Control of Neuroinflammation through Radiation-Induced Microglial Changes
Abstract
:1. Introduction
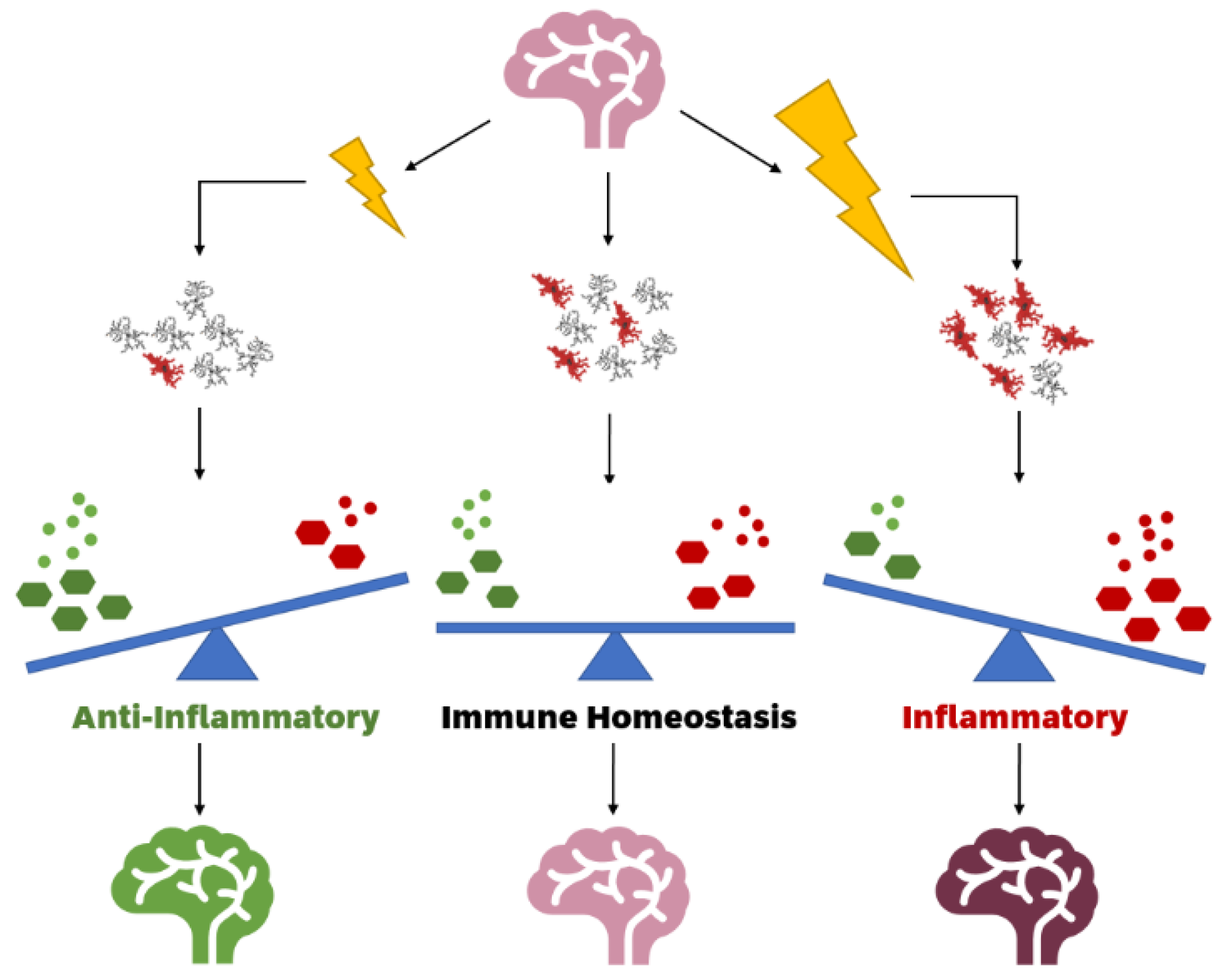
2. Functional States of Microglia Altered by Stressors
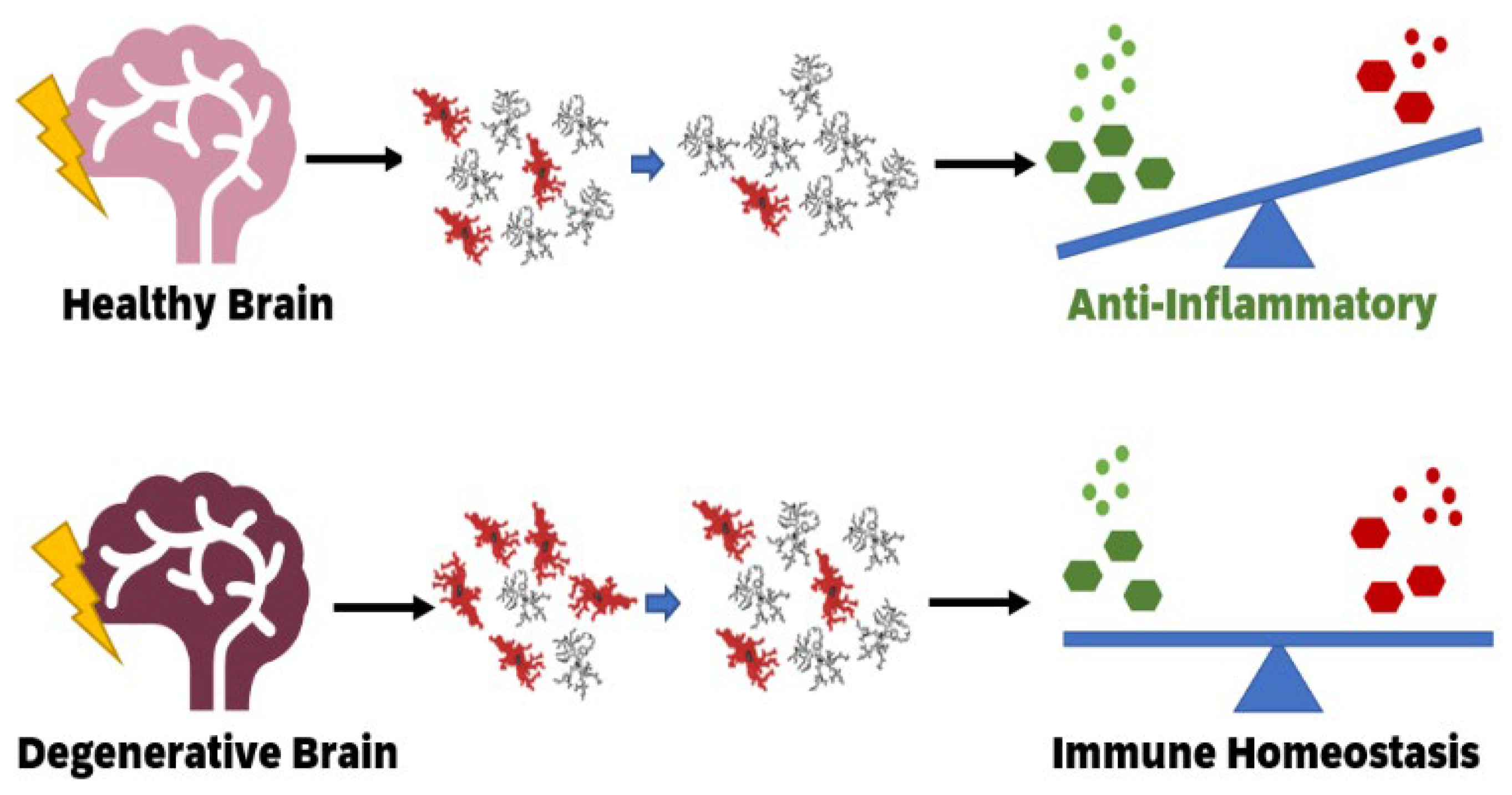
3. Impact of Ionising Radiation on Healthy Brains by Altering Microglial Function States
4. Impact of Low Dose Ionising Radiation on Neurodegenerative Diseases
5. TSPO as a Biomarker for Changes in Microglia
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Furdui, C.M. Ionizing radiation: Mechanisms and therapeutics. Antioxid. Redox Signal. 2014, 21, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, E.C. Radiation Risk From Medical Imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, K.M.; Seo, S.; Lee, D.; Kim, M.; Lee, S.; Parl, S.; Jin, Y.W. Is the Linear No-Threshold Dose-Response Paradigm Still Necessary for the Assessment of Health Effects of Low Dose Radiation? J. Korean Med. Sci. 2016, 31, S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; O’Connor, M.K. Estimating risk of low radiation doses—A critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat. Res. 2014, 182, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbron, R.W. Cancer risks from low dose exposure to ionising radiation—Is the linear no-threshold model still relevant? Radiography 2012, 18, 28–33. [Google Scholar] [CrossRef]
- Nakamura, N. A hypothesis: Radiation carcinogenesis may result from tissue injuries and subsequent recovery processes which can act as tumor promoters and lead to an earlier onset of cancer. Br. J. Radiol. 2020, 93, 20190843. [Google Scholar] [CrossRef]
- Williams, D. Radiation carcinogenesis: Lessons from Chernobyl. Oncogene 2008, 27 (Suppl. 2), S9–18. [Google Scholar] [CrossRef] [Green Version]
- Boice, J.D.; Held, K.D.; Shore, R.E. Radiation epidemiology and health effects following low-level radiation exposure. J. Radiol. Prot. 2019, 39, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, S.; Sreetharan, S.; Brooks, A.L.; Boreham, D.R. Re-evaluation of the linear no-threshold (LNT) model using new paradigms and modern molecular studies. Chem Biol. Interact. 2019, 301, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Reponse 2018, 16, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socol, Y. Reconsidering Health Consequences of the Chernobyl Accident. Dose-Response 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR 2020 REPORT: SOURCES, EFFECTS AND RISKS OF IONIZING RADIATION; United Nations: New York, NY, USA, 2020.
- Sutou, S. Black rain in Hiroshima: A critique to the Life Span Study of A-bomb survivors, basis of the linear no-threshold model. Genes Environ. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Calabrese, E.J. Key studies used to support cancer risk assessment questioned. Environ. Mol. Mutagenesis 2011, 52, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Muller’s Nobel Prize Lecture: When ideology prevailed over science. Toxicol. Sci. 2012, 126, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.-G.; Chen, S.-D. The changing phenotype of microglia from homeostasis to disease. Transl. Neurodegener. 2012, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.M.; Sun, Y.; Burns, T.C.; He, L.; Kee, N.; Oliva-Vilarnau, N.; Alevyzaki, A.; Zhou, K.; Louhivuori, L.; Uhlen, P.; et al. Radiation Triggers a Dynamic Sequence of Transient Microglial Alterations in Juvenile Brain. Cell Rep 2020, 31, 107699. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Egensperger, R.; Maassen, A.; Hager, G.; Kreutzberg, G.W.; Graeber, M.B. Mitochondria in activated microglia in vitro. J. Neurocytol. 2004, 33, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Moneta, M.E.; Gehrmann, J.; Töpper, R.; Banati, R.B.; Kreutzberg, G.W. Cell adhesion molecule expression in the regenerating rat facial nucleus. J. Neuroimmunol. 1993, 45, 203–206. [Google Scholar] [CrossRef]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp. Cell Res. 2016, 340, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.-W.; Yu, S.-W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. Biochem. Mol. Biol. Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef]
- Kim, S.; Chung, H.; Mai, H.N.; Nam, Y.; Shin, S.J.; Park, Y.H.; Chung, M.J.; Lee, J.K.; Rhee, H.Y.; Jahng, G.-H.; et al. Low-Dose Ionizing Radiation Modulates Microglia Phenotypes in the Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 4532. [Google Scholar] [CrossRef] [PubMed]
- Tournier, B.; Tsartsalis, S.; Ceyzériat, K.; Garibotto, V.; Millet, P. In Vivo TSPO Signal and Neuroinflammation in Alzheimer’s Disease. Cells 2020, 9, 1941. [Google Scholar] [CrossRef] [PubMed]
- Nogueria, M.L.; Epelbaum, S.; Steyaert, J.M.; Dubois, B.; Schwartz, L. Mechanical tress models of Alzheimer’s disease pathology. Alzheimers Dement. 2016, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Y.D.; Chen, Q.; Gao, Q.; Zhu, X.C.; Zhou, J.S.; Shi, J.Q.; Lu, H.; Tan, L.; Yu, J.T. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 2016, 105, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2020, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Terada, T.; Yokokura, M.; Yoshikawa, E.; Futatsubashi, M.; Kono, S.; Konishi, T.; Miyajima, H.; Hashizume, T.; Ouchi, Y. Extrastriatal spreading of microglial activation in Parkinson’s disease: A positron emission tomography study. Ann. Nucl. Med. 2016, 30, 579–587. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Filiou, M.D.; Arefin, A.S.; Moscato, P.; Graeber, M.B. ‘Neuroinflammation’ differs categorically from inflammation: Transcriptomes of Alzheimer’s disease, Parkinson’s disease,schizophrenia and inflammatory diseases compared. Neurogenetics 2014, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in CNS inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andoh, M.; Koyama, R. Microglia regulate synaptic development and plasticity. Dev. Neurbiology 2021. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front Pharm. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.C.; Vilalta, A. How Microglia kill neurons. Brain Reserach 2015, 1628, 288–297. [Google Scholar] [CrossRef]
