Biochar Applied in Places Where Its Feedstock Was Produced Mitigated More CO2 Emissions from Acidic Red Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Determination Methods
2.4. Data Analysis
3. Results
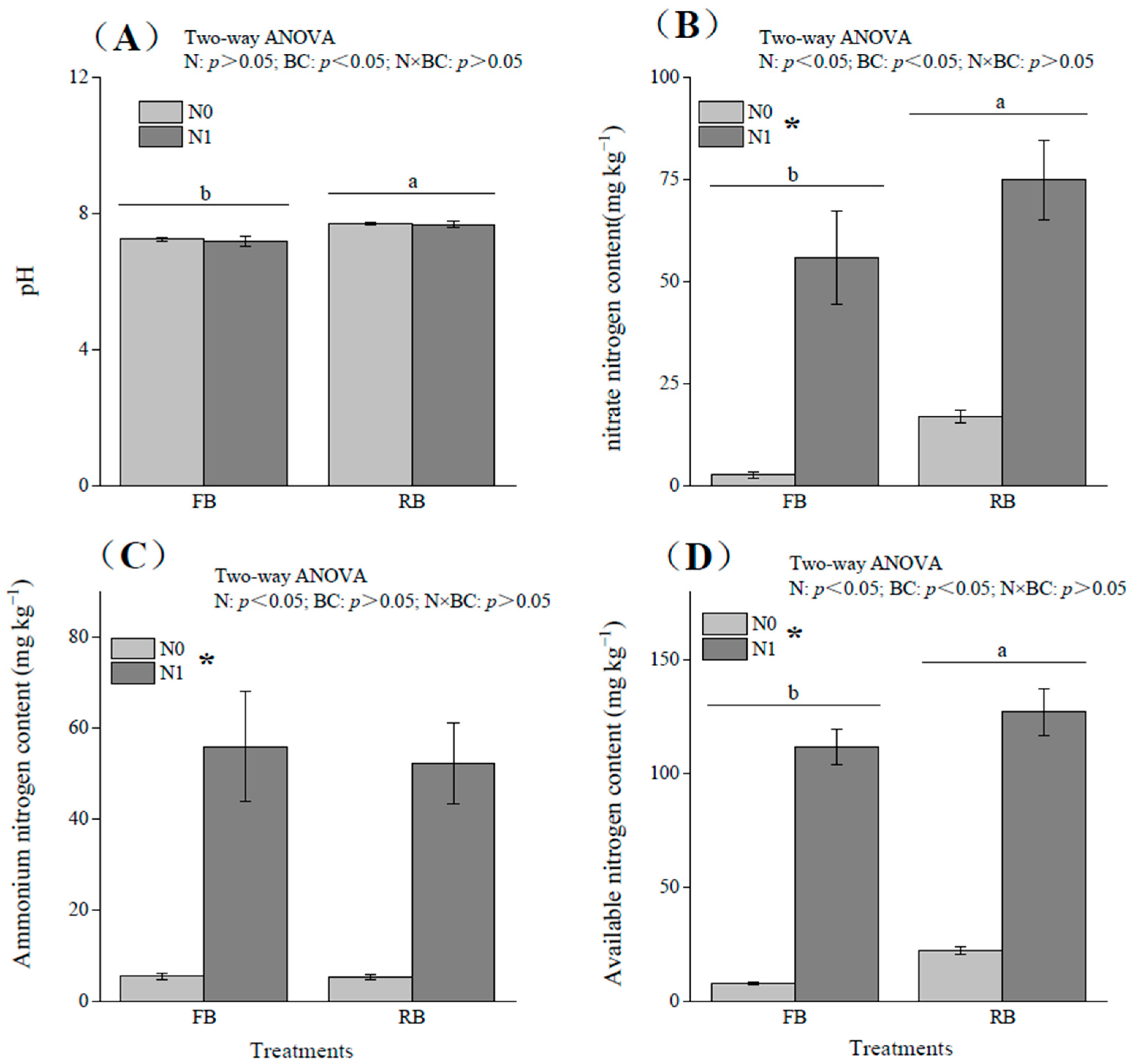
3.1. Effects of Different Treatments on Soil Physicochemical Properties
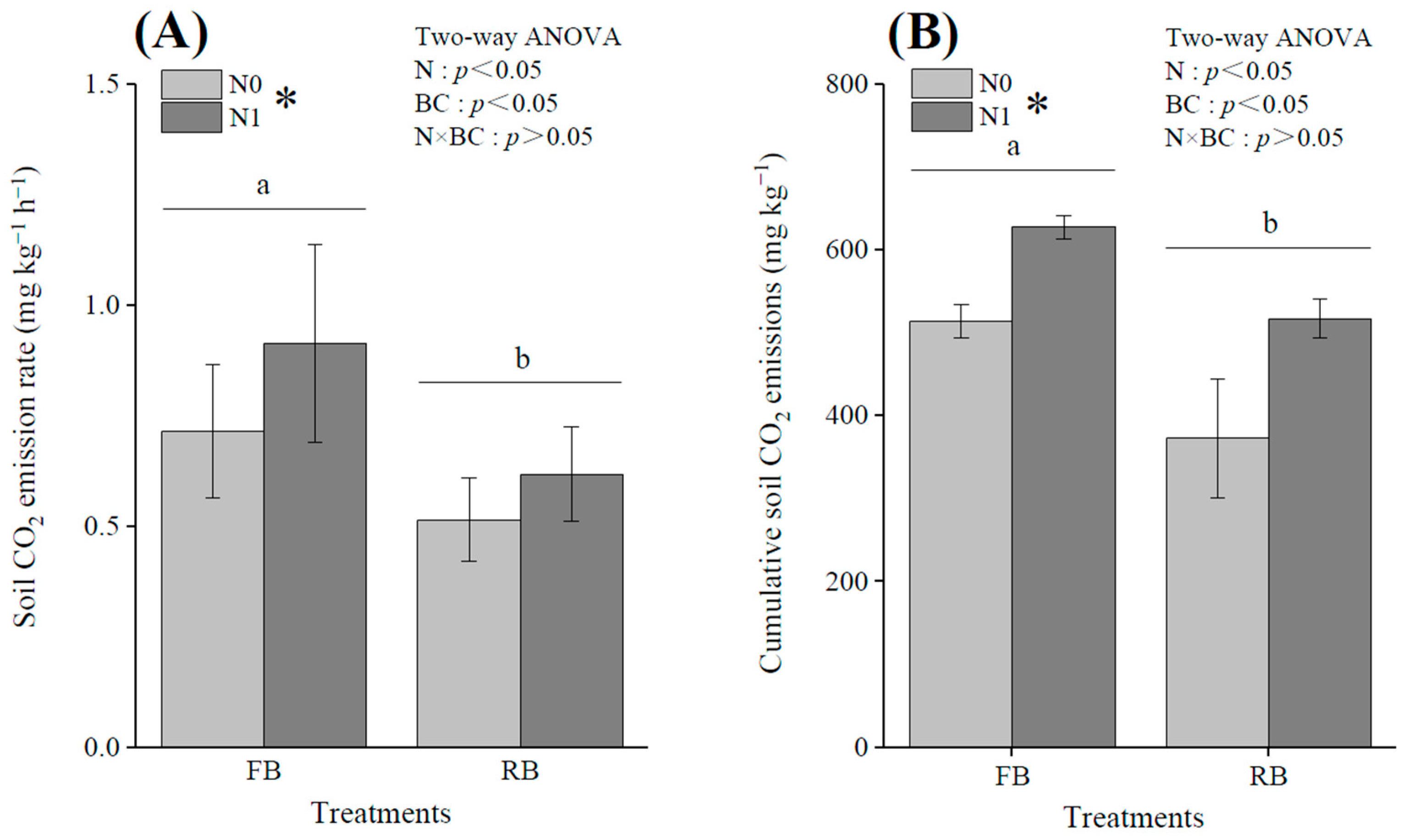
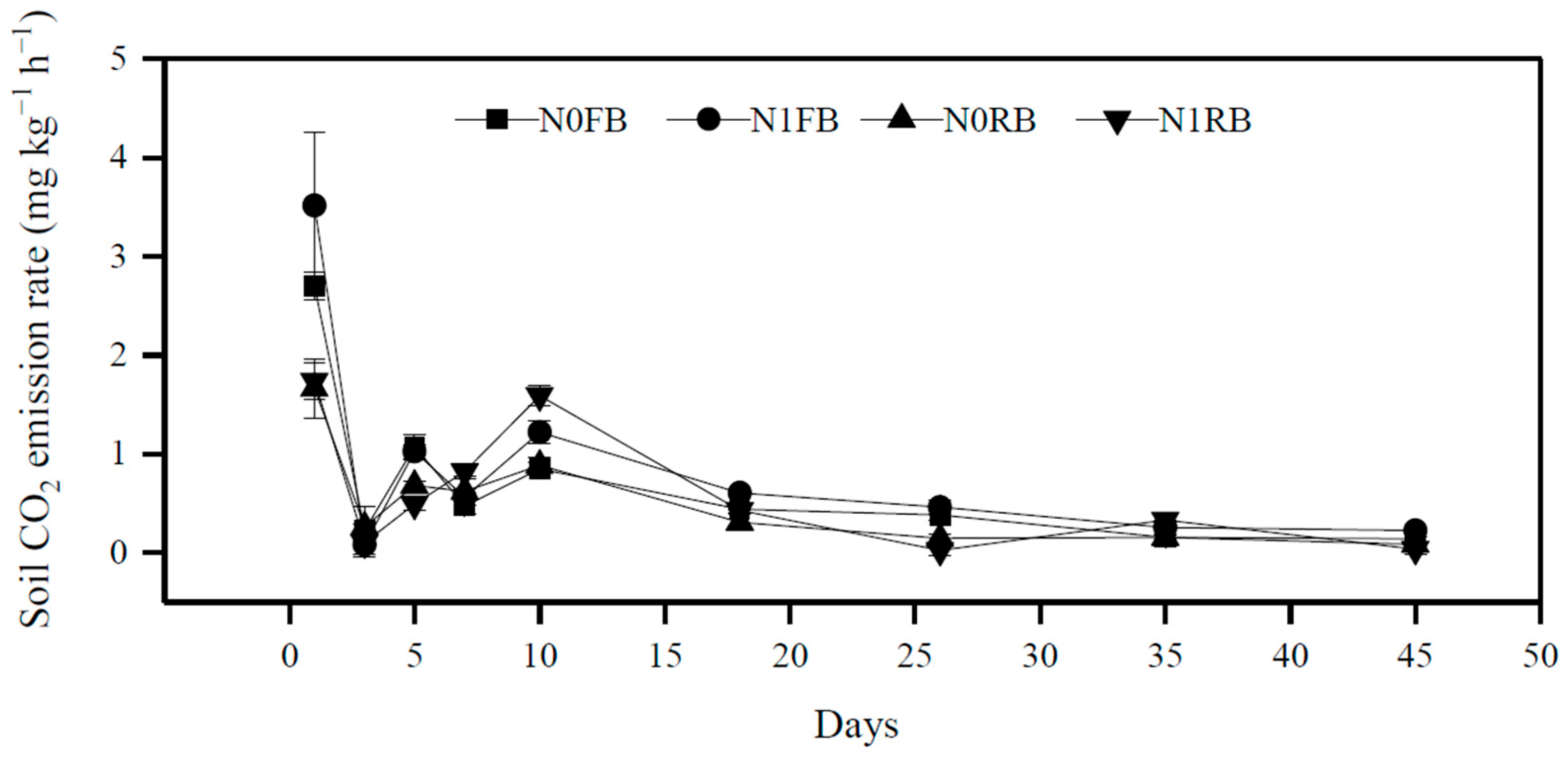
3.2. Effects of Different Treatments on Soil Cumulative CO2 Emissions and CO2 Emission Rate
4. Discussion
4.1. Effects of Different Biochar Additions on Soil CO2 Emissions and Soil Physicochemical Properties in Paddy Field
4.2. Effects of Interaction between Biochar and Nitrogen Fertilizer on Soil CO2 Emissions in Paddy Field
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Dietz, T.; Rosa, E.A. Effects of population and affluence on CO2 emissions. Proc. Natl. Acad. Sci. USA 1997, 94, 175–179. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Agriculture, Forestry and Other Land Use (AFOLU). In Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Guo, Z.; Liu, C.-A.; Hua, K.; Wang, D.; Wu, P.; Wan, S.; He, C.; Zhan, L.; Wu, J. Changing soil available substrate primarily caused by fertilization management contributed more to soil respiration temperature sensitivity than microbial community thermal adaptation. Sci. Total Environ. 2024, 912, 169059. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Hansen, J.E.; Lacis, A.A. Sun and dust versus greenhouse gases: An assessment of their relative roles in global climate change. Nature 1990, 346, 713–719. