke, B. Nitrogen fertilization of Miscanthus × giganteus: Effects on nitrogen uptake, growth, yield and emissions from biomass combustion. Nutr. Cycl. Agroecosyst. 2016, 106, 249–256. [Google Scholar]
- Yan, Z.D.; Guo, P.Y.; Yuan, X.Y.; Xiao, X.L.; Wang, D.M. Effect of foliar application of nitrogen fertilizer on photosynthetic characteristics and yield of foxtail millet cultivar ‘Zhangzagu 10’ Setaria italic (L.) Beauv. J. Shanxi Agric. Univ. (Nat. Sci. Ed.) 2018, 39, 37–41. (In Chinese) [Google Scholar]
- Tian, T.; Wang, J.G.; Wang, H.J.; Cui, J.; Shi, X.Y.; Song, J.H.; Li, W.D.; Zhong, M.T.; Qiu, Y.; Xu, T. Nitrogen application alleviates salt stress by enhancing osmotic balance, ROS scavenging, and photosynthesis of rapeseed seedlings (Brassica napus). Plant Signal. Behav. 2022, 17, 2081419. [Google Scholar]
- Lu, X.K.; Mo, J.M.; Gilliam, F.S.; Zhou, G.Y.; Fang, Y.T. Effects of experimental nitrogen depositions on plant diversity in an old-growth tropical forest. Glob. Chang. Biol. 2010, 16, 2688–2700. [Google Scholar]
- Anning, D.K.; Qiu, H.Z.; Zhang, C.H.; Ghanney, P.; Zhang, Y.J.; Guo, Y.J. Maize straw return and nitrogen rate effects on patato (Solanum tuberosum L.) performance and soil physicochemical characteristics in northwest China. Sustainability 2021, 13, 5508. [Google Scholar]
- Ramteke, P.R.; Vashisht, B.B.; Sharma, S.; Jalota, S.K. Assessing and ranking influence of rates of rice (Oryza sativa L.) straw incorporation and N fertilizer on soil physicochemical properties and wheat (Triticum aestivum L.) yield in rice-wheat system. J. Soil Sci. Plant Nutr. 2022, 22, 515–526. [Google Scholar]
- Behnke, G.D.; David, M.B.; Voigt, T.B. Greenhouse gas emissions, nitrate leaching, and biomass yields from production of miscanthus×giganteus in Illinois, USA. Bioenergy Res. 2012, 5, 801–813. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Wang, C.T.; Song, W.B.; Silang, S.; Garong, R.; Dawa, Z.; Zhaxi, L. Advances on soil enzymatic activities in grassland ecosystem. Pratacult. Sci. 2011, 28, 1801–1806. [Google Scholar]
- Tian, M.Y.; Yu, C.J.; Wang, J.K.; Ding, F.; Chen, Z.H.; Jiang, N.; Jiang, H.; Jiang, L.J. Effect of nitrogen depositions on soil pH, phosphorus contents and phosphatase activities in grassland. Chin. J. Appl. Ecol. 2020, 31, 2985–2992. (In Chinese) [Google Scholar]
- Sekaran, U.; Mccoy, C.; Kumar, S.; Subramanian, S. Soil microbial community structure and enzymatic activity responses to nitrogen management and landscape positions in switchgrass (Panicum virgatum L.). Glob. Chang. Biol. Bioenergy 2019, 11, 836–851. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.M.; Xu, K.; Yang, X.T. Effect of simulated nitrogen deposition on soil enzyme activities in a temperate forest. Acta Ecol. Sin. 2017, 37, 1956–1965. (In Chinese) [Google Scholar]
- Zhang, N.L.; Wan, S.Q.; Li, L.H.; Bi, J.; Zhao, M.M.; Ma, K.P. Impacts of urea N deposition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]
- Yan, G.Y.; Xing, Y.J.; Han, S.J.; Zhang, J.H.; Wang, Q.G.; Mu, C.C. Long-time precipitation and nitrogen deposition increase alter soil nitrogen dynamic by influencing soil bacterial communities and functional groups. Pedosphere 2020, 30, 363–377. [Google Scholar] [CrossRef]