- York, E.M.; Bernier, L.-P.; MacVicar, B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2017, 78, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Z.; Xiong, S.; Sun, F.; Qin, G.; Hu, G.; Wang, J.; Zhao, L.; Liang, Y.-X.; Wu, T.; et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 2018, 21, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.; Young, K.; Qureshi, M.; Rowe, R.K.; Lifshitz, J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep. 2017, 7, 13211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; Fernández-Llebrez, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeluyi, A.; Guerin, L.; Fisher, M.L.; Galloway, A.; Cole, R.D.; Chan, S.S.L.; Wyatt, M.D.; Davis, S.W.; Freeman, L.R.; Ortinski, P.I.; et al. Microglia morphololgy and proinflammatory signalling in the nucleus accumbens during nictoine withdrawal. Sci. Adv. 2019, 5, eaax7031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.S.; Ma, J.; Jegathees, T.; Goldsbury, C. Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer’s disease. Brain Pathol. 2017, 27, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Angelova, D.M.; Brown, D.R. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neuer. 2019, 151, 676–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidehpour, R.K.; Higdon, R.E.; Crawford, N.G.; Nelter, J.H.; Ighodaro, E.T.; Patel, E.; Price, D.; Nelson, P.T.; Bachstetter, A.D. Dystrophic microglia are a disease associated microglia morphology in the human brain. Neurobiol. Aging 2021, 22, 19–27. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Wooden, J.I.; Nixon, K. Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Front. Neuroanat. 2020, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Masao, K. MD-2, a Molecule that Confers Lipopolysaccharide Responsiveness on Toll-like Receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglial activation by microbial neuraminidase through TLR2 and TLR4 receptors. J. Neuroinflammation 2019, 16, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; O’Brien, C.E.; Bieri, G.; Czirr, E.; Mosher, K.I.; Abbey, R.J.; Mastroeni, D.F.; Jospeh, R.; Spencer, B.; Masliah, E.; et al. Microglial Beclin 1 Regulates Retromer Trafficking and Phagocytosis and Is Impaired in Alzheimer’s Disease. Neuron 2013, 79, 873–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Guo, Y.; Wei, X.; Yan, S.; Qin, Y.; Zhang, X.; Jiang, F.; Lou, H. TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp. Neurol. 2018, 302, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Karin, M. Missing Pieces in the NF-κB Puzzle. Cell 2002, 109, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Frakes, A.E.; Ferraiuolo, L.; Haidet-Phillips, A.M.; Schmelzer, L.; Braun, L.; Miranda, C.J.; Ladner, K.J.; Bevan, A.K.; Foust, K.D.; Godbout, J.P.; et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 2015, 81, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Gomes, C.; Vaz, A.R.; Brites, D. Exploring New Inflammatory Biomarkers and Pathways during LPS-Induced M1 Polarization. Mediat. Inflamm. 2016, 2016, 6986175. [Google Scholar] [CrossRef] [PubMed]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavieele, T.; Almeida, M.I. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Hiscott, J.; Marois, J.; Garoufalis, J.; D’Addario, M.; Roulston, A.; Kwan, I.; Pepin, N.; Lacoste, J.; Nguyen, H.; Bensi, G. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: Evidence for a positive autoregulatory loop. Mol. Cell. Biol. 1993, 13. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.-H.; Jeong, Y.-T.; Lee, K.-A.; Choi, K.-H.; Kim, S.-M.; Rhim, B.-Y.; Kim, K. Roles of MAPK and NF-kB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2008, 51, 71–77. [Google Scholar] [CrossRef]
- Baeuerle, P.; Vassalli, P.; Collart, M.A. Regulation of tumor necrosis factor alpha transcription in macrophages: Involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol. 1990, 10, 1498–1506. [Google Scholar]
