Effects of Various Levels of Water Stress on Morpho-Physiological Traits and Spectral Reflectance of Maize at Seedling Growth Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Measurement of Morphological and Physiological Indicators of Maize Leaves
2.3. Acquisition of Corn Leaf Spectra
2.4. Statistical Analysis
3. Results
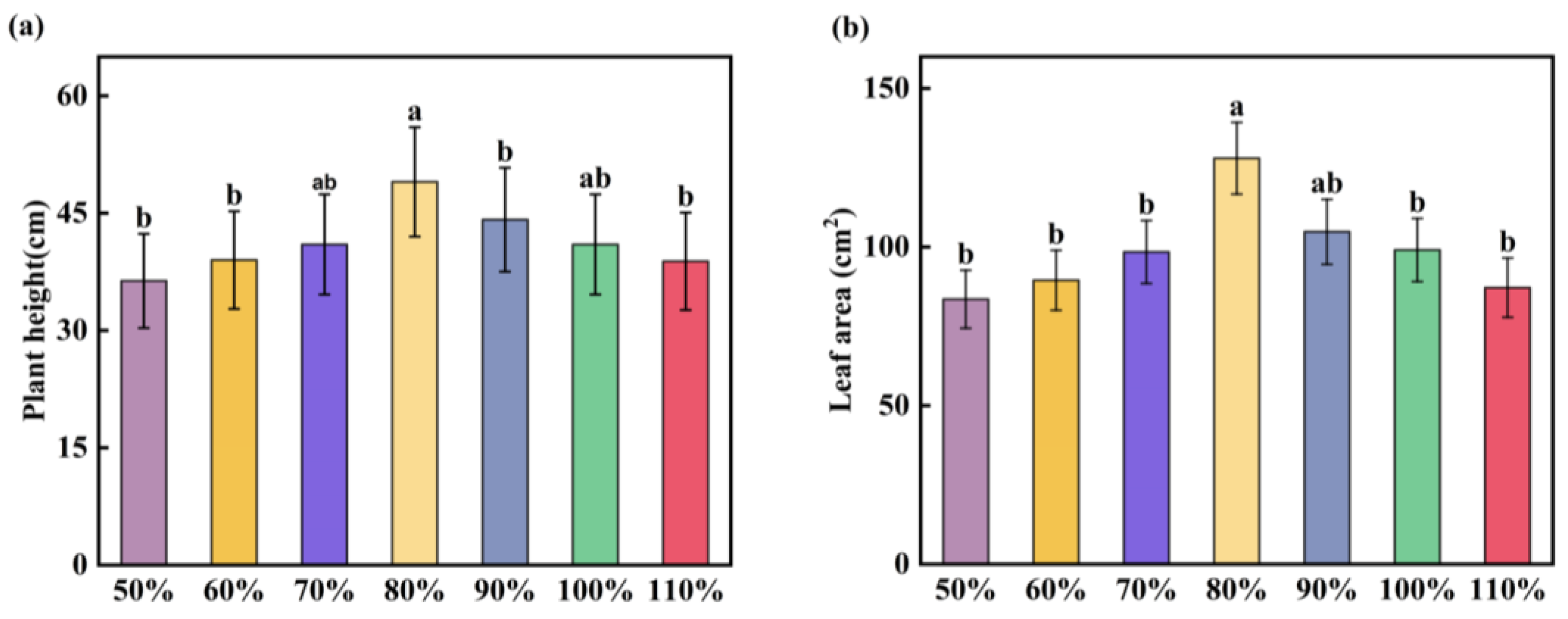
3.1. Phenotypic Response of Corn during the Seedling Stage
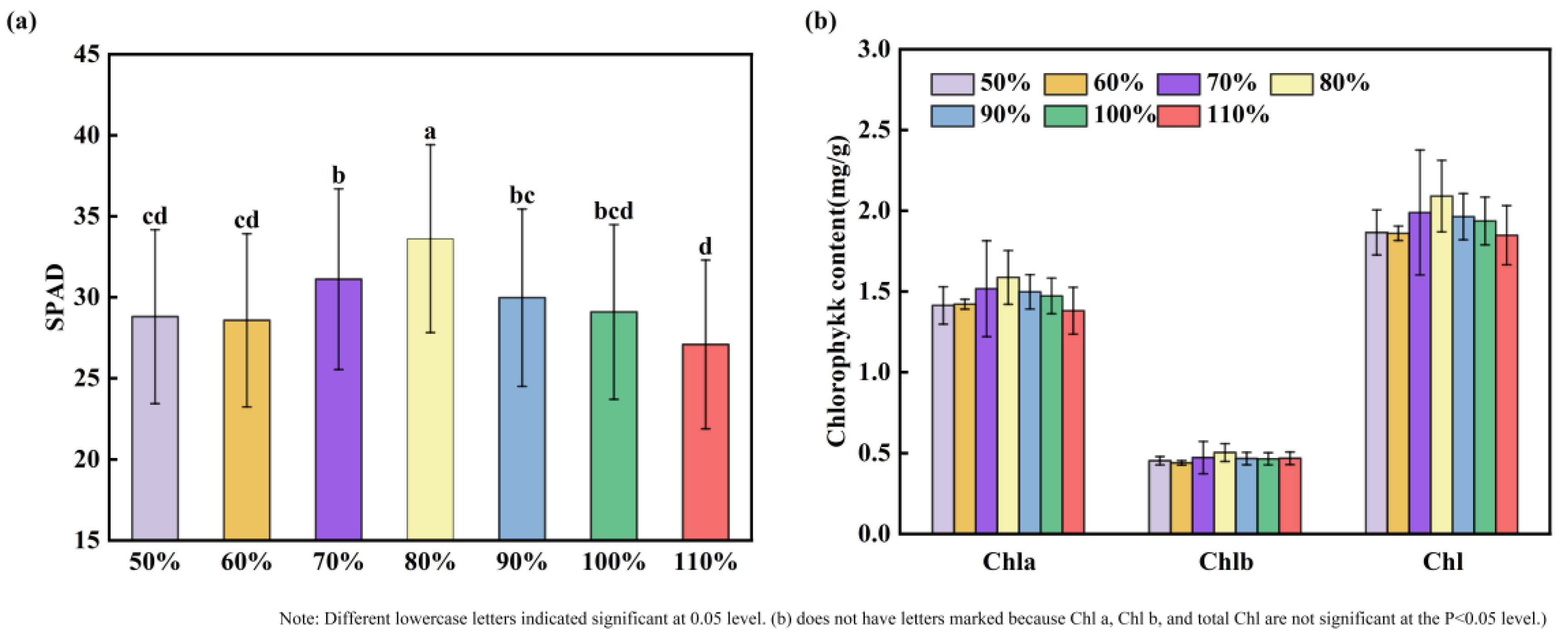
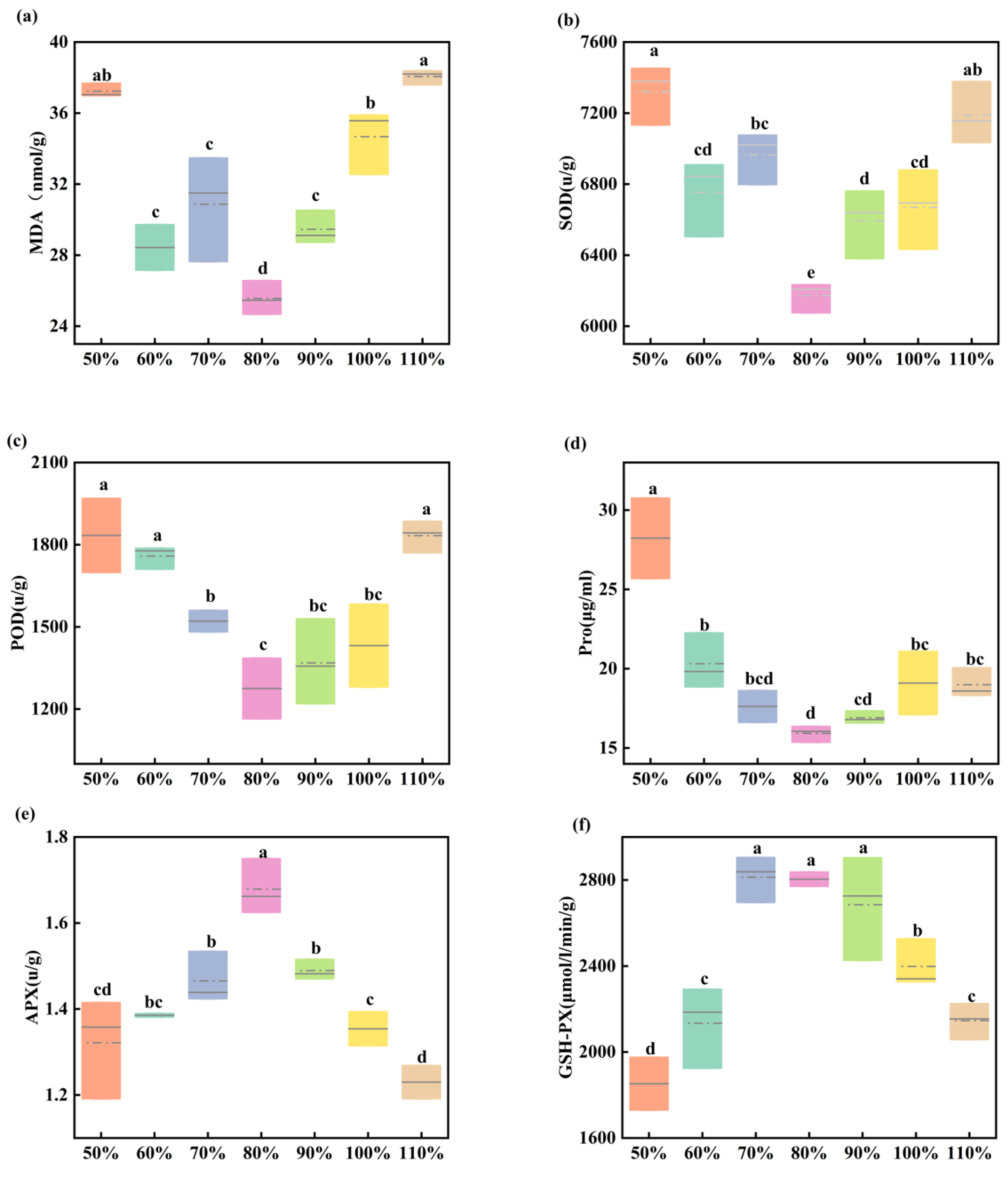
3.2. Effects of Soil Moisture on the Physiological Traits of Maize Seedling Leaves
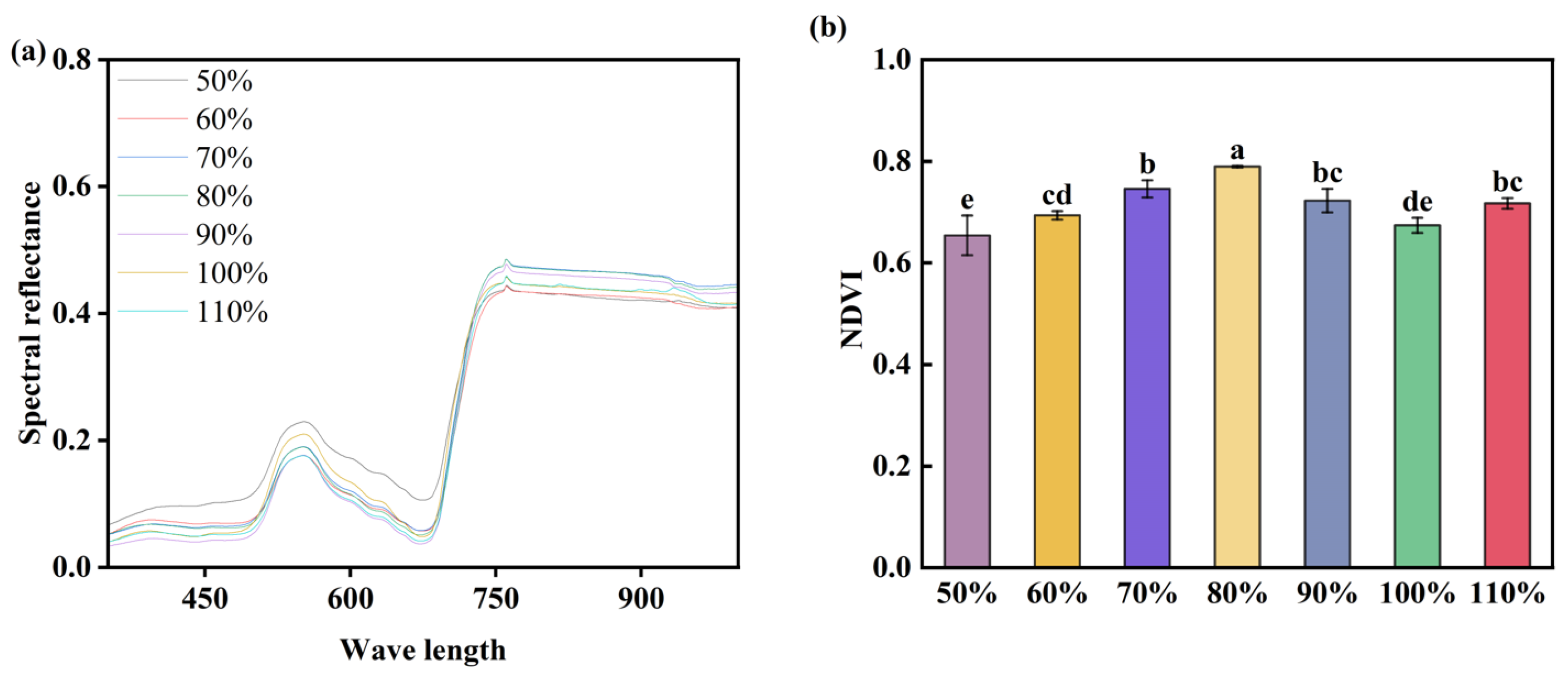
3.3. Influence of Soil Moisture on Maize Leaf Spectra
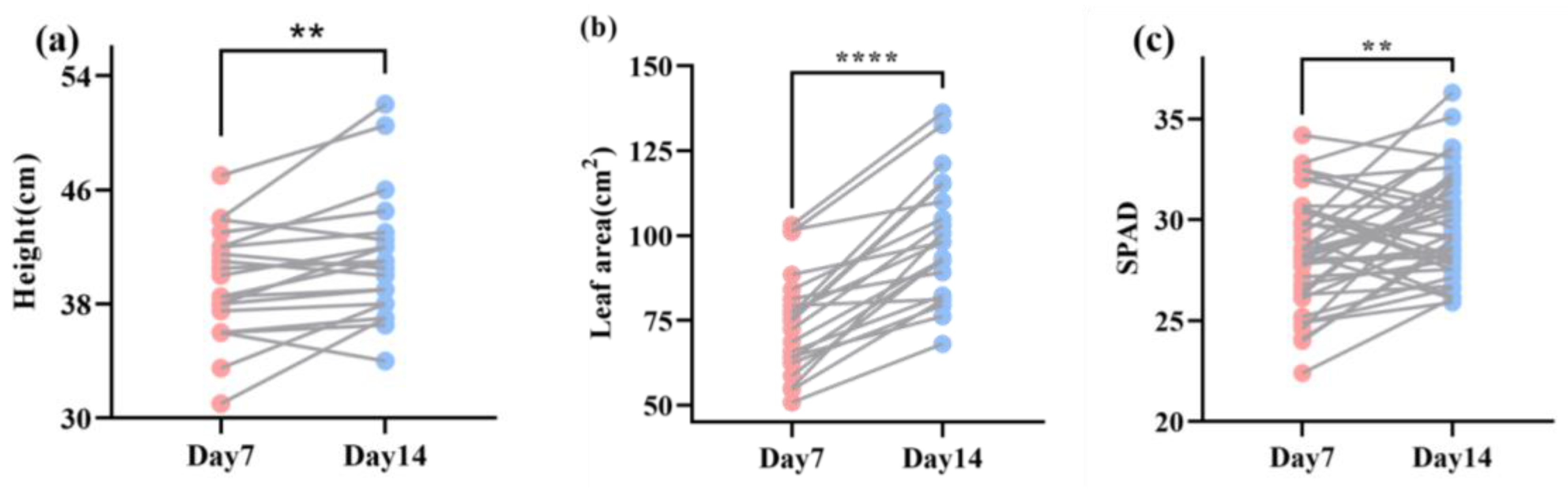
3.4. t-Test of Maize Phenotypic Characteristics
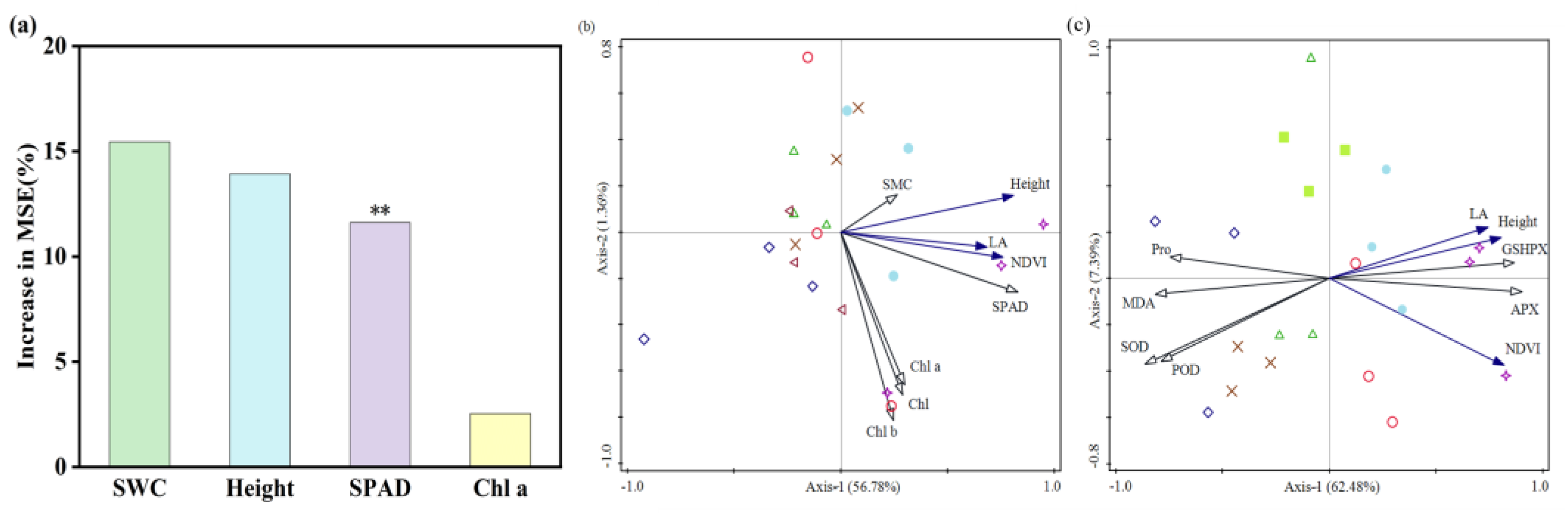
3.5. Contribution of Maize Phenotypic and Physiological Traits to NDVI
4. Discussion
4.1. The Response of Maize Seedling Phenotypic Traits to Soil Moisture
4.2. Impact of Soil Moisture on the Antioxidant System of Maize
4.3. Effects of Soil Moisture on Leaf NDVI of Maize Seedlings
4.4. Contribution of Maize Seedling Phenotypic and Physiological Characteristics to NDVI
5. Conclusions
- (1)
- Phenotypic analysis indicates that both drought and waterlogging stress reduce photosynthesis in maize seedlings, resulting in decreased chlorophyll content (SPAD values). As drought and waterlogging stress intensify, the inhibitory effects also increase, leading to smaller and yellowing seedling leaves. The 50% and 110% treatments have the most significant impact on maize phenotypes, while the 70% and 90% treatments do not produce significant effects on maize phenotypes.
- (2)