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Motsi, H.; Manyevere, A.; Poswa, S.B. Assessing the Potential of Biochar as a Viable Alternative to Synthetic Fertilizers in Sub-Saharan Africa Smallholder Farming: A Review. Agronomy 2024, 14, 1215. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Liu, X.; Huang, Y.; Cai, A.; Xu, H.; Ran, J.; Xiao, J.; Zhang, W. A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 2024, 11, 57. [Google Scholar] [CrossRef]
- Wang, B.; Gao, Y.; Lai, X.; Luo, L.; Zhang, X.; Hu, D.; Shen, Z.; Hu, S.; Zhang, L. The effects of biochar derived from feedstock with different Si and Al concentration on soil N2O and CO2 emissions. Environ. Pollut. 2023, 317, 120731. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Yang, C.; Xin, X.; Zheng, J.; Zong, T.; Dou, C. Effects of Biochar on Gaseous Carbon and Nitrogen Emissions in Paddy Fields: A Review. Agronomy 2024, 14, 1461. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F. Biochar production and characteristics, its impacts on soil health, crop production, and yield enhancement: A review. Plants 2024, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Azzi, E.S.; Li, H.; Cederlund, H.; Karltun, E.; Sundberg, C. Modelling biochar long-term carbon storage in soil with harmonized analysis of decomposition data. Geoderma 2024, 441, 116761. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Jin, L.; Yang, B. Properties of biochars derived from different straw at 500 °C pyrolytic temperature: Implications for their use to improving acidic soil water retention. Agric. Water Manag. 2024, 301, 108953. [Google Scholar] [CrossRef]
