Seed Dormancy and Germination Responses to Different Temperatures of Leptochloa chinensis (L.) Nees: A Case Study with 242 Populations Collected from Rice Fields in East China
Abstract
:1. Introduction
2. Materials and Methods
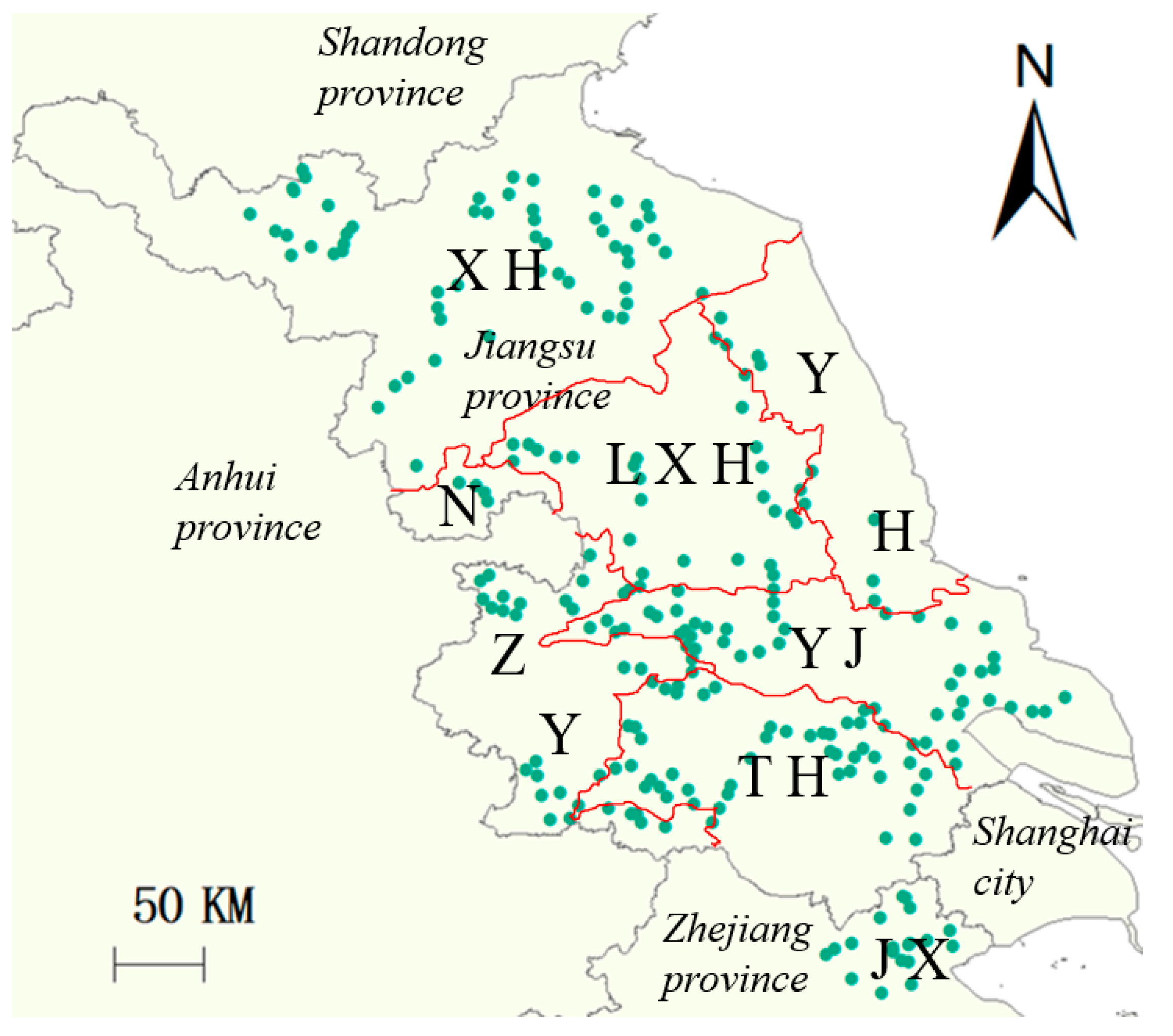
2.1. Plant Material
2.2. Seed Dormancy
2.3. Seed Germination under Different Temperatures
2.4. Statistical Analysis
3. Results
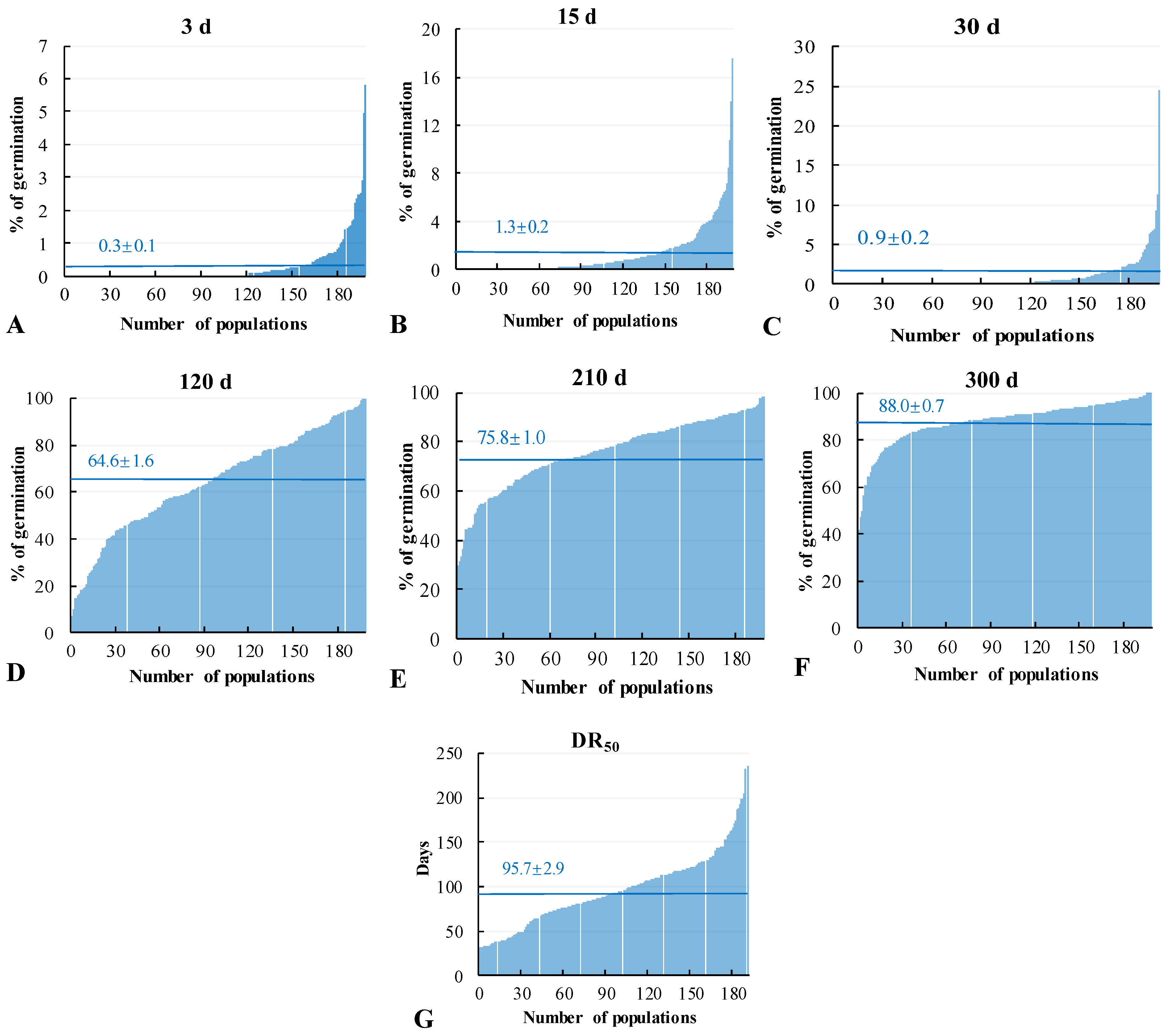
3.1. Seed Dormancy Duration
3.2. Influences of Location and Planting Methods on Seed Dormancy Durations
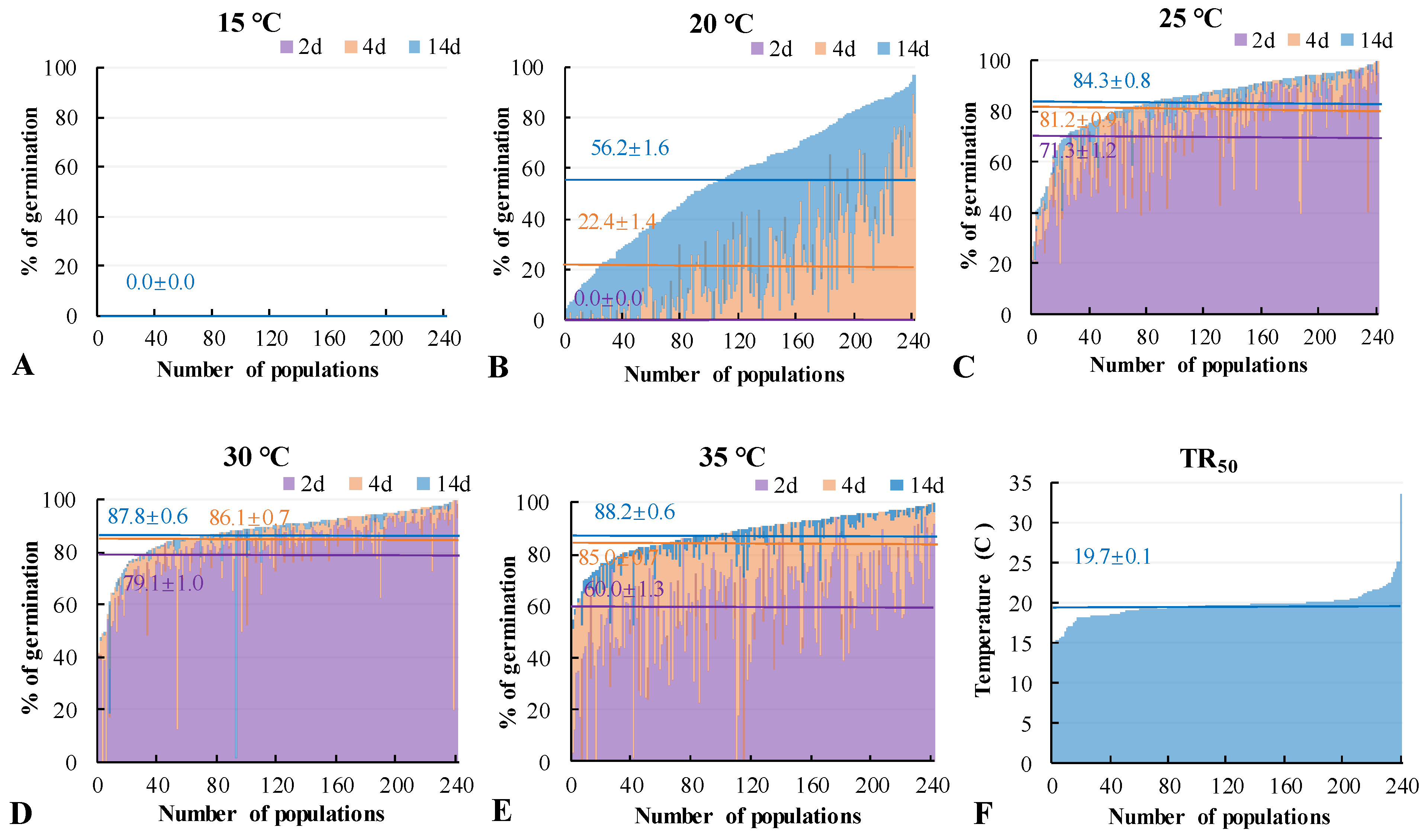
3.3. Seed Germination Percentage under Different Temperatures
3.4. Influences Factors on Seed Germination under Different Temperatures
4. Discussion
4.1. Different Periods Were Required to Release Seed Dormancy in Different Populations
4.2. Different Populations Showed Different Seed Germination Patterns under Different Temperatures