- Physiological reflections indicate that both drought and waterlogging stress affect the antioxidant enzyme system in maize (reducing GSH-PX and APX enzyme activity, while increasing SOD, POD, and Pro enzyme activity). However, APX and GSH-PX exhibit patterns opposite to those of SOD and POD. Therefore, SOD, POD, APX, and GSH-PX may participate in regulating maize growth under water stress. NDVI values also decrease, consistent with changes in SPAD values and photosynthetic rates. This correlates with the phenotype of relatively small and yellowing leaves under water stress treatments.
- (3)
- The results from the Random Forest model and RDA validate the contribution of maize phenotypic and physiological characteristics to NDVI, with SPAD making a significantly meaningful contribution to NDVI.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Li, X.; Lou, Y.; You, S.; Zhao, H. Refined Evaluation of Climate Suitability of Maize at Various Growth Stages in Major Maize-Producing Areas in the North of China. Agronomy 2024, 14, 344. [Google Scholar] [CrossRef]
- Dengxiao, Z.; Hongbin, J.; Wenjing, Z.; Qingsong, Y.; Zhihang, M.; Haizhong, W.; Wei, R.; Shiliang, L.; Daichang, W. Combined biochar and water-retaining agent application increased soil water retention capacity and maize seedling drought resistance in Fluvisols. Sci. Total Environ. 2024, 907, 167885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Williams, A.P.; Berg, A.M.; Cook, B.I.; Zhang, Y.; Hagemann, S.; Lorenz, R.; Seneviratne, S.I.; Gentine, P. Land–atmosphere feedbacks exacerbate concurrent soil drought and atmospheric aridity. Proc. Natl. Acad. Sci. USA 2019, 116, 18848–18853. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, P.; Jiang, B.; Li, M. Learned features of leaf phenotype to monitor maize water status in the fields. Comput. Electron. Agric. 2020, 172, 105347. [Google Scholar] [CrossRef]
- Zhang, L.; Han, W.; Niu, Y.; Chávez, J.L.; Shao, G.; Zhang, H. Evaluating the sensitivity of water stressed maize chlorophyll and structure based on UAV derived vegetation indices. Comput. Electron. Agric. 2021, 185, 106174. [Google Scholar] [CrossRef]