- Wang, Y.S.; Cheng, S.L.; Fang, H.J.; Yu, G.R.; Xu, X.F.; Xu, M.J.; Wang, L.; Li, X.Y.; Si, G.Y.; Geng, J.; et al. Contrasting effects of ammonium and nitrate inputs on soil CO2 emission in a subtropical coniferous plantation of Southern China. Biol. Fertil. Soils 2015, 51, 815–825. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Zheng, M.H.; Jiang, L.F.; Luo, Y.Q. Patterns and mechanisms of responses by soil microbial communities to nitrogen deposition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Van Diepen, L.T.A.; Lilleskov, E.A.; Pregitzer, K.S. Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Mol. Ecol. 2011, 20, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Averill, C.; Dietze, M.C.; Bhatnagar, J.M. Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Glob. Chang. Biol. 2018, 24, 4544–4553. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.; Fei, S.L.; Oswalt, C.M.; Domke, G.M.; Phillips, R.P. Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci. Adv. 2019, 5, eaav6358. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Y.F.; Li, X.L. Effect of external hyphae of arbuscular mycorrhizal plant on water-stable aggregates in sandy soil. J. Soil Water Conserv. 2001, 15, 99–102. (In Chinese) [Google Scholar]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidification caused by nitrogen deposition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.W.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen deposition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Kaspari, M.; Bujan, J.; Weiser, M.D.; Ning, D.L.; Michaletz, S.T.; He, Z.L.; Enquist, B.J.; Waide, R.B.; Zhou, J.Z.; Turner, B.L. Biogeochemistry drives diversity in the prokaryotes, fungi, and invertebrates of a Panama forest. Ecology 2017, 98, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.R.; Zhang, X.; Mao, Q.G.; Li, X.Z.; You, Y.M.; Wang, J.X.; Zheng, M.H.; Zhang, W.; Lu, X.K.; et al. Nitrogen deposition reduces soil bacterial richness, while phosphorus deposition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Yan, G.Y.; Han, S.J.; Wang, Q.G.; Wang, X.C.; Hu, C.Y.; Xing, Y.J. Variations of the effects of reduced precipitation and N deposition on the microbial diversity among different seasons in a temperate forest. Appl. Soil Ecol. 2021, 166, 103995. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X.L. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
| Yield (t·ha−1) | Number of Tillers | Number of Effective Panicles | Grains per Panicle | 1000-Grain Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Root–Shoot Ratio | ||
|---|---|---|---|---|---|---|---|---|---|
| ES | ZN | 6.72 ± 0.05 a | 13.73 ± 1.07 a | 13.13 ± 0.97 a | 76.75 ± 2.52 a | 24.55 ± 0.31 b | 44.52 ± 4.78 a | 2.20 ± 0.49 a | 0.049 ± 0.008 a |
| LN | 5.72 ± 0.20 a | 12.73 ± 0.37 a | 11.60 ± 0.64 a | 84.94 ± 13.10 a | 23.41 ± 1.09 b | 37.57 ± 2.06 a | 1.73 ± 0.32 a | 0.046 ± 0.007 a | |
| HN | 6.72 ± 0.55 a | 13.13 ± 0.13 a | 12.67 ± 0.13 a | 78.50 ± 7.49 a | 29.53 ± 1.18 a | 45.25 ± 2.32 a | 2.37 ± 0.44 a | 0.054 ± 0.013 a | |
| LS | ZN | 7.17 ± 1.11 a | 11.06 ± 0.43 a | 10.17 ± 0.73 a | 50.84 ± 3.53 b | 25.45 ± 0.77 ab | 27.94 ± 0.92 a | 1.73 ± 0.05 a | 0.062 ± 0.002 a |