- Mona, S.; Saini, N.; Kumar, S.; Sharma, A.; Yadav, A.; Yadav, N.; Deepak, B. Characterization and utilization of algal and wheat husk biochar as biofertilizers for sustainable soil amelioration. Bioresour. Technol. Rep. 2024, 27, 101893. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Wu, Y.; Wang, H.; Chen, Y.; Wu, W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments 2011, 11, 930–939. [Google Scholar] [CrossRef]
- Jiang, M.D.; He, Z.L.; Sun, Y.; Zhou, W.; Hu, R.G.; Lin, S. The effect of wheat-straw derived biochar on the soil pH and emissions of CO2 and CH4 from tea garden soil. J. Agro-Environ. Sci. 2018, 37, 196–204. [Google Scholar]
- Sun, Y.; He, Z.L.; Lin, S.; Zhang, S.Q.; Liu, W.Y. Effects of different biochars on N2O and CO2 emission from acidified tea field soil. J. Agro-Environ. Sci. 2017, 36, 2544–2552. [Google Scholar]
- Kong, F.; Jiu, A.; Kan, Z.; Zhou, J.; Yang, H.; Li, F.-M. Deep tillage combined with straw biochar return increases rice yield by improving nitrogen availability and root distribution in the subsoil. Field Crops Res. 2024, 315, 109481. [Google Scholar] [CrossRef]
- Huang, H.; Ge, L.; Zhang, X.; Chen, H.; Shen, Y.; Xiao, J.; Lu, H.; Zhu, Y.; Han, J.; Li, R. Rice straw biochar and lime regulate the availability of heavy metals by managing colloid-associated- but dissolved-heavy metals. Chemosphere 2024, 349, 140813. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, S.; Chen, F.; Xu, X.; Deng, B.; Fang, X.-M.; Liu, Y.; Siemann, E.; Zhang, L. Non-additive effects of Chinese fir leaf litter mixtures of different ages on soil N2O emissions in a monoculture plantation treated with N and P additions. Plant Soil 2024. [Google Scholar] [CrossRef]