- Féret, J.-B.; le Maire, G.; Jay, S.; Berveiller, D.; Bendoula, R.; Hmimina, G.; Cheraiet, A.; Oliveira, J.; Ponzoni, F.; Solanki, T.; et al. Estimating leaf mass per area and equivalent water thickness based on leaf optical properties: Potential and limitations of physical modeling and machine learning. Remote Sens. Environ. 2019, 231, 110959. [Google Scholar] [CrossRef]
- Yi, Q.-X.; Bao, A.-M.; Wang, Q.; Zhao, J. Estimation of leaf water content in cotton by means of hyperspectral indices. Comput. Electron. Agric. 2013, 90, 144–151. [Google Scholar] [CrossRef]
- Mwinuka, P.R.; Mbilinyi, B.P.; Mbungu, W.B.; Mourice, S.K.; Mahoo, H.; Schmitter, P. The feasibility of hand-held thermal and UAV-based multispectral imaging for canopy water status assessment and yield prediction of irrigated African eggplant (Solanum aethopicum L.). Agric. Water Manag. 2021, 245, 106584. [Google Scholar] [CrossRef]
- Bu, L.F.; Zhang, R.H.; Chang, Y.; Xue, J.; Han, M. Response of photosynthetic characteristics to water stress of maize leaf in seeding. Acta Ecol. Sin 2010, 30, 1184–1191. [Google Scholar]
- Ma, X.; Zhou, G.; Li, G.; Wang, Q. Quantitative evaluation of the trade-off growth strategies of maize leaves under different drought severities. Water 2021, 13, 1852. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhu, M.; Li, F. Exogenous 6-benzyladenine improves waterlogging tolerance in maize seedlings by mitigating oxidative stress and upregulating the ascorbate-glutathione cycle. Front. Plant Sci. 2021, 12, 680376. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.B.; Zeng, F.; Shabala, L.; Zhang, G.; Yu, M.; Demidchik, V.; Shabala, S.; Zhou, M. Identification of QTL related to ROS formation under hypoxia and their association with waterlogging and salt tolerance in barley. Int. J. Mol. Sci. 2019, 20, 699. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Vikal, Y.; Kaur, L.; Kalia, A.; Mittal, A.; Kaur, D.; Yadav, I. Elucidating the morpho-physiological adaptations and molecular responses under long-term waterlogging stress in maize through gene expression analysis. Plant Sci. 2021, 304, 110823. