Organic Management Mediates Multifunctionality Responses to Land Conversion from Longan (Dimocarpus longan) to Tea Plantations at the Aggregate Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Land Uses
2.3. Soil Sampling and Aggregate Size Fractionation
2.4. Soil Chemical Analysis
2.5. Soil Microbial Community Analysis
2.6. Soil Extracellular Enzyme Assays
2.7. Soil Multifunctionality Index
2.8. Statistical Analysis
3. Results
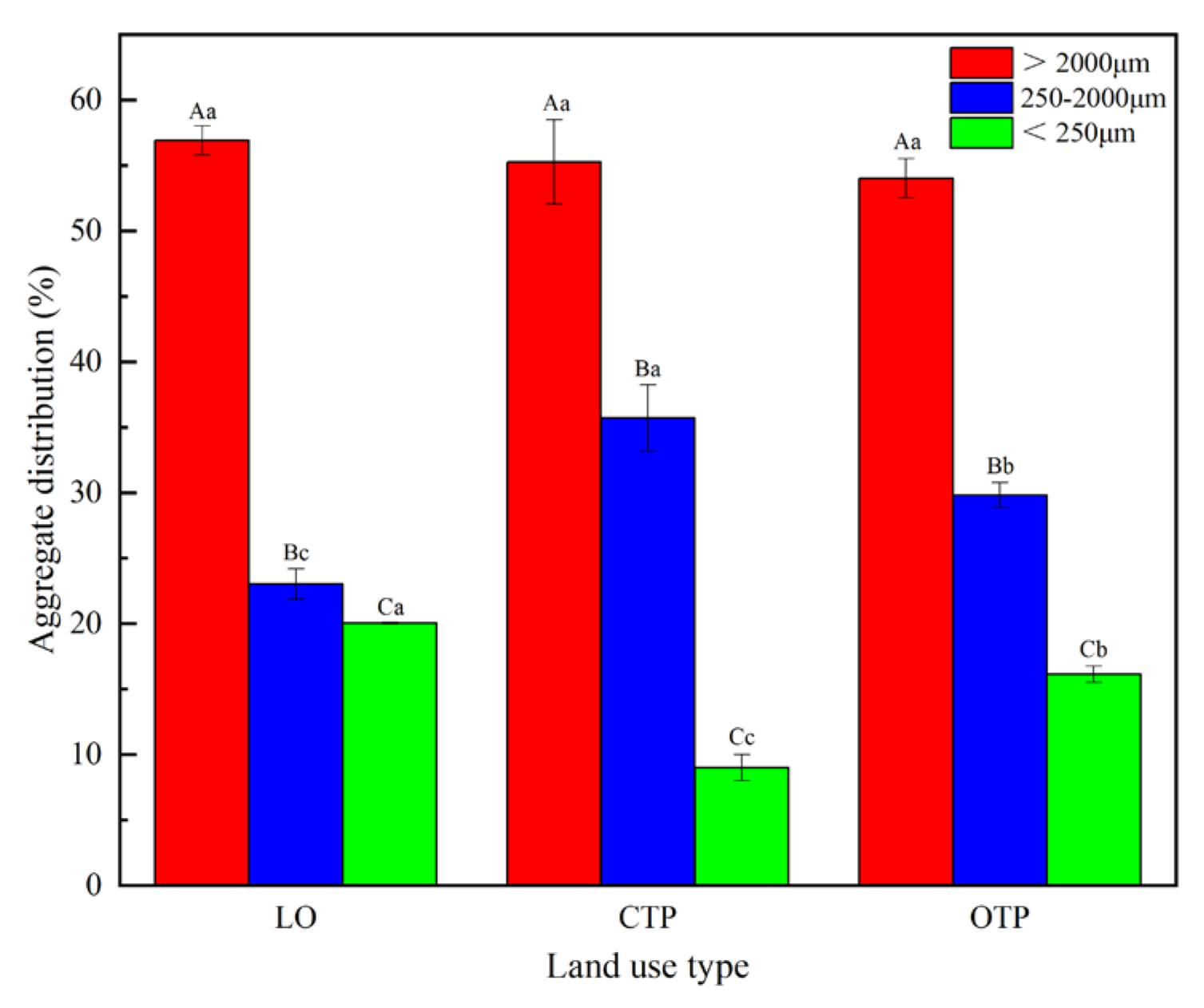
3.1. Soil Aggregate Fractions
3.2. Physicochemical Properties of Soil Aggregates with Different Land Use Types
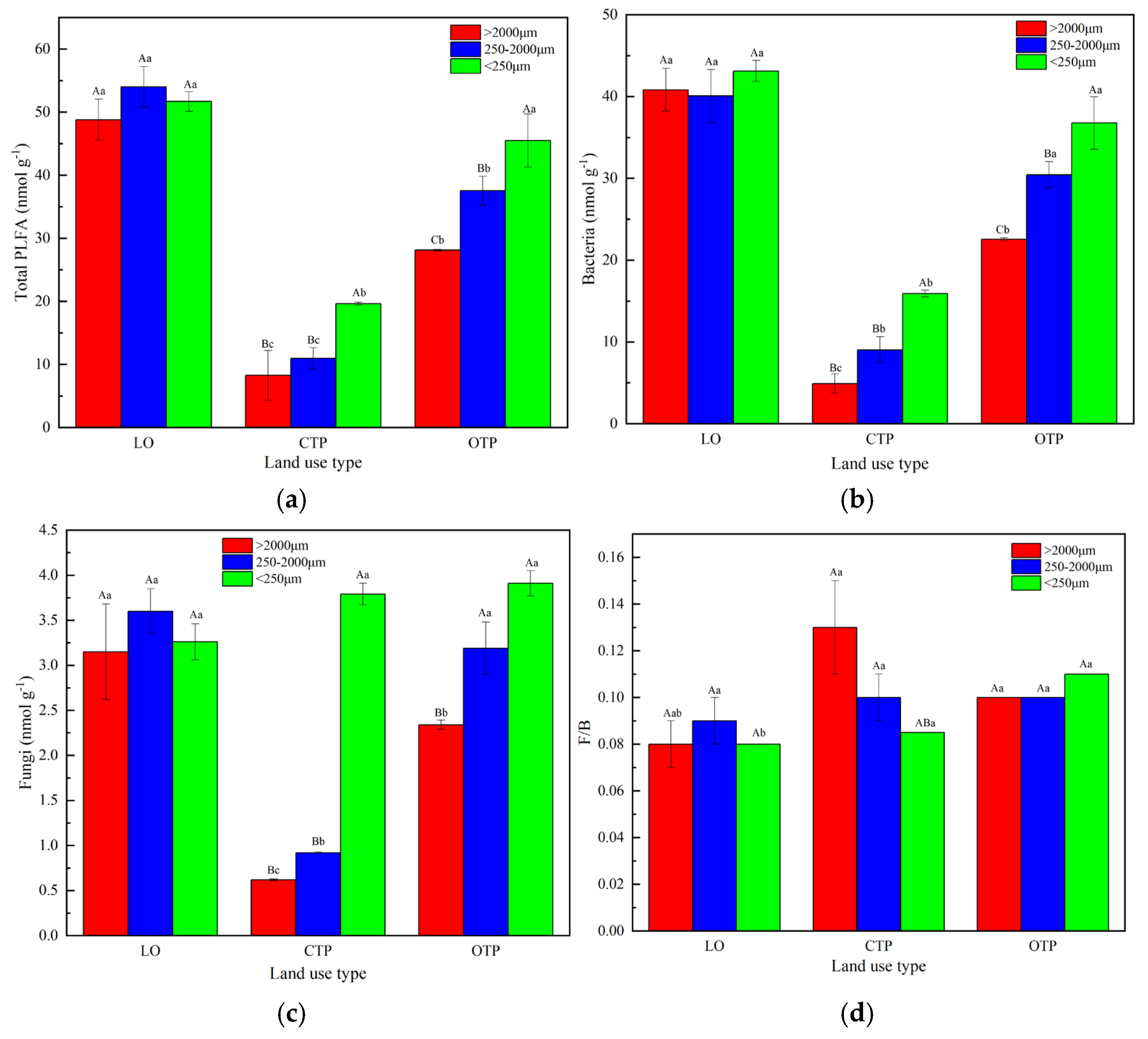
3.3. Soil Microbial Communities within Aggregate Fractions
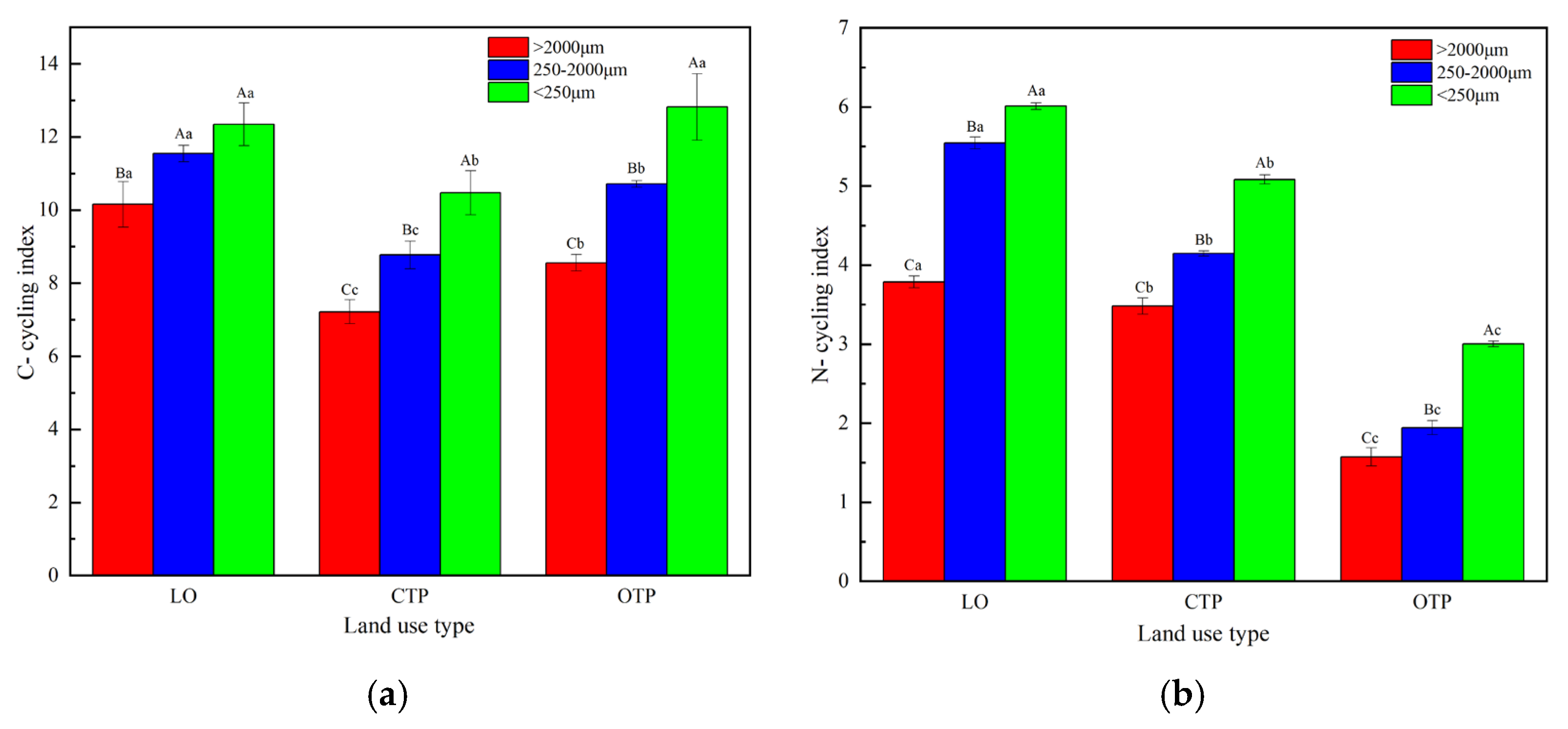
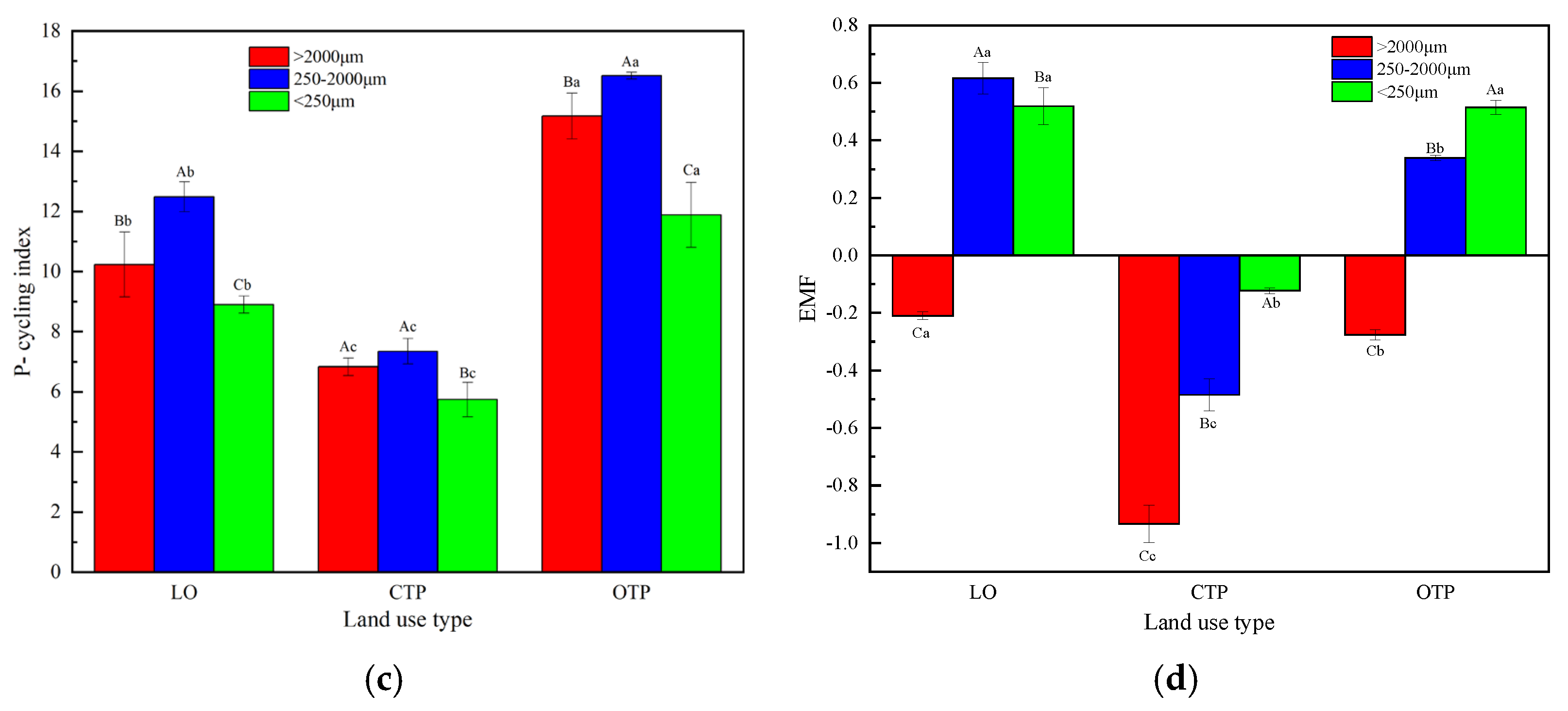
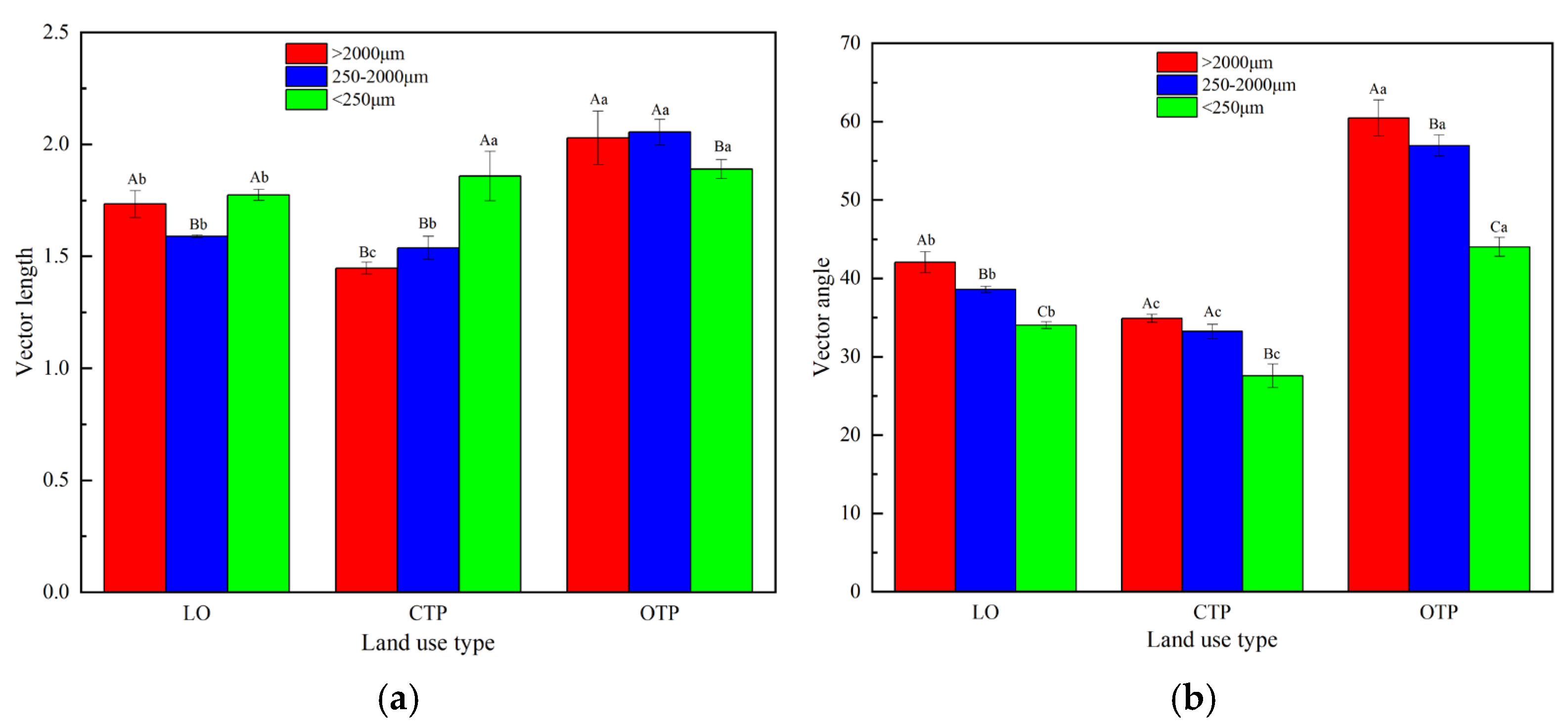
3.4. Soil Aggregate-Related Nutrient Cycling Indices, Extracellular Enzyme Stoichiometries, and Ecosystem Multifunctionality
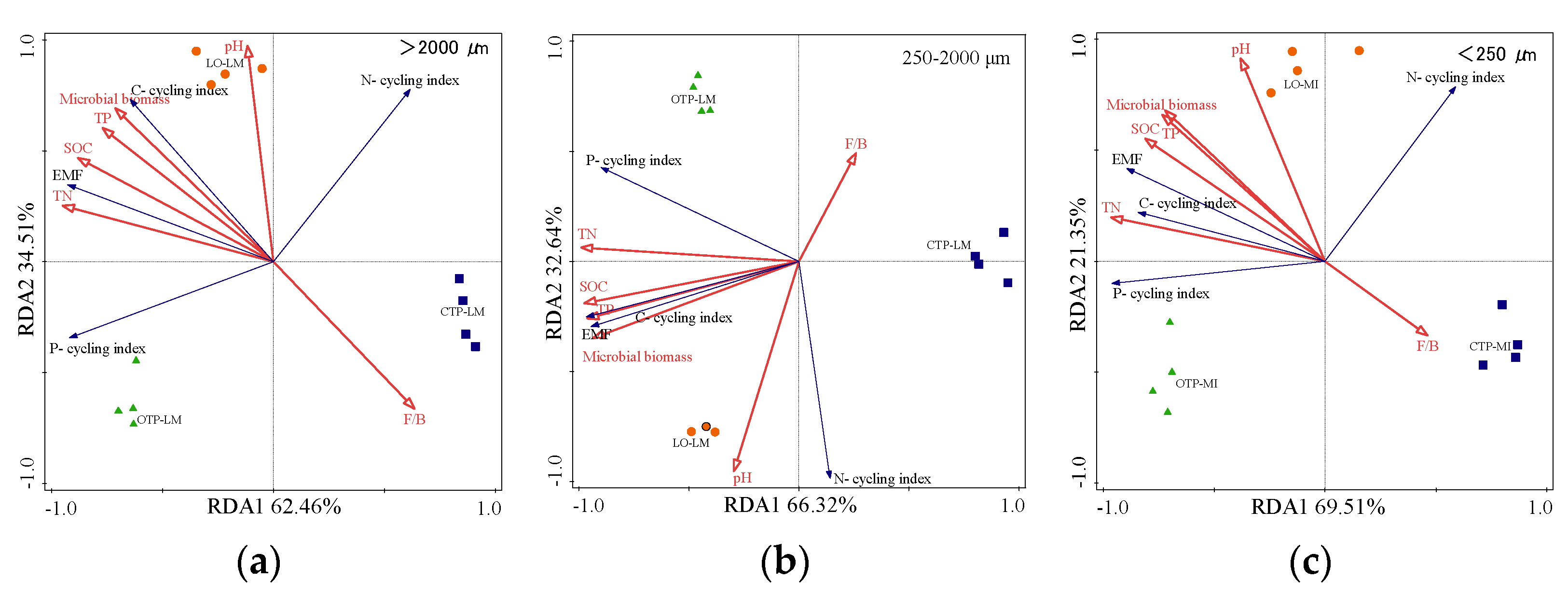
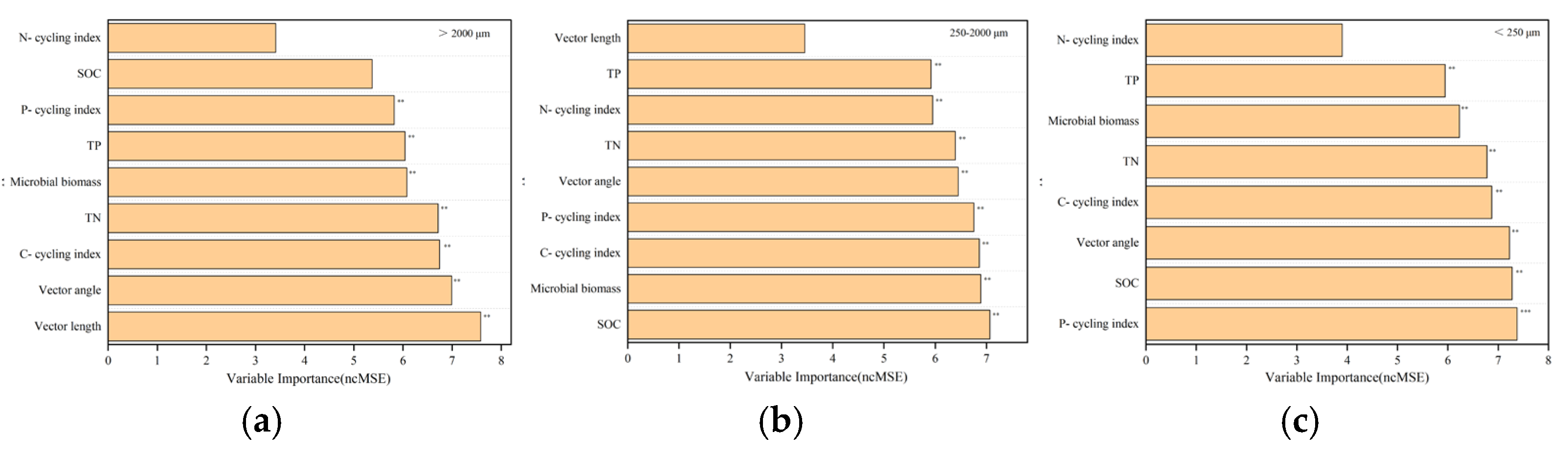
3.5. The Relationship among Soil Ecosystem Multifunctionality, Individual Soil Functions, and Driving Factors within Aggregates
4. Discussion
4.1. Land Conversion Concentrates Microbial Communities within Micro-Aggregates
4.2. Land Conversion Changes Nutrient Cycling Indices within Soil Aggregates
4.3. Biotic and Abiotic Factors Mediating Soil Aggregate EMF under Land Conversion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azadi, H.; Taheri, F.; Burkart, S.; Mahmoudi, H.; Maeyer, P.; Witlox, F. Impact of agricultural land conversion on climate change. Environ. Dev. Sustain. 2021, 23, 3187–3198. [Google Scholar] [CrossRef]
- Guan, P.; Mahamood, M.; Yang, Y.; Wu, D. Land conversion regulates the effects of long-term climate warming on soil micro-food web communities. Agric. Ecosyst. Environ. 2021, 314, 107426. [Google Scholar] [CrossRef]