| LN | 8.28 ± 0.87 a | 11.78 ± 1.20 a | 10.78 ± 0.78 a | 59.60 ± 7.94 ab | 24.06 ± 0.20 b | 26.34 ± 2.25 a | 1.77 ± 0.18 a | 0.068 ± 0.007 a | |
| HN | 7.89 ± 1.36 a | 9.78 ± 0.31 a | 9.39 ± 0.29 a | 95.76 ± 17.07 a | 26.76 ± 1.01 a | 27.51 ± 2.84 a | 1.64 ± 0.09 a | 0.060 ± 0.003 a |
| TC Content (g/kg) | TN Content (g/kg) | TP Content (g/kg) | TC/TN Ratio | TC/TP Ratio | TN/TP ratio | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ES | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | |||||||
| ZN | 196.13 ± 41.15 a | 311.83 ± 33.76 a | 4.88 ± 0.87 a | 7.43 ± 0.79 a | 0.52 ± 0.18 a | 0.40 ± 0.19 b | 39.73 ± 1.94 a | 41.93 ± 0.40 a | 1591.22 ± 983.98 a | 1182.03 ± 558.35 a | 0.64 ± 0.35 a | 32.05 ± 16.73 a | |||||||
| LN | 229.11 ± 12.51 a | 310.41 ± 24.89 a | 5.73 ± 0.50 a | 8.12 ± 1.16 a | 0.40 ± 0.02 a | 0.85 ± 0.04 a | 40.25 ± 1.88 a | 38.89 ± 2.34 a | 1790.68 ± 763.27 a | 2505.75 ± 23.97 a | 0.54 ± 0.06 a | 9.78 ± 1.95 a | |||||||
| HN | 159.30 ± 19.26 a | 367.86 ± 63.55 a | 4.33 ± 0.23 a | 8.56 ± 1.13 a | 0.58 ± 0.24 a | 0.91 ± 0.03 a | 36.51 ± 2.68 a | 42.55 ± 1.84 a | 438.44 ± 225.43 a | 2391.68 ± 346.47 a | 0.88 ± 0.64 a | 9.46 ± 1.44 a | |||||||
| LS | Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | |
| ZN | 355.14 ± 99.65 a | 346.53 ± 4.63 a | 307.68 ± 7.05 a | 10.90 ± 3.47 a | 8.02 ± 0.30 a | 7.22 ± 0.55 a | 0.99 ± 0.13 a | 0.88 ± 0.12 b | 1.76 ± 0.45 a | 33.22 ± 1.62 a | 43.35 ± 2.18 a | 43.02 ± 2.80 a | 392.77 ± 160.65 a | 410.06 ± 56.66 a | 177.46 ± 17.34 a | 12.21 ± 5.45 a | 9.58 ± 1.64 a | 4.20 ± 0.64 a | |
| LN | 260.94 ± 16.50 a | 349.11 ± 6.75 a | 252.48 ± 32.44 a | 8.08 ± 0.40 a | 9.17 ± 0.63 a | 6.83 ± 0.77 a | 0.85 ± 0.04 a | 0.89 ± 0.02 b | 1.55 ± 0.08 ab | 32.28 ± 1.04 a | 38.37 ± 2.37 a | 36.73 ± 1.86 a | 309.79 ± 31.57 a | 392.99 ± 16.26 a | 161.70 ± 13.63 a | 9.56 ± 0.71 a | 10.34 ± 0.88 a | 4.38 ± 0.30 a | |
| HN | 371.11 ± 92.51 a | 352.00 ± 10.91 a | 297.06 ± 24.01 a | 12.67 ± 1.49 a | 9.18 ± 0.48 a | 7.38 ± 0.38 a | 1.02 ± 0.06 a | 1.33 ± 0.07 a | 1.32 ± 0.19 b | 28.35 ± 4.31 a | 38.62 ± 2.77 a | 40.39 ± 3.55 a | 359.85 ± 80.41 a | 265.70 ± 6.05 b | 234.61 ± 39.31 a | 12.41 ± 0.93 a | 6.95 ± 0.52 a | 5.86 ± 1.02 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Kuang, M.; Hei, Z.; Zhong, J.; Abdo, A.I.E.; Wei, H.; Zhang, J.; Xiang, H. Simulated Nitrogen Deposition Decreases the Ratios of Soil C to P and N to P, Changes Soil Enzyme Activity, and Reduces Soil Microbial Biomass in Paddy Soil in Southern China. Agronomy 2023, 13, 2249. https://doi.org/10.3390/agronomy13092249
Deng Y, Kuang M, Hei Z, Zhong J, Abdo AIE, Wei H, Zhang J, Xiang H. Simulated Nitrogen Deposition Decreases the Ratios of Soil C to P and N to P, Changes Soil Enzyme Activity, and Reduces Soil Microbial Biomass in Paddy Soil in Southern China. Agronomy. 2023; 13(9):2249. https://doi.org/10.3390/agronomy13092249
Chicago/Turabian StyleDeng, Yuhao, Meijie Kuang, Zewen Hei, Jiawen Zhong, Ahmed Ibrahim Elsayed Abdo, Hui Wei, Jiaen Zhang, and Huimin Xiang. 2023. "Simulated Nitrogen Deposition Decreases the Ratios of Soil C to P and N to P, Changes Soil Enzyme Activity, and Reduces Soil Microbial Biomass in Paddy Soil in Southern China" Agronomy 13, no. 9: 2249. https://doi.org/10.3390/agronomy13092249






