Trypsin Inhibitor of Ricinus communis L. (Euphorbiaceae) in the Control of Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Castor Oil Plant Leaf Meal (CLM)
2.2. Insects
2.3. In Vitro S. frugiperda Trypsin Inhibition Assays
2.4. Optimization of the Extraction of S. frugiperda Trypsin Inhibitor Present in CLM
2.4.1. 24 Factorial Design
2.4.2. Optimization of the Extraction Parameters for the S. frugiperda Trypsin Inhibitor Present in CLM Using a 22 Central Composite Design (22 CCD)
2.5. Purification and Identification of Substances
2.6. Effects of the CLM Extract on S. frugiperda Development
2.7. Protein Concentration in S. frugiperda Feces
2.8. Statistical Analysis
3. Results and Discussion
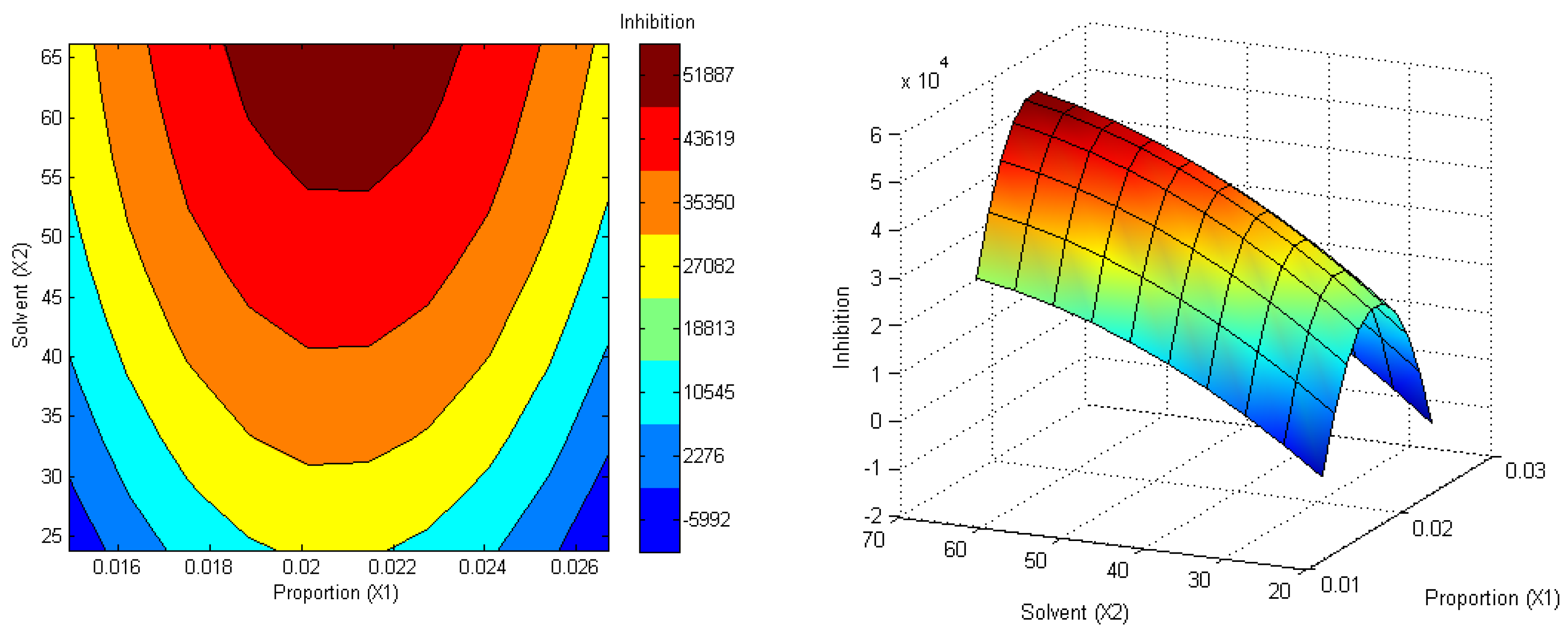
3.1. Optimization of CLM Trypsin Inhibitor Extraction
3.2. Chromatographic Purification
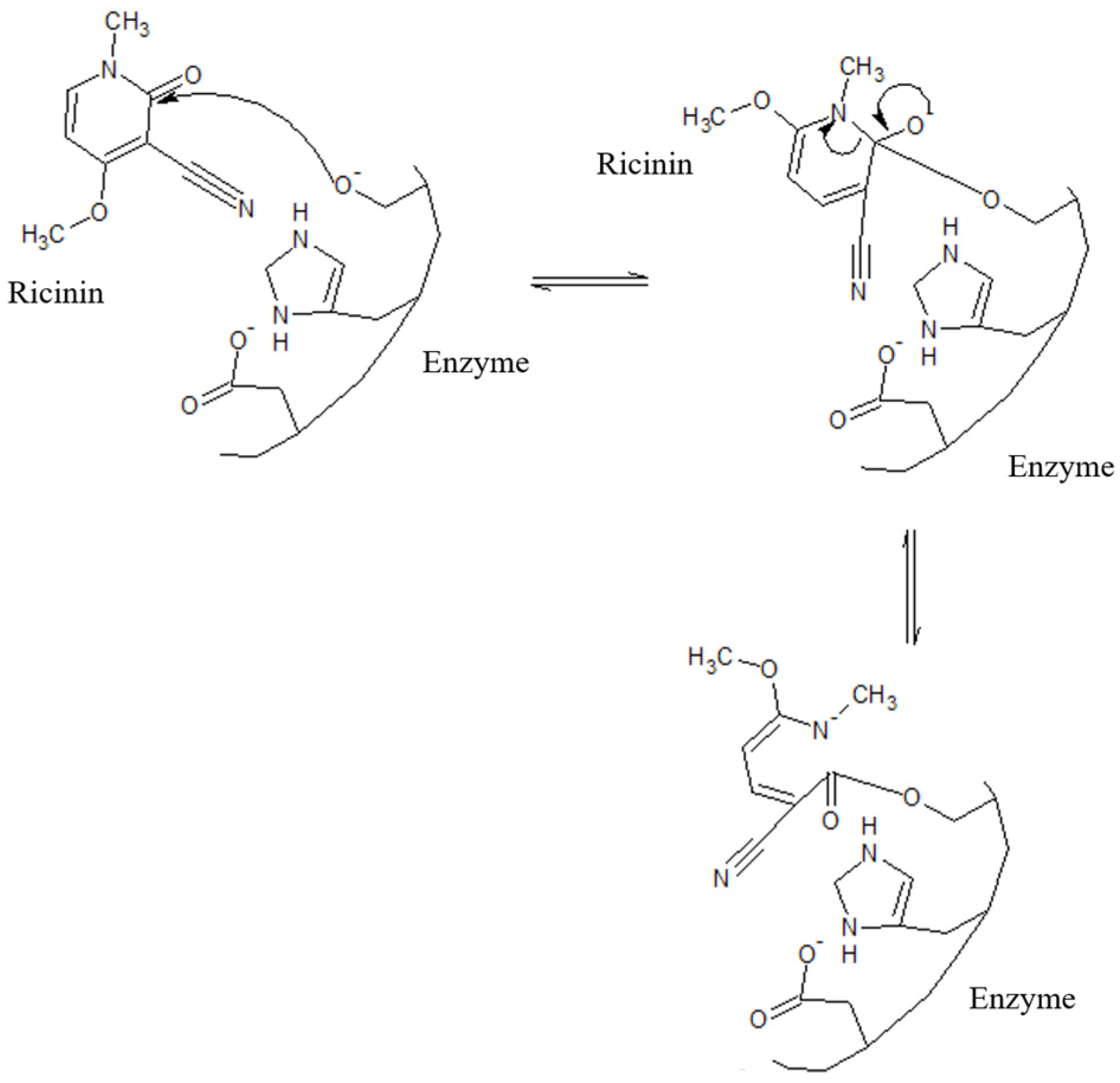
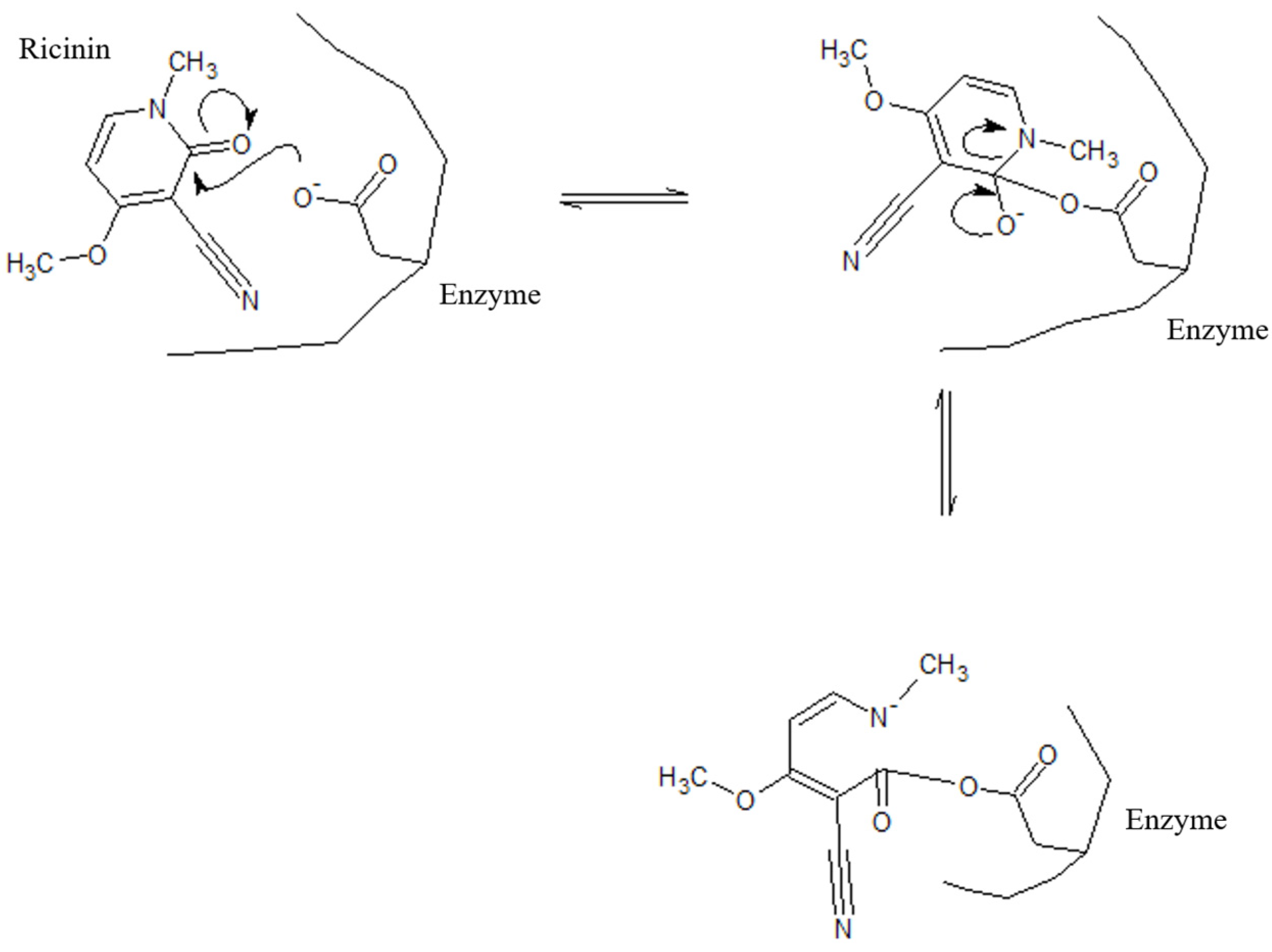
3.3. Identification and Characterization by Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry
3.4. Effect of Castor Oil Plant Leaf Extract on S. frugiperda Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, S.J.; McCord, E. Lack of cross-resistance to indoxacarb in insecticide-resistant Spodoptera frugiperda (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Yponomeutidae). Pest. Manag. Sci. 2007, 63, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.R.B.; do Farias, J.R.; Bernardi, D.; Horikoshi, R.J.; Omoto, C. Genetic basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) resistance to the chitin synthesis inhibitor lufenuron. Pest. Manag. Sci. 2016, 72, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Mayorga, E.V.; Bello-Ruiz, D.G.; Paredes-Sánchez, F.A.; Segovia-Tagle, V.; García-Aguirre, K.K.; Lara-Ramírez, E.E.; Rivera, G. Identification of Snp’s in the Ace-1 Gene of Spodoptera frugiperda associated with resistance to organophosphorus insecticides. S. Entomol. 2018, 43, 855–865. [Google Scholar] [CrossRef]
- Van den Berg, J.; Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef]
- Monnerat, R.; Martins, E.; Macedo, C.; Queiroz, P.; Praça, L.; Soares, M.; Moreira, H.; Grisi, I.; Silva, J.; Soberon, M.; et al. Evidence of field-evolved resistance of Spodoptera frugiperda to Bt corn expressing Cry1F in Brazil that is still sensitive to modified Bt toxins. PLoS ONE 2015, 10, e0119544. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest. Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef]