- Xu, X.; He, C.; Yuan, X.; Zhang, Q.; Wang, S.; Wang, B.; Guo, X.; Zhang, L. Rice straw biochar mitigated more N2O emissions from fertilized paddy soil with higher water content than that derived from ex situ biowaste. Environ. Pollut. 2020, 263, 114477. [Google Scholar] [CrossRef]
- Lin, Y.; Ding, W.; Liu, D.; He, T.; Yoo, G.; Yuan, J.; Chen, Z.; Fan, J. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Gao, W.; Tu, Q.; Tang, X.; Duan, S.; Zeng, J.; Wang, B.; Xu, J. Characteristics of lignin isolated from an agricultural by-product: Camellia oleifera shell. Ind. Crops Prod. 2024, 219, 118973. [Google Scholar] [CrossRef]
- Zeng, B.; Zeng, X.; Hu, L.; Huang, L.; Huang, Y.; Zhou, Y.; Liu, G.; Huang, W. Activated carbon from Camellia oleifera shells for adsorption of Y (iii): Experimental and DFT studies. RSC Adv. 2024, 14, 4252–4263. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, B.; Luo, L.; Deng, B.; Shad, N.; Hu, D.; Aly, H.M.; Zhang, L. Effects of hydroxyapatite and modified biochar derived from Camellia oleifera fruit shell on soil Cd contamination and N2O emissions. Ind. Crops Prod. 2022, 177, 114476. [Google Scholar] [CrossRef]
- Fang, H.; Xu, X.; Zhang, Q.; Wang, S.; Yu, Y.; Wang, H.; Muhammad, A.; Yang, Y.; Aly, H.M.; Hu, D.; et al. Biochar mitigated more N2O emissions from soils with the same origin ecosystem as its feedstocks: Implications for application of biochar. J. Soils Sediments 2024, 24, 2458–2466. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Taylor & Francis: Abingdon, UK, 2024. [Google Scholar]