- Guadagno, J.; Swan, P.; Cregan, S.P. Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis. 2015, 6, e1779. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Huang, Y.; Zhao, L.; Li, Y.; Sun, L.; Zhou, Y.; Qian, G.; Zheng, J.C. IL-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 2013, 125, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.I.; Bogetofte, H.; Ritter, L.; Agergaard, J.B.; Hammerick, D.; Kabiljagic, A.A.; Wlodarczyk, A.; Lopez, S.G.; Sørensen, M.D.; Jørgensen, M.L.; et al. Microglia-Secreted Factors Enhance Dopaminergic Differentiation of Tissueand iPSC-Derived Human Neural Stem Cells. Stem Cell Rep. 2021, 16, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski-Treska, J.; Ulrich, G.; Chasserot-Golaz, S.; Zwiller, J.; Revel, M.-O.; Aunis, D.; Bader, M.-F. Mechanisms Underlying Neuronal Death Induced by Chromogranin A-activated Microglia. J. Biol. Chem. 2001, 276, 13113–13120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.L.; Jones, F.; Kubota, E.S.F.C.S.; Pocock, J.M. Stimulation of Microglial Metabotropic Glutamate Receptor mGlu2 Triggers Tumor Necrosis Factor α-Induced Neurotoxicity in Concert with Microglial-Derived Fas Ligand. J. Neurosci. 2005, 25, 2952–2964. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Sobue, A.; Komine, O.; Hara, Y.; Endo, F.; Mizoguchi, H.; Watanabe, S.; Murayama, S.; Saito, T.; Saido, T.C.; Sahara, N.; et al. Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Emrani, S.; Arain, H.A.; DeMarshall, C.; Nuriel, T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: A systematic review. Alzheimer’s Res. Ther. 2020, 12, 141. [Google Scholar] [CrossRef]
- Wa, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar]
- Qin, L.; Liu, Y.; Wang, T.; Wei, S.-J.; Block, M.L.; Wilson, B.; Liu, B.; Hong, J.-S. NADPH Oxidase Mediates Lipopolysaccharide-induced Neurotoxicity and Proinflammatory Gene Expression in Activated Microglia. J. Biol. Chem. 2004, 279, 1415–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, S.J.; Bermudez-Sabogal, S.L.; Brynes, K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation 2013, 10, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Gao, J.-H.; Yan, Z.-F.; Huang, X.-Y.; Guo, P.; Sun, L.; Liu, Z.; Hu, Y.; Yu, S.-Y.; Cao, C.-J.; et al. Minimally toxic dose of lipopolysaccharide and α-Synuclein oligomer elicit synergistic dopaminergic neurodegeneration: Role and mechanism of microglial NOX2 activation. Mol. Neurobiol. 2018, 55, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; de lure, A.; Giampà, C.; Chiasserini, D.; Tozzi, A.; Orvietani, P.L.; Ghiglieri, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; et al. Persistent activation of microglia and NADPH oxidase drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 2016, 6, 20926. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baydoun, H.H.; Cherian, A.M.; Green, P.; Lee, R. Inducible nitric oxide synthase mediates DNA double strand breaks in Human T-Cell Leukemia Virus Type 1-induced leukemia/lymphoma. Retrovirology 2015, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Folkes, L.K.; O’Neill, P. DNA damage induced by nitric oxide during ionizing radiation is enhanced at replication. Nitric Oxide Chem. Biol. 2013, 34, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Clemons, N.J.; McColl, K.E.L.; Fitzgerald, R.C. Nitric oxide and acid induce double-strand DNA breaks in Barrett’s esophagus carcinogenesis via distinct mechanisms. Gastroentrerology 2007, 133, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Lepoivre, M.; Chenais, B.; Yapo, A.; Lemaire, G.; Thelander, L.; Tenu, J.-P. Alterations of Ribonucleotide Reductase Activity Following Induction of the Nitrite-generating Pathway in Adenocarcinoma Cells. J. Biol. Chem. 1990, 265, 14143–14149. [Google Scholar] [CrossRef]
- Bernier, L.-P.; Bohlen, C.J.; York, E.M.; Choi, H.B.; Kamyabi, A.; Dissing-Olesen, L.; Hefendehl, J.K.; Collins, H.Y.; Stevens, B.; Barres, B.A.; et al. Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep. 2019, 27, 2895–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialas, A.R.; Stevens, B. TGF-β Signaling Regulates Neuronal C1q Expression and Developmental Synaptic Refinement. Nat. Neurosci. 2013, 16, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2015, 136, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Walton, N.M.; Sutter, B.M.; Laywell, E.D.; Levkoff, L.H.; Kearns, S.M.; Marshall, G.P.; Scheffler, B.; Steindler, D.A. Microglia instruct subventricular zone neurogenesis. Glia 2006, 54, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Schafer, D.P.; Stevens, B. Phagocytic glial cells: Sculpting synaptic circuits in the developing nervous system. Curr. Opin. Neurobiol. 2013, 23, 1034–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ge, H.; Liu, W.; Zhu, H.; Chen, Y.; Zhang, X.; Yang, Y.; Yin, Y.; Chen, W.; Wu, W.; et al. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARγ signaling pathway. Oncotarget 2017, 8, 19855–19865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; Nabekura, J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R.; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.-B. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douple, E.B.; Mabuchi, K.; Cullings, H.M.; Preston, D.L.; Kodama, K.; Shimizu, Y.; Fujiwara, S.; Shore, R.E. Long-term Radiation-Related Health Effects in a Unique Human Population: Lessons Learned from the Atomic Bomb Survivors of Hiroshima and Nagasaki. Disaster Med. Public Health Prep. 2011, 5, S122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, A.; Tanigawa, K.; Ohtsuru, A.; Yabe, H.; Maeda, M.; Shigemura, J.; Ohira, T.; Tominaga, T.; Akashi, M.; Hirohashi, N.; et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet 2015, 386, 479–488. [Google Scholar] [CrossRef]
- Tran, L.; Seeram, E. Current Perspectives on the Use of the Linear Non-Threshold (LNT) Model. Int. J. Radiol. Med Imaging 2017, 3, 1–8. [Google Scholar]
