Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain
Abstract
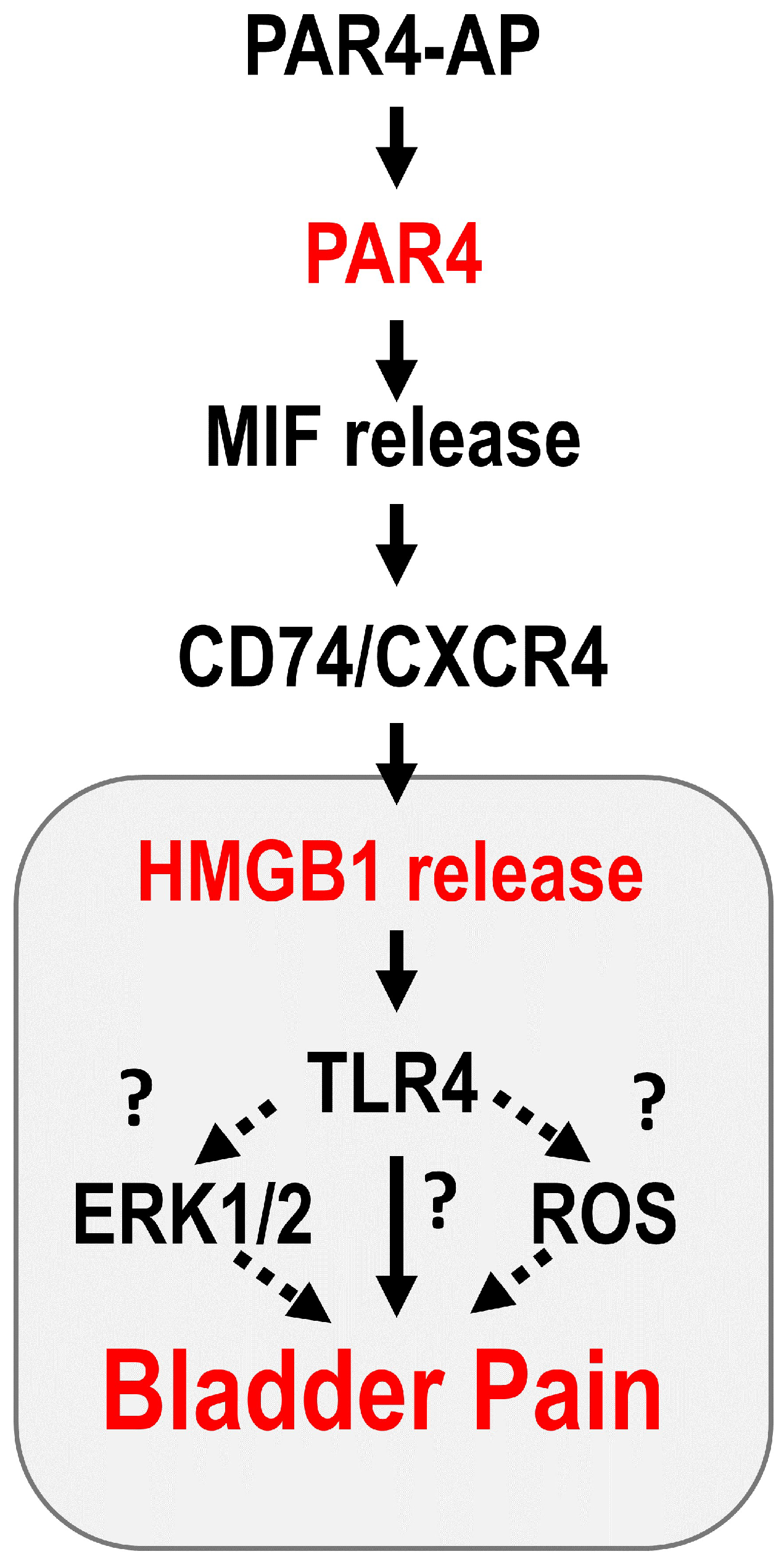
:1. Introduction
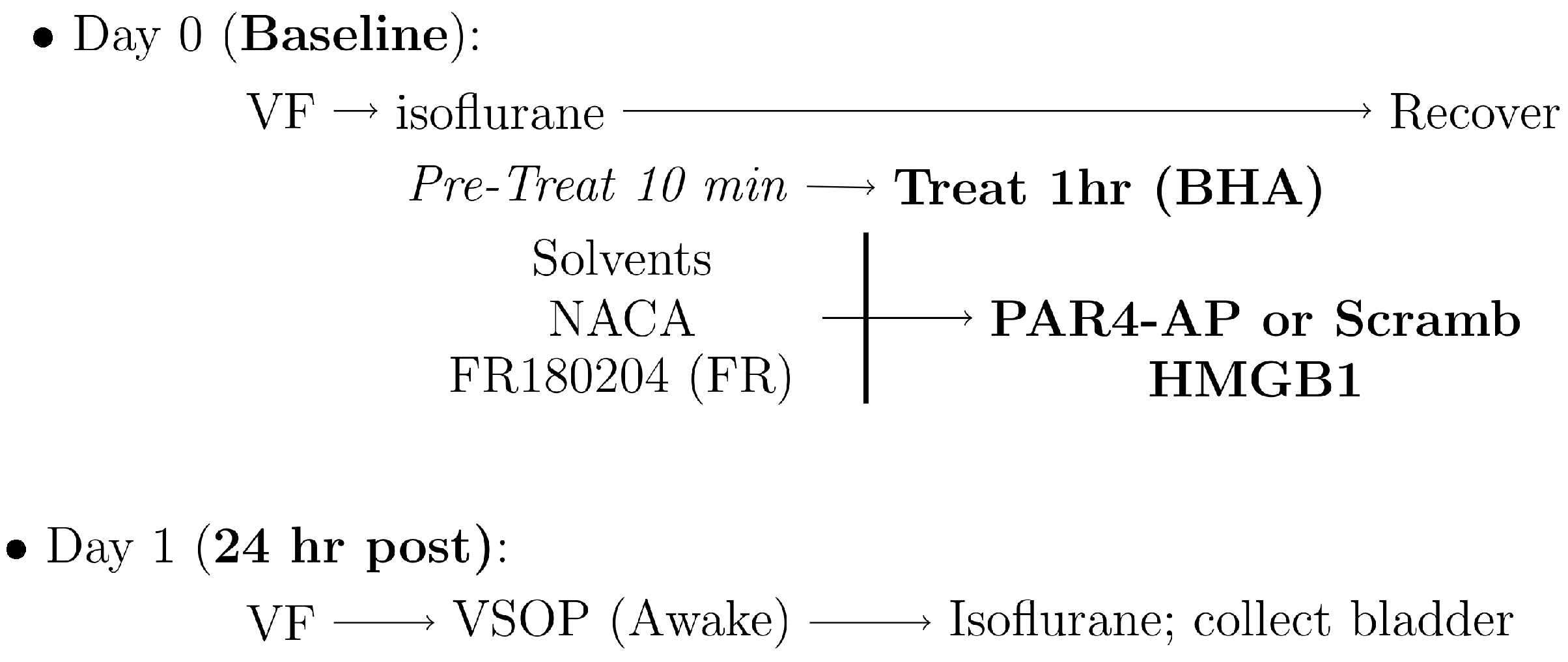
2. Materials and Methods
2.1. Experiment 1: Oxidative Stress and ERK Phosphorylation Changes in Our Bladder Pain Model
2.1.1. Western Blotting
2.1.2. Immunohistochemistry