- Ding, L.; Ren, X.; Zhou, Z.; Zhu, D.; Zhu, Y. Forest-to-cropland conversion reshapes microbial hierarchical interactions and degrades ecosystem multifunctionality at a national scale. Environ. Sci. Technol. 2024, 58, 11027–11040. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Y.; Xie, H.; Zhang, L.; Zhang, Z.; Yang, X.; Hao, S.; Gai, J.; Chen, Y. Responses of soil microbial diversity, network complexity and multifunctionality to three land-use changes. Sci. Total Environ. 2023, 859, 160255. [Google Scholar] [CrossRef]
- Zwetsloot, M.J.; Leeuwen, J.V.; Hemerik, L.; Martens, H.; Iolanda, S.; Broek, M.; Debeljak, M.; Rutgers, M.; Sandén, T.; Wall, D. Soil multifunctionality: Synergies and trade-offs across European climatic zones and land uses. Eur. J. Soil Sci. 2021, 72, 1640–1654. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Byrnes, J.E.; Isbell, F.; Gamfeldt, L.; Griffin, J.N.; Eisenhauer, N.; Hensel, M.J.; Hector, A.; Cardinale, B.J.; Duffy, J.E. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 2015, 6, 6936. [Google Scholar] [CrossRef]
- Zhang, L.; Du, H.; Song, T.; Yang, Z.; Peng, W.; Gong, J.; Huang, G.; Li, Y. Conversion of farmland to forest or grassland improves soil carbon, nitrogen, and ecosystem multi-functionality in a subtropical karst region of southwest China. Sci. Rep. 2024, 14, 17745. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Annabi, M.; Raclot, D.; Bahri, H.; Bailly, J.S.; Gomez, C.; Le Bissonnais, Y. Spatial variability of soil aggregate stability at the scale of an agricultural region in Tunisia. Catena 2017, 153, 157–167. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Luo, X.; Hao, X.; Ouyang, Y.; Zeng, L.; Wang, L.; Wen, S.; Wang, B.; Van Nostrand, J.D.; Chen, W.; et al. Microscale heterogeneity of the soil nitrogen cycling microbial functional structure and potential metabolism. Environ. Microbiol. 