- Cramer, C.K.; McKee, N.; Case, L.D.; Chan, M.D.; Cummings, T.L.; Lesser, G.J.; Shaw, E.G.; Rapp, S.R. Mild cognitive impairment in long-term brain tumor survivors following brain irradiation. J. Neuro-Oncol. 2019, 141, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Barani, I.J.; Cuttino, L.W.; Benedict, S.H.; Todor, D.; Bump, E.A.; Wu, Y.; Chund, T.D.; Broaddus, W.C.; Lin, P.-S. Neural stem cell-preserving external-beam radiotherapy of central nervous system malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 978–985. [Google Scholar] [CrossRef]
- Acharya, M.M.; Green, K.N.; Allen, B.D.; Najafi, A.R.; Syage, A.; Minasyan, H.; Le, M.T.; Kawashita, T.; Giedzinski, E.; Parihar, V.K.; et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci. Rep. 2016, 6, 31545. [Google Scholar] [CrossRef]
- Farjam, R.; Pramanik, P.; Aryal, M.P.; Srinivasan, A.; Chapman, C.H.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. A radiation-induced hippocampal vascular injury surrogate marker predicts late neurocognitive dysfunction. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Raber, J.; Rola, R.; LeFevour, A.; Morhardt, D.; Curley, J.; Mizumatsu, S.; VandenBerg, S.R.; Fike, J.R. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004, 162, 39–47. [Google Scholar] [CrossRef]
- Rola, R.; Raber, J.; Rizk, A.; Otsuka, S.; VandenBerg, S.R.; Morhardt, D.R.; Fike, J.R. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. 2004, 188, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme Sensitivity of Adult Neurogenesis to Low Doses of X-Irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar]
- Tallet, A.V.; Azria, D.; Barlesi, F.; Spano, J.-P.; Carpentier, A.F.; Goncalves, A.; Metellus, P. Neurocognitive function impairment after whole brain radiotherapy for brain metastases: Actual assessment. Radiat. Oncol. 2012, 7, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casciati, A.; Dobos, K.; Antonelli, F.; Benedek, A.; Kempf, S.J.; Belles, M.; Balogh, A.; Tanori, M.; Heredia, L.; Atkinson, M.J.; et al. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 2016, 7, 28040–28058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helson, L. Radiation-induced Demyelination and Remyelination in the Central Nervous System: A Literature Review. Anticancer Res. 2018, 38, 4999–5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, M. Cranial Radiation Therapy and Damage to Hippocampal Neurogenesis. Dev. Disabil. Res. Rev. 2008, 14, 238–242. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Rola, R.; Fike, J.R. Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res. 2008, 331, 251–262. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, Z.; Han, L.; Yang, Y.; Li, J.; Liu, S.; Lv, X. Network-level dysconnectivity in patients with nasopharyngeal carcinoma (NPC) early post-radiotherapy: Longitudinal resting state fMRI study. Brain Imaging Behav. 2018, 12, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.; Ruffer, J.; Corn, B.; DeVries, K.; Mollman, J. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: Neuropsychologic outcome and proposed mechanisms. J. Clin. Oncol. 1995, 13, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Khan, A.R.; Modi, S.; Kumar, B.S.H.; Javed, S.; Tripathi, R.P.; Kushu, S. Altered brain metabolism after whole body irradiation in mice: A preliminary in vivo 1H MRS study. Int. J. Radiat. Biol. 2013, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Jenrow, K.A.; Brown, S.L.; Lapanowski, K.; Naei, H.; Kolozsvary, A.; Kim, J. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat. Res. 2013, 179, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Schindler, M.K.; McQuail, J.A.; Forbes, M.E.; Riddle, D.R. Regionally distinct responses of microglia and glial progenitor cells to whole brain irradiation in adult and aging rats. PLoS ONE 2012, 7, e52728. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Chong, Z.Z.; De Toledo, S.M.; Azzam, E.I.; Elkabes, S.; Souayah, N. Delayed activation of human microglial cells by high dose ionizing radiation. Brain Res. 2016, 1646, 193–198. [Google Scholar] [CrossRef]
- Ung, M.-C.; Garrett, L.; Dalke, C.; Leitner, V.; Dragosa, D.; Hladik, D.; Neff, F.; Wagner, F.; Zitzelsberger, H.; Miller, G.; et al. Dose-dependent long-term effects of a single radiation event on behaviour and glial cells. Int. J. Radiat. Biol. 2020, 97, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Boström, M.; Ek, C.J.; Li, T.; Xie, C.; Xu, Y.; Sun, Y.; Blomgren, K.; Zhu, C. Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum. Sci. Rep. 2017, 7, 46181. [Google Scholar] [CrossRef]
- Ismail, A.F.M.; El-Sonbaty, S.M. Fermentation enhances Ginkgo biloba protective role on gamma-irradiation induced neuroinflammatory gene expression and stress hormones in rat brain. J. Photochem. Photobiol. B: Biol. 2016, 158, 154–163. [Google Scholar] [CrossRef]
- Limoli, C.L.; Giedzinski, E.; Rola, R.; Otsuka, S.; Palmer, T.D.; Fike, J.R. Radiation response of neural precursor cells: Linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat. Res. 2004, 161, 17–27. [Google Scholar] [CrossRef]