4.3. Intraspecific Variations in Seed Dormancy and Germination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.J.; Pan, L.; Liu, D.C.; Cheng, X.M.; Ma, G.L.; Li, S.F.; Liu, X.Y.; Wang, L.F.; Bai, L.Y. Confirmation and characterization of cyhalofop-butyl-resistant Chinese sprangletop (Leptochloa chinensis) populations from China. Weed Sci. 2020, 68, 253–259. [Google Scholar] [CrossRef]
- Chin, D. Biology and management of barnyardgrass, red sprangletop and weedy rice. Weed Biol. Manag. 2001, 1, 37–41. [Google Scholar] [CrossRef]
- Zheng, H.H.; Fan, G.M.; Han, R.Y.; Huang, G.Y.; Pan, X.Z. Occurrence and control of Leptochloa chinensis in paddy field. Plant Prot. 1997, 23, 49–50. (In Chinese) [Google Scholar]
- Hayyat, M.S.; Safdar, M.E.; Javaid, M.M.; Ullah, S.; Chauhan, B.S. Estimation of the economic threshold of Leptochloa chinensis (Chinese sprangletop) in direct-seeded fine grain rice (Oryza sativa). Semin.-Cienc. Agrar. 2023, 44, 803–822. [Google Scholar] [CrossRef]