2021, 2, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jin, C.; Sun, B. Soil aggregate stratification of nematodes and ammonia oxidizers affects nitrification in an acid soil. Environ. Microbiol. 2014, 16, 3083–3094. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Y.; Wang, K.; Hao, X.; Chen, W.; Huang, Q. Complexity of bacterial and fungal network increases with soil aggregate size in an agricultural Inceptisol. Appl. Soil Ecol. 2020, 154, 103640. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, H.; Yu, M.; Cheng, X. Large macroaggregate properties are sensitive to the conversion of pure plantation to uneven-aged mixed plantations. Catena 2020, 194, 104724. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, M.; Liu, G.; Ma, L.; Zhang, S.; Xiao, T.; Peng, G. Effect of vegetation type on microstructure of soil aggregates on the Loess Plateau, China. Agric. Ecosyst. Environ. 2017, 242, 1–8. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, S.; Lu, X.; Ren, Z.; Wu, Q.; Xu, M.; Ren, C.; Yang, G.; Han, X. Organic carbon, nitrogen accumulation, and soil aggregate dynamics as affected by vegetation restoration patterns in the Loess Plateau of China. Catena 2021, 196, 104867. [Google Scholar] [CrossRef]
- Zhou, H.; Peng, X.; Perfect, E.; Xiao, T.; Peng, G. Effects of organic and inorganic fertilization on soil aggregation in an Ultisol as characterized by synchrotron based X-ray micro-computed tomography. Geoderma 2013, 195–196, 23–30. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Ma, J.; Zhang, Y.; Li, X.; Pang, D. Aggregate-associated carbon contributes to soil organic carbon accumulation along the elevation gradient of Helan Mountains. Soil Biol. Biochem. 2023, 178, 108926. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Zhang, X.; Liang, W. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Wang, H.; Lv, G.; Cai, Y.; Zhang, X.; Jiang, L.; Yang, X. Determining the effects of biotic and abiotic factors on the ecosystem multifunctionality in a desert-oasis ecotone. Ecol. Indic. 2021, 128, 107830. [Google Scholar] [CrossRef]