- Kam, W.W.-Y.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridharan, D.M.; Asaithamby, A.; Bailey, S.M.; Costes, S.V.; Doestch, P.W.; Dynan, W.S.; Kronenberg, A.; Rithidech, K.N.; Saha, J.; Snijders, A.M. Understanding cancer development processes after HZE-particle exposure: Roles of ROS, DNA damage repair and inflammation. Radiat. Res. 2015, 183, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Rodriguez, O.C.; Winters, T.A.; Fornace, A.J.; Albanese, C.; Datta, K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging 2013, 5, 607–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fishman, K.; Baure, J.; Zou, Y.; Huang, T.-T.; Andres-Mach, M.; Rola, R.; Suarez, T.; Acharya, M.; Limoli, C.L.; Lamborn, K.R.; et al. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic. Biol. Med. 2009, 47, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Singla, N.; Chadha, V.D.; Dhawan, D.K. A concept of radiation hormesis. Stimulation of antioxidant machinery in rats by low dose ionizing radiation. Hell. J. Nucl. Med. 2019, 22, 43–48. [Google Scholar]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Jopson, T.D.; Liu, S.; Gupta, N.; Rosi, S. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE 2014, 9, e93650. [Google Scholar] [CrossRef]
- Acharya, M.M.; Patel, N.H.; Craver, B.M.; Tran, K.K.; Giedzainski, E.; Tseng, B.P.; Parihar, V.K.; Limoli, C.L. Consequences of low dose ionizing radiation exposure on the hippocampal microenvironment. PLoS ONE 2015, 10, e1028316. [Google Scholar] [CrossRef] [PubMed]
- Kalm, M.; Fukuda, A.; Fukuda, H.; Ohrfelt, A.; Lannering, B.; Björk-Eriksson, T.; Blennow, K.; Márky, I.; Blomgran, K. Transient inflammation in neurogenic regions after irradiation of the developing brain. Radiat. Res. 2009, 171, 66–76. [Google Scholar] [CrossRef]
- Leach, J.K.; Van Tuyle, G.; Lin, P.-S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar] [PubMed]
- Takács, S.F.; Benedek, A.; Mán, I.; Ozsvári, B.; Puskás, L.G.; Neefs, M.; Benotmane, M.A.; Sáfrány, G.; Lumniczky, K. Analysis of radiation-induced blood-brain barrier damage in mice by in vivo bio-imaging technique. Cent. Eur. J. Occup. Environ. Med. 2015, 21, 87–95. [Google Scholar]
- Liao, H.; Wang, H.; Rong, X.; Li, E.; Xu, R.-H.; Peng, Y. Mesenchymal Stem Cells Attenuate Radiation-Induced Brain Injury by Inhibiting Microglia Pyroptosis. BioMed Res. Int. 2017, 2017, 1948985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, B.A.; Fernades, J.P.; Doan, M.A.L.; Schmitt, L.M.; Branton, W.G.; Power, C. Activation of the executioner caspases-3 and -7 promotes microglial pyroptosis in models of multiple sclerosis. J. Neuroinflammation 2020, 17, 253. [Google Scholar] [CrossRef]
- Li, M.D.; Burns, T.C.; Kumar, S.; Morgan, A.A.; Sloan, S.A.; Palmer, T.D. Aging-like Changes in the Transcriptome of Irradiated Microglia. Glia 2015, 63, 754–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krukowski, K.; Feng, X.; Paladini, M.S.; Chou, A.; Sacramento, K.; Grue, K.; Riparip, L.-K.; Jones, T.; Campbell-Beachler, M.; Nelson, G.; et al. Temporary microglia-depletion after cosmic radiation modifies phagocytic activity and prevents cognitive deficits. Sci. Rep. -Nat. 2018, 8, 7857. [Google Scholar] [CrossRef]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkle, J.J.; Olschowka, J.A.; Love, T.M.; Williams, J.P.; O’Banion, M.K. Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Sci. Rep. 2019, 9, 18800. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Radiation and Pregnancy: Infomation for Clinicians; CDC: Altanta, GA, USA, 2019. [Google Scholar]
- Preston, D.L.; Cullings, H.; Suyama, A.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K.; Kasagi, F.; Shore, R.E. Solid Cancer Incidence in Atomic Bomb Survivors Exposed In Utero or as Young Children. J. Natl. Cancer Inst. 2008, 100, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, M.; Cesari, E.; Nobili, E.; Straface, G.; Cavaliere, A.F.; Caruso, A. Radiation effects on development. Birth Defects Res. Part C 2007, 81, 177–182. [Google Scholar] [CrossRef]
- Guilbaud, L.; Beghin, D.; Dhombres, F.; Blondiaux, E.; Friszer, S.; Le Pointe, H.D.; Éléfant, E.; Jouannic, J.-M. Pregnancy outcome after first trimester exposure to ionizing radiations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 232, 18–21. [Google Scholar] [CrossRef]
- Schull, W.J.; Otake, M. Cognitive Function and Prenatal Exposure to Ionising Radiation. Teratology 1999, 59, 222–226. [Google Scholar] [CrossRef]
- Verreet, T.; Rangaraja, J.R.; Quintens, R.; Verslegers, M.; Lo, A.C.; Govaerts, K.; Neefs, M.; Leysen, L.; Baatout, S.; Maes, F.; et al. Persistent Impact of In utero Irradiation on Mouse Brain Structure and Function Characterized by MR Imaging and Behavioral Analysis. Front. Behav. Neurosci. 2016, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Barazzuol, L.; Rickett, N.; Ju, L.; Jeggo, P.A. Low levels of endogenous or X-ray-induced DNA double-strand breaks activate apoptosis in adult neural stem cells. J. Cell Sci. 2015, 128, 3597–3606. [Google Scholar] [PubMed] [Green Version]
- Roque, T.; Haton, C.; Etienne, O.; Chicheportiche, A.; Rousseau, L.; Martin, L.; Mouthon, M.A.; Boussin, F.D. Lack of a p21waf1/cip-Dependent G1/S Checkpoint in Neural Stem and Progenitor Cells After DNA Damage In Vivo. Stem Cells 2011, 30, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.C.; Pinto, M.; Antonelli, F.; Amicarelli, F.; Balata, M.; Belli, M.; Conti Devirgiliis, L.; Ioannucci, L.; Nisi, S.; Sapora, O.; et al. The Cosmic Silence experiment: On the putative adaptive role of environmental ionizing radiation. Radiat. Environ. Biophys. 2009, 48, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.M.; Kim, C.S.; Lee, B.-S.; Nam, S.Y.; Kang, K.H.; Kim, J.-Y.; Park, J.-J.; Min, K.-J.; Jin, Y.-W. Low-dose radiation induces Drosophila innate immunity through Toll pathway activation. J. Radiat. Res. 2012, 53, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, K.M.; Kim, C.S.; Seo, S.-W.; Jeon, H.Y.; Lee, B.-S.; Nam, S.Y.; Yang, K.H.; Kim, J.-Y.; Kim, C.-S.; Min, K.-J.; et al. Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology 2011, 12, 93–107. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Iavicoli, I.; Calabrese, V. Hormesis: Why it is important to biogerontologists. Biogerontology 2012, 13, 215–235. [Google Scholar] [CrossRef]