- Ghorbani, M.; Amirahmadi, E. Insights into soil and biochar variations and their contribution to soil aggregate status–A meta-analysis. Soil Tillage Res. 2024, 244, 106282. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci. Total Environ. 2014, 487, 26–36. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Allison, S.D.; Czimczik, C.I.; Treseder, K.K. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob. Change Biol. 2008, 14, 1156–1168. [Google Scholar] [CrossRef]
- Cheng, X.; Kang, F.; Han, H.; Liu, H.; Zhang, Y. Effect of thinning on partitioned soil respiration in a young Pinus tabulaeformis plantation during growing season. Agric. For. Meteorol. 2015, 214–215, 473–482. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.; Yi, M.; Xie, H.; Chen, T.; Xie, W.; He, L.; Wang, X.; Liu, L.; Zhang, L. Biochar Applied in Places Where Its Feedstock Was Produced Mitigated More CO2 Emissions from Acidic Red Soils. Agronomy 2024, 14, 2193. https://doi.org/10.3390/agronomy14102193
Lai M, Yi M, Xie H, Chen T, Xie W, He L, Wang X, Liu L, Zhang L. Biochar Applied in Places Where Its Feedstock Was Produced Mitigated More CO2 Emissions from Acidic Red Soils. Agronomy. 2024; 14(10):2193. https://doi.org/10.3390/agronomy14102193
Chicago/Turabian StyleLai, Meng, Min Yi, Haiping Xie, Tingxuan Chen, Wenlei Xie, Lei He, Xiaodong Wang, Liangying Liu, and Ling Zhang. 2024. "Biochar Applied in Places Where Its Feedstock Was Produced Mitigated More CO2 Emissions from Acidic Red Soils" Agronomy 14, no. 10: 2193. https://doi.org/10.3390/agronomy14102193