- Yu, T.; Yang, R.; Jie, X.; Lian, T.; Zang, H.; Zeng, Y.; Yang, Y. Organic management improved the multifunctionality in recolonization soil by increasing microbial diversity and function. Funct. Eco. 2024. [Google Scholar] [CrossRef]
- Shu, X.; Liu, W.; Hu, Y.; Xia, L.; Fan, K.; Zhang, Y.; Zhang, Y.; Zhou, W. Ecosystem multifunctionality and soil microbial communities in response to ecological restoration in an alpine degraded grassland. Front. Plant Sci. 2023, 14, 1173962. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Rensing, C.; Chen, H.; Liu, M.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct. Ecol. 2018, 32, 1103–1116. [Google Scholar] [CrossRef]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Padalia, K.; Bargali, S.S.; Bargali, K.; Manral, V. Soil microbial biomass phosphorus under different land use systems of Central Himalaya. Trop. Ecol. 2022, 63, 30–48. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Li, Y.; Liu, X.; Wang, Y.; Qin, J.; Wu, J. Effects of land-use conversion from Masson pine forests to tea plantations on net ecosystem carbon and greenhouse gas budgets. Agric. Ecosyst. Environ. 2021, 320, 107578. [Google Scholar] [CrossRef]
- Xu, H.; Wei, X.; Cheng, X. Fungal diversity dominates the response of multifunctionality to the conversion of pure plantations into two-aged mixed plantations. Sci. Total Environ. 2023, 866, 161384. [Google Scholar] [CrossRef]
- Aaron, F.; Israel, I.; Gemma, T.S.; Gary, L.; Achim, S.; Steve, W.; Rachel, C. The influence of aggregate size fraction and horizon position on microbial community composition. Appl. Soil Ecol. 2018, 127, 19–29. [Google Scholar]