- Chen, G.Q.; Yuan, S.Z.; Guo, B.W.; Dai, Q.G.; Huo, Z.Y.; Gao, H.; Wei, H.Y. Safe and Efficient Use Technology of Herbicide in Paddy Field, 1st ed.; China Agriculture Press: Beijing, China, 2021; pp. 105–134. [Google Scholar]
- Zhang, Y.; Chen, L.P.; Song, W.; Cang, T.; Xu, M.F.; Wu, C.X. Diverse mechanisms associated with cyhalofop-butyl resistance in Chinese sprangletop (Leptochloa chinensis (L.) Nees): Characterization of target-site mutations and metabolic resistance-related genes in two resistant populations. Front. Plant Sci. 2022, 13, 990085. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Y.; Yao, S.; Wu, J.W.; Zhu, A.X.; Yang, Q.; Yuan, S.Z. Current status of cyhalofop-butyl and metamifop resistance and diversity of the ACCase gene mutations in Chinese sprangletop (Leptochloa chinensis) from China. Pest. Biochem. Physiol. 2023, 197, 105648. [Google Scholar] [CrossRef]
- Benvenuti, S.; Dinelli, G.; Bonetti, A. Germination ecology of Leptochloa chinensis: A new weed in the Italian rice agro-environment. Weed Res. 2004, 44, 87–96. [Google Scholar] [CrossRef]
- Dong, L.Y.; Wu, S.W.; Shen, J.L. Study on dormancy release method and germination conditions of Leptochloa chinensis seeds. Jiangsu Agric. Sci. 2005, 5, 48–51. (In Chinese) [Google Scholar]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef]
- Dong, L.Y.; Wang, H.C.; Chen, G.Q. Weed Management Technology of Direct-Seeded Rice Fields; China Agriculture Press: Beijing, China, 2016; pp. 24–26. (In Chinese) [Google Scholar]
- Agricultural Regionalization of Jiangsu Province. Available online: http://zrzy.jiangsu.gov.cn/jsbzdt/dtll/18NY/index.html (accessed on 10 April 2024).