- Dalke, C.; Neff, F.; Bains, S.K.; Bright, S.; Lord, D.; Reitmeir, P.; Rößler, U.; Samaga, D.; Unger, K.; Braselmann, H.; et al. Lifetime study in mice after acute low-dose ionizing radiation: A multifactorial study with special focus on cataract risk. Radiat. Environ. Biophys. 2018, 57, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Gori, T.; Münzel, T. Biological effects of low-dose radiation: Of harm and hormesis. Eur. Heart J. 2012, 33, 292–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, L.; Chen, W.-K.e.; Liu, S.-T.; Chang, C.-R.; Kao, M.-C.; Chen, K.-W.; Chiu, S.-C.; Hsu, M.-L.; Hsiang, I.-C.; Chen, Y.-J.; et al. Low-dose ionizing radiation induces mitochondrial fusion and increases expression of mitochondrial complexes I and III in hippocampal neurons. Oncotarget 2015, 6, 30628–30629. [Google Scholar] [CrossRef] [Green Version]
- Baulch, J.E.; Craver, B.M.; Tran, K.K.; Yu, L.; Chmielewski, N.; Allen, B.D.; Limoli, C.L. Persistent oxidative stress in human neural stem cells exposed to low fluences of charged particles. Redox. Biol. 2015, 5, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.L.; Tedesco, I.; Russo, M.; Cioppa, A.; Anreassi, M.G.; Picano, E. Cellular adaptive response to chronic radiation exposure in interventional cardiologists. Eur. Heart J. 2012, 33, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eken, A.; Aydin, A.; Erdem, O.; Akay, C.; Sayal, A.; Somuncu, I. Induced antioxidant activity in hospital staff occupationally exposed to ionizing radiation. Int. J. Radiat. Biol. 2012, 88, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Kang, H.; Hong, E.-H.; Kim, J.Y.; Nam, S.Y. Transcriptome analysis of low-dose ionizing radiation-impacted genes in CD4+ T-cells undergoing activation and regulation of their expression of select cytokines. J. Immunotoxicol. 2018, 15, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukimoto, M.; Nakatsukasa, H.; Sugawara, K.; Yamashita, K.; Kojima, S. Repeated 0.5-Gy gamma irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL17 production. Radiat. Res. 2008, 170, 429–436. [Google Scholar] [CrossRef]
- Shimura, N.; Kojima, S. Effects of low-dose-gamma rays on the immune system of different animal models of disease. Dose Response 2014, 12, 429–465. [Google Scholar] [CrossRef] [Green Version]
- Sekihara, K.; Saitoh, K.; Yang, H.; Kawashima, H.; Kazuno, S.; Kikkawa, M.; Arai, H.; Miida, T.; Hayashi, N.; Sasai, K.; et al. Low-dose ionizing radiation exposure represses the cell cycle and protein synthesis pathways in in vitro human primary keratinocytes and U937 cell lines. PLoS ONE 2018, 13, e0199117. [Google Scholar]
- Sandor, N.; Walter, F.R.; Bocsik, A.; Santha, P.; Schilling-Toth, B.; Lener, V.; Varga, Z.; Kahan, Z.; Deli, M.A.; Sáfrány, G.; et al. Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS ONE 2014, 9, e112397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hladik, D.; Dalke, C.; von Toerne, C.; Hauck, S.M.; Azimzadeh, O.; Philipp, J.; Ung, M.-C.; Schlattl, H.; Rößler, U.; Graw, J.; et al. CREB Signaling Mediates Dose-Dependent Radiation Response in the Murine Hippocampus Two Years after Total Body Exposure. J. Proteome Res. 2020, 19, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Howell, N.R.; Storer, B.; Davis, E.; Davies, J.; Banati, R.B.; Liu, G.-J. Mitochondrial translocator protein (TSPO) expression in the brain after whole body gamma irradiation. Front. Cell Dev. Biol. 2021, in press. [Google Scholar]
- Matthews, J.D.; Forsythe, A.V.; Brady, Z.; Bulter, M.W.; Goegen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A.; et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. Br. Med. J. 2013, 346, f2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.L.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J. Natl. Cancer Inst. Monogr. 2020, 2020, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Pasqual, E.; de Basea, M.B.; López-Vicente, M.; Thierry-Chef, I.; Cardis, E. Neurodevelopmental effects of low dose ionizing radiation exposure: A systematic review of the epidemiological evidence. Environ. Int. 2020, 136, 105371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Liu, A.; Gurvitz, M.; Guo, L.; Therrien, J.; Laprise, C.; Kaufman, J.S.; Abrahamowicz, M.; Marelli, A.J. Exposure to Low-Dose Ionizing Radiation From Cardiac Procedures and Malignancy Risk in Adults With Congenital Heart Disease. Circulation 2018, 137, 1334–1345. [Google Scholar] [CrossRef]
- Lumniczky, K.; Szatmari, T.; Safrany, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front Immunol 2017, 8, 517. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.R.; Loganovsky, K. Low dose or low dose rate ionizing radiation-induced health effect in the human. J. Environ. Radioact. 2018, 192, 32–47. [Google Scholar] [CrossRef]
- Safrany, G.; Lumniczky, K.; Manti, L. New Discoveries in Radiation Science. Cancers 2021, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Okabe, A.; Uchihori, Y.; Kitamura, H.; Sekine, E.; Ebisawa, S.; Suzuki, M.; Okayasu, R. Single extreme low dose/low dose rate irradiation causes alteration in lifespan and genome instability in primary human cells. Br. J. Cancer 2007, 96, 1707–1710. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freji, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 2013, 4, e00264-13. [Google Scholar] [CrossRef] [Green Version]