- Han, S.; Luo, X.; Tan, S.; Wang, J.; Chen, W.; Huang, Q. Soil aggregates impact nitrifying microorganisms in a Vertisol under diverse fertilization regimes. Eur. J. Soil Sci. 2019, 71, 536–547. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, G.; Wang, X.; Li, P.; Dang, X.; Jiang, W.; Ma, T.; Wang, B.; Gu, F.; Li, Z. Contribution of soil aggregate particle size to organic carbon and the effect of land use on its distribution in a typical small watershed on Loess Plateau, China. Ecol. Indic. 2023, 155, 110988. [Google Scholar] [CrossRef]
- Panettieri, M.; Rumpel, C.; Dignac, M.F.; Chabbi, A. Does grassland introduction into cropping cycles affect carbon dynamics through changes of allocation of soil organic matter within aggregate fractions? Sci. Total Environ. 2017, 576, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Dorji, T.; Field, D.J.; Odeh, I.O.A.; Bhogal, A. Soil aggregate stability and aggregate-associated organic carbon under different land use or land cover types. Soil Use Manag. 2020, 36, 308–319. [Google Scholar] [CrossRef]
- Briar, S.S.; Fonte, S.J.; Park, I.; Six, J.; Scow, K.; Ferris, H. The distribution of nematodes and soil microbial communities across soil aggregate fractions and farm management systems. Soil Biol. Biochem. 2011, 43, 905–914. [Google Scholar] [CrossRef]
- Han, S.; Delgado-Baquerizo, M.; Luo, X.; Liu, Y.; Van Nostrand, J.D.; Chen, W.; Zhou, J.; Huang, Q. Soil aggregate size-dependent relationships between microbial functional diversity and multifunctionality. Soil Biol. Biochem. 2021, 154, 108143. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Liang, Y.; Liang, Y.; Zhao, Y.; Wang, Z.; Li, Y.; Liu, W.; Wang, X.; Yang, G.; et al. The multifunctionality of soil aggregates is related to the complexity of aggregate microbial community during afforestation. Catena 2024, 236, 107737. [Google Scholar] [CrossRef]
- Wu, T.; Liu, W.; Wang, D.; Zou, Y.; Lin, R.; Yang, Q.; Gbokie, T.; Bughio, M.A.; Li, Q.; Wang, J. Organic management improves soil phosphorus availability and microbial properties in a tea plantation after land conversion from longan (Dimocarpus longan). Appl. Soil Ecol. 2020, 154, 103642. [Google Scholar] [CrossRef]