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size in studies on the germination process. Botany 2016, 94, 103–115. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Zhu, F.; Gao, T.J.; Chen, G.Q. Proliferative capacity in relation to metamifop resistance in Echinochloa glabrescens: A case study. Chil. J. Agric. Res. 2023, 83, 408–417. [Google Scholar] [CrossRef]
- Zhou, L.F.; Yu, H.W.; Yang, K.W.; Chen, L.; Yin, W.D.; Ding, J.Q. Latitudinal and Longitudinal Trends of Seed Traits Indicate Adaptive Strategies of an Invasive Plant. Front. Plant Sci. 2021, 12, 657813. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Luo, X.P.; Yuan, Z.; Bai, M.J.; Hu, X.W. Seed dormancy release of Halenia elliptica in response to stratification temperature, duration and soil moisture content. BMC Plant Biol. 2020, 20, 352. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Wu, H.W.; Ren, X.L.; Hu, H.Y.; Wang, L.; Ma, Y. Seasonal changes in germinability, dormancy and viability of field bindweed (Convolvulus arvensis) seeds as affected by storage and duration. Adv. Weed Sci. 2023, 41, e020220082. [Google Scholar] [CrossRef]
- Toorop, P.E.; Cuerva, R.C.; Begg, G.S.; Locardi, B.; Squire, G.R.; Iannetta, P.P.M. Co-adaptation of seed dormancy and flowering time in the arable weed Capsella bursa-pastoris (shepherd’s purse). Ann. Bot. 2012, 109, 481–489. [Google Scholar] [CrossRef]
- Chen, G.Q.; An, K.; Chen, Y.; Zhuang, X.X. Double-spraying with different routes significantly improved control efficacies of herbicides applied by unmanned aerial spraying system: A case study with rice herbicides. Crop. Prot. 2023, 167, 106203. [Google Scholar] [CrossRef]
- Bradford, K.J.; Bello, P. Population-based models for environmental factors affecting seed germination. In Applying Population-Based Threshold Models to Quantify and Improve Seed Quality Attributes, 2nd ed.; Buitink, J., Leprince, O., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; Volume 4, pp. 19–23. [Google Scholar]
- Schmuths, H.; Bachmann, K.; Weber, W.E.; Horres, R.; Hoffmann, M.H. Effects of preconditioning and temperature during germination of 73 natural accessions of Arabidopsis thaliana. Ann. Bot. 2006, 97, 623–634. [Google Scholar] [CrossRef]
- Liu, X.J.; Xu, D.P.; Yang, Z.J.; Zhang, N.N. Geographic variations in seed germination of Dalbergia odorifera T. Chen in response to temperature. Ind. Crop. Prod. 2017, 102, 45–50. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, D.L.; Liu, H.Z.; Guo, C.L.; Tang, L.; Wang, H.G.; Chen, Y.H.; Luo, K. Effect of temperature and water potential on the germination of seeds from three different populations of Bidens pilosa as a potential Cd hyperaccumulator. BMC Plant Biol. 2022, 22, 487. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Chen, J.J.; Clark, D.; Perez, H.; Huo, H.Q. Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia x hybrida under Different Abiotic Stresses. Plants 2021, 10, 581. [Google Scholar] [CrossRef]
- Chen, D.L.; Yuan, Z.; Wei, Z.C.; Hu, X.W. Effect of maternal environment on seed germination and seed yield components of Thlaspi arvense. Ind. Crops Prod. 2022, 181, 114790. [Google Scholar] [CrossRef]