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Wang, S.; Guan, K.; Wang, Z.; Ainsworth, E.A.; Zheng, T.; Townsend, P.A.; Liu, N.; Nafziger, E.; Masters, M.D.; Li, K.; et al. Airborne hyperspectral imaging of nitrogen deficiency on crop traits and yield of maize by machine learning and radiative transfer modeling. Int. J. Appl. Earth Obs. Geoinf. 2021, 105, 102617. [Google Scholar] [CrossRef]
- Faiçal, B.S.; Freitas, H.; Gomes, P.H.; Mano, L.Y.; Pessin, G.; de Carvalho, A.C.; Krishnamachari, B.; Ueyama, J. An adaptive approach for UAV-based pesticide spraying in dynamic environments. Comput. Electron. Agric. 2017, 138, 210–223. [Google Scholar] [CrossRef]
- Caballero, D.; Calvini, R.; Amigo, J.M. Hyperspectral Imaging in Crop Fields: Precision Agriculture, in Data Handling in Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 453–473. [Google Scholar]
- Elsherbiny, O.; Fan, Y.; Zhou, L.; Qiu, Z. Fusion of feature selection methods and regression algorithms for predicting the canopy water content of rice based on hyperspectral data. Agriculture 2021, 11, 51. [Google Scholar] [CrossRef]
- Agilandeeswari, L.; Prabukumar, M.; Radhesyam, V.; Phaneendra, K.L.N.B.; Farhan, A. Crop classification for agricultural applications in hyperspectral remote sensing images. Appl. Sci. 2022, 12, 1670. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Wu, S.; Yang, C. Analyzing ecological environment change and associated driving factors in China based on NDVI time series data. Ecol. Indic. 2021, 129, 107933. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Sinha, P. Soil salinity and vegetation cover change detection from multi-temporal remotely sensed imagery in Al Hassa Oasis in Saudi Arabia. Geocarto Int. 2018, 33, 830–846. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Yang, G.; Zhu, C.; Huo, L.; Feng, H. Assessment of defoliation during the Dendrolimus tabulaeformis Tsai et Liu disaster outbreak using UAV-based hyperspectral images. Remote Sens. Environ. 2018, 217, 323–339. [Google Scholar] [CrossRef]