- Wang, S.; Ye, S. Dynamics of bacterial function characteristics in soil aggregates during the tea-planting process. Geoderma 2020, 377, 114609. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Gui, H.; Fan, L.; Wang, D.; Yan, P.; Li, X.; Zhang, L.; Han, W. Organic management practices shape the structure and associations of soil bacterial communities in tea plantations. Appl. Soil Ecol. 2021, 163, 103975. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods of Soil Agrochemistry, 1st ed.; Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Frostegard, A.; Tunlid, A.; Bååth, E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types exposed to different heavy metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Francisco, R.; Stone, D.; Creamer, R.E.; Sousa, J.P.; Morais, P.V. European scale analysis of phospholipid fatty acid composition of soils to establish operating ranges. Appl. Soil Ecol. 2016, 97, 49–60. [Google Scholar] [CrossRef]
- McKinley, V.L.; Peacock, A.D.; White, D.C. Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol. Biochem. 2005, 37, 1946–1958. [Google Scholar] [CrossRef]
- Bach, L.H.; Grytnes, J.A.; Halvorsen, R.; Ohlson, M. Tree influence on soil microbial community structure. Soil Biol. Biochem. 2010, 42, 1934–1943. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 37, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- He, H. Linking soil depth to aridity effects on soil microbial community composition, diversity and resource limitation. Catena 2023, 232, 107393. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Grinyer, J.; Reich, P.B.; Singh, B.K. Relative importance of soil properties and microbial community for soil functionality: Insights from a microbial swap experiment. Funct. Ecol. 2016, 30, 1862–1873. [Google Scholar] [CrossRef]