- Chandrasena, D.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis derived Cry1Fδ endotoxin in Spodoptera frugiperda populations from Argentina. Pest. Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Blanco, C.A.; Portilla, M.; Adamczyk, J.; Luttrell, R.; Huang, F. Evidence of multiple/cross resistance to Bt and organophosphate insecticides in Puerto Rico population of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 2015, 122, 15–21. [Google Scholar] [CrossRef]
- de Tavares, W.S.; Cruz, I.; Petacci, F.; de Assis Júnior, S.L.; de Sousa Freitas, S.; Zanuncio, J.C.; Serrão, J.E.; de Assis, S.L.; Freitas, S.D.; Zanuncio, J.C.; et al. Potential use of Asteraceae extracts to control Spodoptera frugiperda (Lepidoptera: Noctuidae) and selectivity to their parasitoids Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) and Telenomus remus (Hymenoptera: Scelionidae). Ind. Crops Prod. 2009, 30, 384–388. [Google Scholar] [CrossRef]
- Freitas, A.F.; Pereira, F.F.; Formagio, A.S.N.; Lucchetta, J.T.; Vieira, M.C.; Mussury, R.M. Effects of methanolic extracts of Annona species on the development and reproduction of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 446–452. [Google Scholar] [CrossRef]
- Bibiano, C.S.; Alves, D.S.; Freire, B.C.; Bertolucci, S.K.V.; Carvalho, G.A. Toxicity of EOs and pure compounds of Lamiaceae species against Spodoptera frugiperda (Lepidoptera: Noctuidae) and their safety for the nontarget organism Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Crop Protect. 2022, 158, 106011. [Google Scholar] [CrossRef]
- Oliveira, J.A.C.; Fernandes, L.A.; Figueiredo, K.G.; Correa, E.J.A.; Lima, L.H.F.; Alves, D.S.; Bertoucci, S.K.; Carvalho, G.A. Effects of essential oils on biological characteristics and potential molecular targets in Spodoptera frugiperda. Plants 2024, 13, 1801. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Corigliano, M.; Pariani, S.A.; Sánches-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant serine protease inhibitors: Biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a Kunitz-type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef]