- Brainard, D.C.; DiTommaso, A.; Mohler, C.L. Intraspecific variation in seed characteristics of Powell amaranth (Amaranthus powellii) from habitats with contrasting crop rotation histories. Weed Sci. 2007, 55, 218–226. [Google Scholar] [CrossRef]
- Gafni, R.; Blank, L.; Eizenberg, H. Variability in germination fractions of Amaranthus albus in response to weed management and abiotic maternal conditions. Eur. J. Agron. 2024, 152, 127009. [Google Scholar] [CrossRef]
- Yu, J.; Gao, H.; Li, W.X.; Bai, Y.C.; Wang, J.D.; Wang, X.K. Evaluation of cultivated land quality in Jiangsu province based on GIS. Soil Fert. Sci. China 2022, 9, 222–230. (In Chinese) [Google Scholar]
- Zhang, H.; Wang, X.K.; Xu, J.P.; Zhang, Y.C.; Ai, Y.C. Distribution characteristics of soil carbon and nitrogen and its influencing factors in different farming regions of Jiangsu province. Jiangsu J. Agric. Sci. 2014, 30, 1028–1036. (In Chinese) [Google Scholar]
- Cheng, J.Y.; Huang, H.; Liu, W.W.; Zhou, Y.; Han, W.P.; Wang, X.Y.; Zhang, Y.H. Unraveling the Effects of Cold Stratification and Temperature on the Seed Germination of Invasive Spartina alterniflora across Latitude. Front. Plant Sci. 2022, 13, 911804. [Google Scholar] [CrossRef]
- Shihan, A.; Barre, P.; Copani, V.; Kallida, R.; Ostrem, L.; Testa, G.; Norton, M.R.; Sampoux, J.P.; Volaire, F. Induction and potential role of summer dormancy to enhance persistence of perennial grasses under warmer climates. J. Ecol. 2022, 110, 1283–1295. [Google Scholar] [CrossRef]
- Mojtabedi, N.; Hiramatsu, M.; Mizunoe, Y.; Okubo, H. Inheritance of bulb dormancy and early flowering ability in F1 progenies of intra- and interspecific crosses of Lilium formosanum and L. longiflorum. J. Fac. Agric. Kyushu Univ. 2013, 58, 23–25. [Google Scholar]
- Wagmann, K.; Hautekèete, N.C.; Piquot, Y.; Meunier, C.; Schmitt, S.E.; Van Dijk, H. Seed dormancy distribution: Explanatory ecological factors. Ann. Bot. 2012, 110, 1205–1219. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Unexpected intraspecific variability of perennial ryegrass (Lolium perenne L.) in response to constant temperature during germination and initial heterotrophic growth. Front. Plant Sci. 2022, 13, 856099. [Google Scholar] [CrossRef]
- Dwiyanti, M.S.; Stewart, J.R.; Nishiwaki, A.; Yamada, T. Natural variation in Miscanthus sinensis seed germination under low temperatures. Grassl. Sci. 2014, 60, 194–198. [Google Scholar] [CrossRef]
- Weng, J.H.; Hsu, F.H. Variation of germination response to temperature in Formosan lily (Lilium formosanum Wall.) collected from different latitudes and elevations in Taiwan. Plant. Prod. Sci. 2006, 9, 281–286. [Google Scholar] [CrossRef]

| Influencing Factors | F |
|---|---|
| Seed storage | 128.5 * |
| Agricultural region | 21.1 * |
| Rice planting method | 0.1 |
| Seed storage × agricultural region | 7.4 * |
| Seed storage × rice planting method | 2.1 |
| Agricultural region × rice planting method | 0.8 |
| Seed storage × agricultural region × rice planting method | 1.6 |
| Agricultural Region | Days after Storage | ||
|---|---|---|---|
| 120 | 210 | 300 | |
| XH | 71.0 ± 2.9 ab | 80.3 ± 1.3 b | 92.0 ± 0.6 a |
| YH | 80.5 ± 5.7 a | 89.2 ± 1.5 a | 92.2 ± 0.8 a |
| LXH | 59.7 ± 3.9 b | 78.9 ± 2.0 b | 90.2 ± 1.0 ab |
| NZY | 66.5 ± 3.1 b | 68.5 ± 2.4 d | 87.6 ± 1.1 bc |