- Rillig, M.C.; Muller, L.A.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, Y.; Zhang, B.; Luo, Y.; Yi, X.; Wu, J.; Chen, Y.; Sarker, T.C.; Cai, Y.; Chang, S.X. Land-use-driven change in soil labile carbon affects microbial community composition and function. Geoderma 2022, 426, 116056. [Google Scholar] [CrossRef]
- Fan, L.; Shao, G.; Pang, Y.; Dai, H.; Zhang, L.; Yan, P.; Zou, Z.; Zhang, Z.; Xu, J.; Zamanian, K.; et al. Enhanced soil quality after forest conversion to vegetable cropland and tea plantations has contrasting effects on soil microbial structure and functions. Catena 2022, 211, 106029. [Google Scholar] [CrossRef]
- Upton, R.N.; Bach, E.M.; Hofmockel, K.S. Spatio-temporal microbial community dynamics within soil aggregates. Soil Biol. Biochem. 2019, 132, 58–68. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, L.; Zhao, J.; Niu, Y.; Xiao, H.; Wang, Z.; Yu, S.; Shi, Z. Forty-year-old orchards promote carbon storage by changing aggregate-associated enzyme activities and microbial communities. Catena 2022, 213, 106195. [Google Scholar] [CrossRef]
- Liao, H.; Hao, X.; Zhang, Y.; Qin, F.; Xu, M.; Cai, P.; Chen, W.; Huang, Q. Soil aggregate modulates microbial ecological adaptations and community assemblies in agricultural soils. Soil Biol. Biochem. 2022, 172, 108769. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Lin, F.; Jiang, C.; Zeng, X.; Feng, D.; Huang, F.; Tang, J. Land use change alters carbon and nitrogen dynamics mediated by fungal functional guilds within soil aggregates. Sci. Total Environ. 2023, 902, 166080. [Google Scholar] [CrossRef]