- Italiani, P.; Mazza, E.M.C.; Lucchesi, D.; Cifola, I.; Gemelli, C.; Grande, A.; Battaglia, C.; Bicciato, S.; Boraschi, D. Transcriptomic Profiling of the Development of the Inflammatory Response in Human Monocytes In Vitro. PLoS ONE 2014, 9, e87680. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hinshaw, R.G.; Le, K.X.; Park, M.-A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Shi, Q.; Holton, P.; et al. Space-like 56Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer’s-like transgenic mice. Nat. Sci. Rep. 2019, 9, 12118. [Google Scholar] [CrossRef]
- Ceyzériat, K.; Zilli, T.; Fall, A.B.; Millet, P.; Koutsouvelis, N.; Dipasquale, G.; Frisoni, G.B.; Tournier, B.B.; Garibotto, V. Treatment by low-dose brain radiation therapy improves memory performances without changes of the amyloid load in the TgF344-AD rat model. Neurobiol. Aging 2021, 103, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Treatment of Alzheimer Disease With CT Scans: A Case Report. Dose-Response 2016, 14, 1559325816640073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Update on a Patient With Alzheimer Disease Treated With CT Scans. Dose-Response 2017, 15, 1559325817693167. [Google Scholar] [CrossRef] [Green Version]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Second Update on a Patient With Alzheimer Disease Treated by CT Scans. Dose-Response 2018, 16, 1559325818756461. [Google Scholar] [CrossRef] [Green Version]
- Cuttler, J.M.; Abdellah, E.; Goldberg, Y.; Al-Shamaa, S.; Symons, S.P.; Black, S.E.; Freedman, M. Low Doses of Ionizing Radiation as a Treatment for Alzheimer’s Disease: A Pilot Study. J. Alzheimers Dis. 2021, 80, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Devereux, C.; Troiano, R.; Zito, G.; Hafstein, M.; Lavenhar, M.; Hernandez, E.; Dowling, P.C. Total lymphoid irradiation in multiple sclerosis: Blood lymphocytes and clinical course. Ann. Neurol. 1987, 22, 634–638. [Google Scholar] [CrossRef]
- Devereux, C.K.; Vidaver, R.; Hafstein, M.P.; Zito, G.; Troiano, R.; Dowling, P.C.; Cook, S.D. Total lymphoid irradiation for multiple sclerosis. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 197–203. [Google Scholar] [CrossRef]
- Cook, S.D.; Troiano, R.; Zito, G.; Rohowsky-Kochan, C.; Sheffit, A.; Dowling, P.C.; Devereux, C.K. Deaths after Total Lymphoid Irradiation for Multiple Sclerosis. Lancet 1989, 334, 277–278. [Google Scholar] [CrossRef]
- Shaygannejad, V.; Zare, M.; Maghzi, H.; Emami, P. Brain radiation and possible presentation of multiple sclerosis. J. Res. Med Sci. 2013, 18, S93–S95. [Google Scholar] [PubMed]
- Murphy, C.B.; Hashimoto, S.A.; Graeb, D.; Thiessen, B.A. Clinical Exacerbation of Multiple Sclerosis Following Radiotherapy. Achives Neurol. 2003, 60, 273–275. [Google Scholar] [CrossRef] [Green Version]
- McMeekin, R.R.; Hardman, J.M.; Kempe, L.G. Multiple Sclerosis After X-radiation. Achives Otolaryngol. -Head Neck Surg. 1969, 90, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Axelson, O.; Landtblom, A.-M.; Flodin, U. Multiple sclerosis and ionizing radiation. Neuroepidemiology 2001, 20, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.C.; Lachance, D.H.; Lucchinetti, C.F.; Keegan, B.M.; Gavrilova, R.H.; Brown, P.D.; Weinshenker, B.G.; Rodriguez, M. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: The Mayo Clinic experience. Int. J. Radiat. Oncol. -Biol. -Phsyics 2006, 66, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Motamed, M.R.; Fereshtehnejad, S.-M.; Abbasi, M.; Sanei, M.; Abbaslou, M.; Meysami, S. X-ray radiation and the risk of multiple sclerosis: Do the site and dose of exposure matter? Med. J. Islamic Repub. Iran 2014, 28, 145. [Google Scholar]
- DeLuca, H.F.; Plum, L. UVB radiation, vitamin D and multiple sclerosis. Photochem. Photobiol. Sci. 2017, 16, 411–415. [Google Scholar] [CrossRef]
- Irving, A.A.; Marling, S.J.; Seeman, J.; Plum, L.A.; DeLuca, H.F. UV light suppression of EAE (a mouse model of multiple sclerosis) is independent of vitamin D and its receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 22552–22555. [Google Scholar] [CrossRef] [PubMed]
- Helis, C.A.; McTyre, E.; Munley, M.T.; Bourland, J.D.; Lucas, J.T.; Cramer, C.K.; Tatter, S.B.; Laxton, A.W.; Chan, M.D. Gamma Knife Radiosurgery for Multiple Sclerosis-Associated Trigeminal Neuralgia. Neurosurgery 2019, 85, E933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, S.; Allan, R.S.; Patanjali, N.; Barnett, M.H.; Jonker, B.P. Neurological deficit following stereotactic radiosurgery for trigeminal neuralgia. J. Clin. Neurosci. 2016, 34, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tago, F.; Fang, S.-P.; Shimura, N.; Kojima, S. Repeated 0.5-Gy γ-ray irradiation attenuates autoimmune manifestations in MRL-lpr/lpr mice. Int. J. Radiat. Biol. 2005, 81, 731–740. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Korzhevskii, D.E.; Kirik, O.V. Brain Microglia and Microglial Markers. Neurosci. Behav. Physiol. 2015, 46, 284–290. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, X.; Zhao, L.; Ma, W.; Rodriguez, I.R.; Fariss, R.N.; Wong, W.T. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J. Neurosci. 2014, 34, 3793–3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banati, R.B.; Goerres, G.W.; Myers, R.; Gunn, R.N.; Turkheimer, F.E.; Kreutzberg, G.W.; Brooks, D.J.; Jones, T.; Duncan, J.S. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen’s encephalitis. Neurology 1999, 53, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Pappata, S.; Levasseur, M.; Gunn, R.N.; Myers, R.; Crouzel, C.; Syrota, A.; Jones, T.; Kreatuzberg, G.T.; Banati, R.B. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [(11)C]PK11195. Neurology 2000, 55, 1052–1054. [Google Scholar] [CrossRef]