| YJ | 65.4 ± 3.6 b | 72.6 ± 1.3 cd | 87.4 ± 1.0 bc |
| TH | 62.8 ± 3.1 b | 77.6 ± 1.4 bc | 83.8 ± 1.1 c |
| JX | 41.9 ± 6.4 c | 67.2 ± 2.5 d | 85.7 ± 1.4 c |
| Seed Storage Period (Day) | Longitude | Latitude | ||
|---|---|---|---|---|
| Formula | R2 | Formula | R2 | |
| 120 | y = −2.92x + 414.59 | 0.014 | y = 5.35x − 109.21 | 0.077 * |
| 210 | y = −0.54x + 140.22 | 0.001 | y = 3.30x − 31.37 | 0.069 * |
| 300 | y = −0.94x + 200.55 | 0.008 | y = 2.39x + 10.33 | 0.081 * |
| Influencing Factors | F |
|---|---|
| Temperature | 205.6 * |
| Agricultural region | 21.0 * |
| Rice planting method | 4.3 * |
| Temperature × agricultural region | 10.3 * |
| Temperature × rice planting method | 0.8 |
| Agricultural region × rice planting method | 1.6 |
| Temperature × agricultural region × rice planting method | 0.8 |
| Agricultural Region | Treated Temperature (°C) | |||
|---|---|---|---|---|
| 20 | 25 | 30 | 35 | |
| XH | 55.5 ± 3.2 b | 87.7 ± 1.9 ab | 90.1 ± 1.0 ab | 91.8 ± 0.8 a |
| 0.8YH | 84.6 ± 2.2 a | 90.8 ± 1.5 a | 92.8 ± 0.8 a | 91.9 ± 1.0 a |
| LXH | 65.7 ± 6.6 b | 89.6 ± 1.4 a | 89.3 ± 1.4 ab | 90.6 ± 1.3 a |
| NZY | 63.0 ± 3.0 b | 85.7 ± 2.3 ab | 86.5 ± 1.4 b | 86.9 ± 1.4 ab |
| YJ | 57.9 ± 3.6 b | 81.4 ± 1.8 bc | 85.5 ± 1.5 b | 88.4 ± 1.5 a |
| TH | 37.8 ± 2.7 c | 79.8 ± 2.3 bc | 88.7 ± 1.1 ab | 82.4 ± 1.8 b |
| JX | 63.4 ± 3.2 b | 76.2 ± 3.7 c | 87.2 ± 2.6 b | 86.3 ± 2.1 ab |
| Temperature °C | Days | Longitude | Latitude | ||
|---|---|---|---|---|---|
| Formula | R2 | Formula | R2 | ||
| 20 °C | 4 | y = −3.06x + 388.89 | 0.016 * | y = 2.78x − 67.99 | 0.020 * |
| 14 | y = −4.96x + 649.83 | 0.035 * | y = 2.87x − 36.96 | 0.018 * | |
| 25 °C | 2 | y = −6.18x + 811.15 | 0.093 * | y = 6.57x − 142.16 | 0.160 * |
| 4 | y = −3.11x + 453.99 | 0.039 * | y = 3.90x − 45.51 | 0.092 * | |
| 14 | y = −3.04x + 448.15 | 0.044 * | y = 3.78x − 38.50 | 0.104 * | |
| 30 °C | 2 | y = −0.12x + 92.98 | 0.000 | y = 3.52x − 35.19 | 0.058 * |
| 4 | y = −0.10x + 97.49 | 0.000 | y = 2.57x + 2.60 | 0.055 * | |
| 14 | y = −0.48x + 145.46 | 0.002 | y = 2.64x + 1.84 | 0.089 * | |
| 35 °C | 2 | y = −2.85x + 401.40 | 0.017 * | y = 4.47x − 85.23 | 0.064 * |
| 4 | y = −0.40x + 132.80 | 0.001 | y = 0.86x + 57.13 | 0.009 | |
| 14 | y = −0.24x + 116.90 | 0.001 | y = 0.98x + 56.51 | 0.020 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, K.; Chen, L.; Liu, Y.; Wei, H.; Chen, G. Seed Dormancy and Germination Responses to Different Temperatures of Leptochloa chinensis (L.) Nees: A Case Study with 242 Populations Collected from Rice Fields in East China. Agronomy 2024, 14, 2177. https://doi.org/10.3390/agronomy14092177
An K, Chen L, Liu Y, Wei H, Chen G. Seed Dormancy and Germination Responses to Different Temperatures of Leptochloa chinensis (L.) Nees: A Case Study with 242 Populations Collected from Rice Fields in East China. Agronomy. 2024; 14(9):2177. https://doi.org/10.3390/agronomy14092177
Chicago/Turabian StyleAn, Kai, Ling Chen, Yiyang Liu, Haiyan Wei, and Guoqi Chen. 2024. "Seed Dormancy and Germination Responses to Different Temperatures of Leptochloa chinensis (L.) Nees: A Case Study with 242 Populations Collected from Rice Fields in East China" Agronomy 14, no. 9: 2177. https://doi.org/10.3390/agronomy14092177