- Sleep, B.; Mason, S.; Janik, L.; Mosley, L. Application of visible near-infrared absorbance spectroscopy for the determination of Soil pH and liming requirements for broad-acre agriculture. Precis. Agric. 2022, 23, 194–218. [Google Scholar] [CrossRef]
- Sun, Z.J.; Li, J.S.; Jia, Y.H.; Li, H. Effects of Wetting-Drying Cycles on Bulk Density and Saturated Hydraulic Conductivity of Soils. J. Irrig. Drain. 2022, 41, 89–96. [Google Scholar]
- Tao, S.; Liu, J.; Miao, X.; Chen, Z.; Chen, Y.; Gong, H.; Xue, D.; Ackermann, K. Determination the total amount of nitrogen, phosphorus and potassium in the soil by perchloric acid-hydrofluoric acid digesting system. China Meas. Test 2022, 48, 78–83. [Google Scholar]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Regulations of 6-benzyladenine (6-BA) on leaf ultrastructure and photosynthetic characteristics of waterlogged summer maize. J. Plant Growth Regul. 2017, 36, 743–754. [Google Scholar] [CrossRef]
- Nurkemayi, M.; Yang, Y.; Abdukerim, P.; Usman, M. Comparison of Methods for Measuring Chlorophyll Content in Wheat. Jiangsu Agric. Sci. 2021, 49, 156–159. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, C.; Meng, Z.; Li, Y.; Abid, M.A.; Askari, M.; Wang, P.; Wang, Y.; Sun, G.; Cai, Y.; et al. Leveraging Atriplex hortensis choline monooxygenase to improve chilling tolerance in cotton. Environ. Exp. Bot. 2019, 162, 364–373. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-T.; Pan, X.-B.; Xi, Z.-M. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: Toward regulating the ascorbate–glutathione cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35 (Suppl. S4), 1011–1019. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Shen, W.; Li, M.; Huang, C.; Wei, A. Quantifying live aboveground biomass and forest disturbance of mountainous natural and plantation forests in Northern Guangdong, China, based on multi-temporal Landsat, PALSAR and field plot data. Remote Sens. 2016, 8, 595. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, H.; Sun, X.; Rymen, B.; Jikumaru, Y.; Kojima, M.; Takebayashi, Y.; Abbeloos, R.; Demuynck, K.; Storme, V.; Vuylsteke, M.; et al. The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol. J. 2018, 16, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Vandoorne, B.; Descamps, C.; Mathieu, A.S.; Ende, W.V.D.; Vergauwen, R.; Javaux, M.; Lutts, S. Long term intermittent flooding stress affects plant growth and inulin synthesis of Cichorium intybus (var. sativum). Plant Soil 2014, 376, 291–305. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, M.; Wei, Y.; Xia, Z. Overexpression of the maize psbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 2016, 6, 1223. [Google Scholar] [CrossRef]
- Min, H.; Chen, C.; Wei, S.; Shang, X.; Sun, M.; Xia, R.; Liu, X.; Hao, D.; Chen, H.; Xie, Q. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, 1080. [Google Scholar] [CrossRef]
- Hu, J.; Yu, W.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. Responses of canopy functionality, crop growth and grain yield of summer maize to shading, waterlogging, and their combination stress at different crop stages. Eur. J. Agron. 2023, 144, 126761. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 2016, 11, e0161424. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Wang, H.; Gao, Y.; Ma, S.; Qin, A.; Liu, Z.; Zhao, B.; Ning, D.; Zheng, H.; et al. Effects of waterlogging at different stages on growth and ear quality of waxy maize. Agric. Water Manag. 2022, 266, 107603. [Google Scholar] [CrossRef]
- Nawaz, M.; Anjum, S.A.; Ashraf, U.; Azeem, F.; Wang, Z. Antioxidant defense system and reactive oxygen species (ROS) interplay in plants under drought condition. In Handbook of Climate Change Management: Research, Leadership, Transformation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–25. [Google Scholar]