- Hamza, R.; Pérez-Hedo, M.; Urbaneja, A.; Rambla, J.L.; Granell, A.; Gaddour, K.; Beltrán, J.P.; Cañas, L.A. Expression of two barley proteinase inhibitors in tomato promotes endogenous defensive response and enhances resistance to Tuta absoluta. BMC Plant Biol. 2018, 18, 24. [Google Scholar] [CrossRef]
- Ramos-Lopez, M.A.; Perez-G, S.; Rodriguez-Hernandez, C.; Guevara-Fefer, P.; Zavala-Sanchez, M.A. Activity of Ricinus communis (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Afr. J. Biotechnol. 2010, 9, 1359–1365. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr. Protein Pept. Sci. 2011, 12, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.D.; Santos, C.D.; Carvalho, G.A.; Alves, D.S.; Pereira, L.L.S.; Carvalho, G.A. Biochemical analysis of a castor oil plant bean leaf extract and its insecticidal effects against Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2012, 41, 503–509. [Google Scholar] [CrossRef]
- Ramos, V.; Alves, D.; Braga, M.; Carvalho, G.; Santos, C. Extraction and isolation of anti-tryptic castor oil plant-bean (Ricinus communis L.) substances and their effects on Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2013, 73, 128–134. [Google Scholar] [CrossRef]
- Tirelli, A.A.; Alves, D.S.; Carvalho, G.A.; Samia, R.R.; Brum, S.S.; Guerreiro, M.C. Effect of tanical fractions on biological and nutritional parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae). Cienc. E Agrotecnologia 2010, 34, 1417–1424. [Google Scholar] [CrossRef]
- Divekar, P.A.; Rani, V.; Majumder, S.; Karkute, S.G.; Molla, K.A.; Pandey, K.K.; Behera, T.K.; Pandi, G.P. Protease inhibitors: An induced plant defense mechanism against herbivores. J. Plant. Growth. Regul. 2023, 42, 6057–6073. [Google Scholar] [CrossRef]
- Ye, M.; Liu, C.; Li, N.; Yuan, C.; Liu, M.; Xin, Z.; Lei, S.; Sun, X. A constitutive serine protease inhibitor suppresses herbivore performance in tea (Camellia sinensis). Hortic. Res. 2023, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.A.; Beekwilder, J. Co-evolution of insect proteases and plant protease inhibitors. Curr. Protein Pept. Sci. 2011, 12, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.A.; Santos, C.D.; Alves, D.S.; Carvalho, G.A.; Cardoso, M.G.; Haro, M.M. Toxic effects of Ricinus communis non-protein trypsin inhibitor on Spodoptera frugiperda (J. E. Smith) (Lepidoptera:Noctuidae). Afr. J. Biotechnol. 2015, 14, 2928–2936. [Google Scholar]