- Cagnin, A.; Kassiou, M.; Meikle, S.R.; Banati, R.B. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol. Scand. 2006, 114, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Gerhard, A.; Banati, R.B. In vivo imaging of neuroinflammation. Eur. Neuropsychopharmacol. 2002, 12, 581–586. [Google Scholar] [CrossRef]
- Banati, R.B. Visualising microglial activation in vivo. Glia 2002, 40, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Pannell, M.; Economopoulos, V.; Wilson, T.C.; Kersemans, V.; Isenegger, P.G.; Larkin, J.R.; Smart, S.; Gilchrist, S.; Gouverneur, V.; Sibson, N.R. Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 2019, 68, 280–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betlazar, C.; Harrison-Brown, M.; Middleton, R.J.; Banati, R.; Liu, G.-J. Cellular Sources and Regional Variations in the Expression of the Neuroinflammatory Marker Translocator Protein (TSPO) in the Normal Brain. Int. J. Mol. Sci. 2018, 19, 2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral Benzodiazepine Receptor/Translocator Protein Global Knock-out Mice Are Viable with No Effects on Steroid Hormone Biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef] [Green Version]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.-Y.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014, 5, 5452. [Google Scholar] [CrossRef]
- Liu, G.J.; Middleton, R.J.; Hatty, C.R.; Kam, W.W.; Chan, R.; Pham, T.; Harrison-Brown, M.; Dodson, E.; Veale, K.; Banati, R.B. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014, 24, 631–653. [Google Scholar] [CrossRef] [Green Version]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.J. The impact of high and low dose ionising radiation on the central nervous system. Redox. Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Yao, R.; Pan, R.; Shang, C.; Li, X.; Cheng, J.; Xu, J.; Li, Y. Translocator Protein 18 kDa (TSPO) Deficiency Inhibits Microglial Activation and Impairs Mitochondrial Function. Front. Pharmacol. 2020, 11, 986. [Google Scholar] [CrossRef]
- Meng, Y.; Tian, M.; Yin, S.; Lai, S.; Zhou, Y.; Chen, J.; He, M.; Liao, Z. Downregulation of TSPO expression inhibits oxidative stress and maintains mitochondrial homeostasis in cardiomyocytes subjected to anoxia/reoxygenation injury. Biomed. Pharmacother. 2020, 121, 109588. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, T.R.; Loth, M.K.; Guariglia, S.R. TSPO Finds NOX2 in Microglia for Redox Homeostasis. Trends Pharmacol. Sci. 2016, 37, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.; Caramoy, A.; Bhuckory, M.B.; Rashid, K.; Chen, M.; Xu, H.; Grimm, C.; Langmann, T. Targeting translocator protein (18 kda) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. J. Neuroinflammation 2015, 12, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monga, S.; Nagler, R.; Amara, R.; Weizman, A.; Gavish, M. Inhibitory Effects of the Two Novel TSPO Ligands 2-Cl-MGV-1 and MGV-1 on LPS-induced Microglial Activation. Cells 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Liu, Y.; Zhang, R.; Liang, Y.; Lan, N.; Ma, B. TSPO Ligands PK11195 and Midazolam Reduce NLRP3 Inflammasome Activation and Proinflammatory Cytokine Release in BV-2 Cells. Front. Cell. Neurosci. 2020, 14, 544431. [Google Scholar] [CrossRef] [PubMed]
- Notter, T.; Schalbetter, S.M.; Clifton, N.E.; Mattei, D.; Richetto, J.; Thomas, K.; Meyer, U.; Hall, J. Neuronal activity increases translocator protein (TSPO) levels. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, J.; Perrin, R.J.; Mach, R.H.; Bales, K.R.; Morris, J.C.; Benzinger, T.L.S.; Holtzman, D.M. Translocator protein in late stage Alzheimer’s disease and Dementia with Lewy bodies brains. Ann. Clin. Transl. Neurol. 2019, 6, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.; Ory, D.; Geric, I.; Declercq, L.; Koole, M.; Kassiou, M.; Bormans, G.; Baes, M. Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Mol. Imaging Biol. 2018, 20, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nettis, M.A.; Veronese, M.; Nikkheslat, N.; Mariani, N.; Lombardo, G.; Sforzini, L.; Enache, D.; Harrison, N.A.; Turkheimer, F.E.; Modelli, V.; et al. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Transl. Psychiatry 2020, 10, 89. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, A.; Byrne, S.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. Control of Neuroinflammation through Radiation-Induced Microglial Changes. Cells 2021, 10, 2381. https://doi.org/10.3390/cells10092381
Boyd A, Byrne S, Middleton RJ, Banati RB, Liu G-J. Control of Neuroinflammation through Radiation-Induced Microglial Changes. Cells. 2021; 10(9):2381. https://doi.org/10.3390/cells10092381
Chicago/Turabian StyleBoyd, Alexandra, Sarah Byrne, Ryan J. Middleton, Richard B. Banati, and Guo-Jun Liu. 2021. "Control of Neuroinflammation through Radiation-Induced Microglial Changes" Cells 10, no. 9: 2381. https://doi.org/10.3390/cells10092381