- Salah, A.; Zhan, M.; Cao, C.; Han, Y.; Ling, L.; Liu, Z.; Li, P.; Ye, M.; Jiang, Y. γ-Aminobutyric acid promotes chloroplast ultrastructure, antioxidant capacity, and growth of waterlogged maize seedlings. Sci. Rep. 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Sohrabi, F.; Fasihfar, E.; Baniasadi, F.; Riasat, M.; Mozafari, A.A. Superoxide dismutase (SOD) as a selection criterion for triticale grain yield under drought stress: A comprehensive study on genomics and expression profiling, bioinformatics, heritability, and phenotypic variability. BMC Plant Biol. 2021, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2022, 27, 699–716. [Google Scholar] [CrossRef]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Nie, G.P.; Chen, M.M.; Yang, L.Y.; Cai, Y.; Xu, F.; Zhang, Y. Plant response to waterlogging stress: Research progress. Chin. Agric. Sci. Bull. 2021, 37, 57–64. [Google Scholar]
- Seyednasrollah, B.; Young, A.M.; Hufkens, K.; Milliman, T.; Friedl, M.A.; Frolking, S.; Richardson, A.D. Tracking vegetation phenology across diverse biomes using Version 2.0 of the PhenoCam Dataset. Sci. Data 2019, 6, 222. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional genomics in plant abiotic stress responses and tolerance: From gene discovery to complex regulatory networks and their application in breeding. Proc. Jpn. Acad. Ser. B 2022, 98, 470–492. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Li, S.; Zhang, L.; Peng, L.; Xie, W.; Liu, F. Photosynthetic response of an alpine plant, Rhododendron delavayi Franch, to water stress and recovery: The role of mesophyll conductance. Front. Plant Sci. 2015, 6, 1089. [Google Scholar] [CrossRef]
- Yang, Y.; Massa, G.D.; Mitchell, C.A. Temperature DIP at the beginning of the photoperiod reduces plant height but not seed yield of maize grown in controlled environments. Ind. Crop. Prod. 2014, 53, 120–127. [Google Scholar] [CrossRef]