- Seidel, V. Initial and bulk extraction of natural products isolation. In Natural Products Isolation; Methods in Molecular Biology; Sarker, S.D., Nahar, L., Eds.; Springer Science: New York, NY, USA, 2012; Volume 864, pp. 27–41. [Google Scholar]
- Parra, J.R.P. Técnicas de Criação de Insetos para Programas de Controle Biológico; ESALQ/FEALQ: Piracicaba, Brasil, 2001. [Google Scholar]
- Erlanger, B.F.; Cohen, W.; Kokowsky, N. Preparation and properties of 2 new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Teofilo, R.F.; Ferreira, M.M.C. Chemometrics II: Spreadsheets for experimental design calculations, a tutorial. Quim. Nova 2006, 29, 338–350. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nunes, C.A.; Freitas, M.P.; Pinheiro, A.C.M.; Bastos, S.C. Chemoface: A Novel Free User-Friendly Interface for Chemometrics. J. Braz. Chem. Soc. 2012, 23, 2003–2010. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Zhao, H.-Q.; Wang, X.; Li, H.-M.; Yang, B.; Yang, H.-J.; Huang, L. Characterization of nucleosides and nucleobases in natural Cordyceps by HILIC-ESI/TOF/MS and HILIC-ESI/MS. Molecules 2013, 18, 9755–9769. [Google Scholar] [CrossRef]
- Li, W.-L.; Yu, L.; Ji, Y.-B. Chemical constituents of n-butanol extract of Capparis spinosa L. Asian J. Chem. 2014, 26, 3435–3437. [Google Scholar] [CrossRef]
- Peng, J.; Cai, S.; Wang, L.; Zhao, N.; Zhang, T.; Chen, Z.; Meng, F. A metabonomic analysis of serum from rats treated with ricinine using ultra performance liquid chromatography coupled with mass spectrometry. PLoS ONE 2014, 9, e90416. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wei, D.; Wang, J.; Dong, G.; Zhang, Q.; Yang, M.; Kong, L. Chronic toxicity of crude ricinine in rats assessed by 1 H NMR metabolomics analysis. RSC Adv. 2015, 5, 27018–27028. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhary, A.K.; Sharma, R. Analgesic and anti-inflammatory potential of Ricinus communis Linn.: Evidence from pharmacology to clinical studies. Curr. Pharmacol. Rep. 2024, 10, 27–67. [Google Scholar] [CrossRef]