- Gnankambary, Z.; Ilstedt, U.; Nyberg, G.; Hien, V.; Malmer, A. Nitrogen and phosphorus limitation of soil microbial respiration in two tropical agroforestry parklands in the south-Sudanese zone of Burkina Faso: The effects of tree canopy and fertilization. Soil Biol. Biochem. 2008, 40, 350–359. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgadobaquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Mcnee, M.; Flower, K.; Pal, S.B.; Minkey, D.; Singh, B.K. Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef] [PubMed]

| Soil Aggregate Size | EMF | ||
|---|---|---|---|
| >2000 μm | 250–2000 μm | <250 μm | |
| pH | 0.459 | 0.992 ** | 0.727 ** |
| SOC | 0.982 ** | 0.941 ** | 0.965 ** |
| TN | 0.979 ** | 0.996 ** | 0.956 ** |
| TP | 0.936 ** | 0.526 | 0.938 ** |
| Microbial biomass | 0.904 ** | 0.992 ** | 0.946 ** |
| F/B | −0.831 ** | −0.374 | −0.607 * |
| G+/G− | −0.868 ** | −0.606 * | −0.585 * |
| C-cycling index | 0.852 ** | 0.992 ** | 0.843 ** |
| N-cycling index | −0.300 | 0.115 | −0.207 |
| P-cycling index | 0.741 ** | 0.765 ** | 0.847 ** |
| Vector length | 0.815 ** | 0.363 | −0.235 |
| Vector angle | 0.644 * | 0.478 | 0.779 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Y.; Yue, Z.; Zhou, G.; Wei, C.; Wu, D.; Liu, B.; Li, Q.; Wang, J.; Zou, Y. Organic Management Mediates Multifunctionality Responses to Land Conversion from Longan (Dimocarpus longan) to Tea Plantations at the Aggregate Level. Agronomy 2024, 14, 2224. https://doi.org/10.3390/agronomy14102224
Shan Y, Yue Z, Zhou G, Wei C, Wu D, Liu B, Li Q, Wang J, Zou Y. Organic Management Mediates Multifunctionality Responses to Land Conversion from Longan (Dimocarpus longan) to Tea Plantations at the Aggregate Level. Agronomy. 2024; 14(10):2224. https://doi.org/10.3390/agronomy14102224
Chicago/Turabian StyleShan, Ying, Zhengfu Yue, Guangfan Zhou, Chaoxian Wei, Dongming Wu, Beibei Liu, Qinfen Li, Jinchuang Wang, and Yukun Zou. 2024. "Organic Management Mediates Multifunctionality Responses to Land Conversion from Longan (Dimocarpus longan) to Tea Plantations at the Aggregate Level" Agronomy 14, no. 10: 2224. https://doi.org/10.3390/agronomy14102224