- Shu, M.; Li, Q.; Ghafoor, A.; Zhu, J.; Li, B.; Ma, Y. Using the plant height and canopy coverage to estimation maize aboveground biomass with UAV digital images. Eur. J. Agron. 2023, 151, 126957. [Google Scholar] [CrossRef]
- Dinç, E.; Ceppi, M.G.; Tóth, S.Z.; Bottka, S.; Schansker, G. The chl a fluorescence intensity is remarkably insensitive to changes in the chlorophyll content of the leaf as long as the chl a/b ratio remains unaffected. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Qin, X.; Lyu, D.; Qin, S.; Zhang, P. ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Sci. Hortic. 2019, 257, 108647. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Gu, L.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
| Soil Texture | Soil pH | Dry Soil Bulk Density g·cm−3 | Soil Field Capacity | Soil Organic Matter g·kg−1 | Total Nitrogen g·kg−1 | Total Phosphorus g·kg−1 |
|---|---|---|---|---|---|---|
| silty clay loam | 8.80 | 1.47 | 27.00% | 18.85 | 0.73 | 0.94 |
| Soil Water Content | Lower Limit of Soil Moisture Control | Upper Limit of Soil Moisture Control |
|---|---|---|
| CK | 75 | 85 |
| 50% | 45 | 55 |
| 60% | 55 | 65 |
| 70% | 65 | 75 |
| 90% | 85 | 95 |
| 100% | 95 | 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Feng, Y.; Sun, X.; Liu, W.; Yang, W.; Ge, X.; Jia, Y. Effects of Various Levels of Water Stress on Morpho-Physiological Traits and Spectral Reflectance of Maize at Seedling Growth Stage. Agronomy 2024, 14, 2173. https://doi.org/10.3390/agronomy14092173
Li X, Feng Y, Sun X, Liu W, Yang W, Ge X, Jia Y. Effects of Various Levels of Water Stress on Morpho-Physiological Traits and Spectral Reflectance of Maize at Seedling Growth Stage. Agronomy. 2024; 14(9):2173. https://doi.org/10.3390/agronomy14092173
Chicago/Turabian StyleLi, Xuemin, Yayang Feng, Xiulu Sun, Wentao Liu, Weiyue Yang, Xiaoyang Ge, and Yanhui Jia. 2024. "Effects of Various Levels of Water Stress on Morpho-Physiological Traits and Spectral Reflectance of Maize at Seedling Growth Stage" Agronomy 14, no. 9: 2173. https://doi.org/10.3390/agronomy14092173