- Almeida-Castro, V.T.; Sobrinho, T.J.; Corrêa, A.J.; Araújo, T.A.S.; Silva, T.G.; Amorin, E.L. The anticholinesterase properties of plants from the northeast of Brazil selected by an ethnopharmacological study for disorders relating to the nervous system. Pharmacogn. Mag. 2016, 12, S195–S200. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.C.; Angelucci, M.E.M.; Da Costa, M.L.; Batista, I.R.; De Oliveira, B.H.; Da Cunha, C. Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from Ricinus communis. Pharmacol. Biochem. Behav. 1999, 63, 367–375. [Google Scholar] [CrossRef]
- Khan, B.; Ghous, T.; Rasheed, A.; Yasin, K.A.; Ahmed, M.N.; Andleeb, S. Evaluation of anti-acetylcholinesterase activity and antioxidant potential of ricinine (a Central Nervous System Stimulant) isolated from Ricinius communis L. J. Chem. Soc. Pak. 2016, 38, 326. [Google Scholar]
- Bullangpoti, V.; Khumrungsee, N.; Pluempanupat, W.; Kainoh, Y.; Saguanpong, U. Toxicity of ethyl acetate extract and ricinine from Jatropha gossypifolia senescent leaves against Spodoptera exigua Hübner (Lepidoptera: Noctuidae). J. Pestic. Sci. 2011, 36, 260–263. [Google Scholar] [CrossRef]
- Flores-Macías, A.; Vela-Correa, G.; de Rodríguez-Gamiño, M.L.; Akhtar, Y.; Figueroa-Brito, R.; Pérez-Moreno, V.; Rico-Rodríguez, M.A.; Ramos-López, M.A. Effect of potassium nitrate on the production of ricinine by Ricinus communis and its insecticidal activity against Spodoptera frugiperda. Rev. Fitotec. Mex. 2016, 39, 41–47. [Google Scholar] [CrossRef]
- Lin, S.J.; Dong, K.C.; Eigenbrot, C.; van Lookeren Campagne, M.; Kirchhofer, D. Structures of neutrophil serine protease 4 reveal an unusual mechanism of substrate recognition by a Trypsin-fold protease. Structure 2014, 22, 1333–1340. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, S. A trypsin homolog in amphioxus: Expression, enzymatic activity and evolution. Mol. Biol. Rep. 2012, 39, 1745–1753. [Google Scholar] [CrossRef]
- Brioschi, D.; Nadalini, L.D.; Bengtson, M.H.; Sogayar, M.C.; Moura, D.S.; Silva-Filho, M.C. General up regulation of Spodoptera frugiperda trypsins and chymotrypsins allows its adaptation to soybean proteinase inhibitor. Insect Biochem. Mol. Biol. 2007, 37, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Paulillo, L.C.M.S.; Lopes, A.R.; Cristofoletti, P.T.; Parra, J.R.P.; Terra, W.R.; Silva-Filho, M.C. Changes in midgut endopeptidase activity of Spodoptera frugiperda (Lepidoptera: Noctuidae) are responsible for adaptation to soybean proteinase inhibitors. J. Econ. Entomol. 2009, 93, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.O.; Silva-Filho, M.C. Plant-insect interactions: An evolutionary arms race between two distinct defense mechanisms. Braz. J. Plant Physiol. 2002, 14, 71–78. [Google Scholar] [CrossRef]
- Moon, J.; Salsman, R.A.; Ahn, J.E.; Koiwa, H.; Zhu-Salzman, K. Transcriptional regulation in cowpea bruched guts during adaptation to a plant defense protease inhibitor. Insect Mol. Biol. 2004, 13, 283–297. [Google Scholar] [CrossRef]
- Hivrale, V.K.; Lomate, P.R.; Basaiyye, S.S.; Kalve, N.D. Compensatory proteolytic responses to dietary proteinase inhibitors from Albizia lebbeck seeds in the Helicoverpa armigera larvae. Arthropod. Plant. Interact. 2013, 7, 259–266. [Google Scholar] [CrossRef]



| Fraction 4 | Fraction 5 | ||
|---|---|---|---|
| Peak Retention Time | % Inhibition | Peak Retention Time | % Inhibition |
| 7.00 | 70.69 | 13.01 | 62 |
| 10.92 | 68.68 | 15.73 | 43.18 |
| 13.38 | 69.35 | 17.07 | 63.87 |
| 14.30 | 70.47 | - | - |
| 16.93 | 61.07 | - | - |
| 21.33 | 62.98 | - | - |
| 24.61 | 68.68 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, V.O.; Alves, D.S.; Carvalho, G.A.; Santos, C.D.; Cavalheiro, A.J.; Oliveira, J.A.C.; Marques, T.R.; Simão, A.A.; Saczk, A.A. Trypsin Inhibitor of Ricinus communis L. (Euphorbiaceae) in the Control of Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae). Agronomy 2024, 14, 2222. https://doi.org/10.3390/agronomy14102222
Ramos VO, Alves DS, Carvalho GA, Santos CD, Cavalheiro AJ, Oliveira JAC, Marques TR, Simão AA, Saczk AA. Trypsin Inhibitor of Ricinus communis L. (Euphorbiaceae) in the Control of Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae). Agronomy. 2024; 14(10):2222. https://doi.org/10.3390/agronomy14102222
Chicago/Turabian StyleRamos, Vinicius O., Dejane S. Alves, Geraldo A. Carvalho, Custódio D. Santos, Alberto J. Cavalheiro, Júlia A. C. Oliveira, Tamara R. Marques, Anderson A. Simão, and Adelir A. Saczk. 2024. "Trypsin Inhibitor of Ricinus communis L. (Euphorbiaceae) in the Control of Spodoptera frugiperda (Smith, 1797) (Lepidoptera: Noctuidae)" Agronomy 14, no. 10: 2222. https://doi.org/10.3390/agronomy14102222