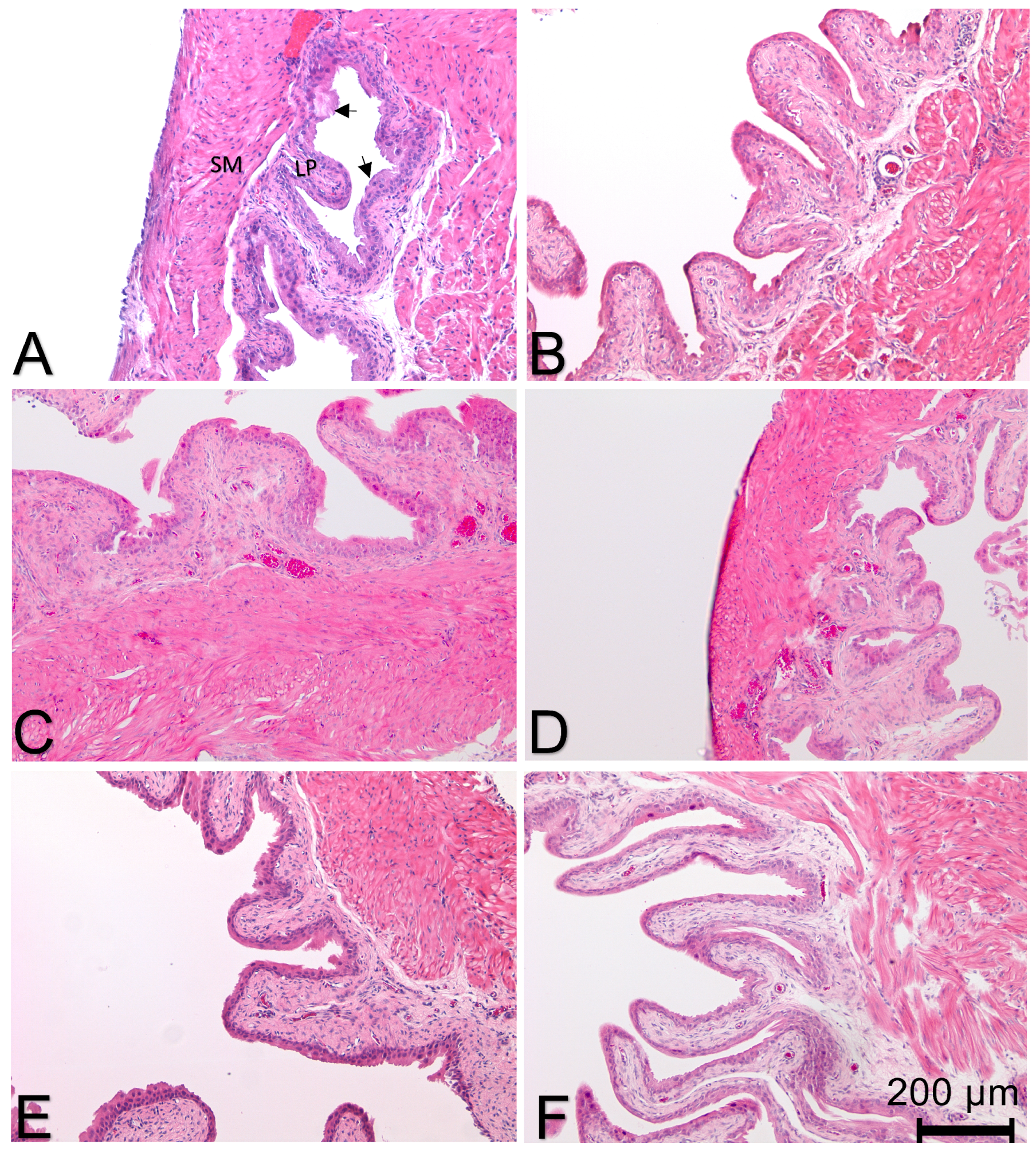
2.1.3. Histological Measurements
2.2. Experiment 2: Oxidative Stress and ERK Phosphorylation Blockage in HMGB1-Induced Bladder Hyperalgesia
2.2.1. Abdominal Mechanical Sensitivity
- (Days 1, 2) Acclimation to testing room:
- –
- Mice placed in testing room and left undisturbed for 3 h;
- (Days 3, 4) Acclimation to testing chamber:
- –
- Mice placed in testing chamber and left undisturbed for 2 h;
- (Day 5) Acclimation to von Frey (VF) monofilaments:
- –
- Mice placed in testing chamber and VF monofilaments applied to lower abdominal area;
- (Day 7) Baseline VF testing.
2.2.2. Micturition Parameters in Awake Mice
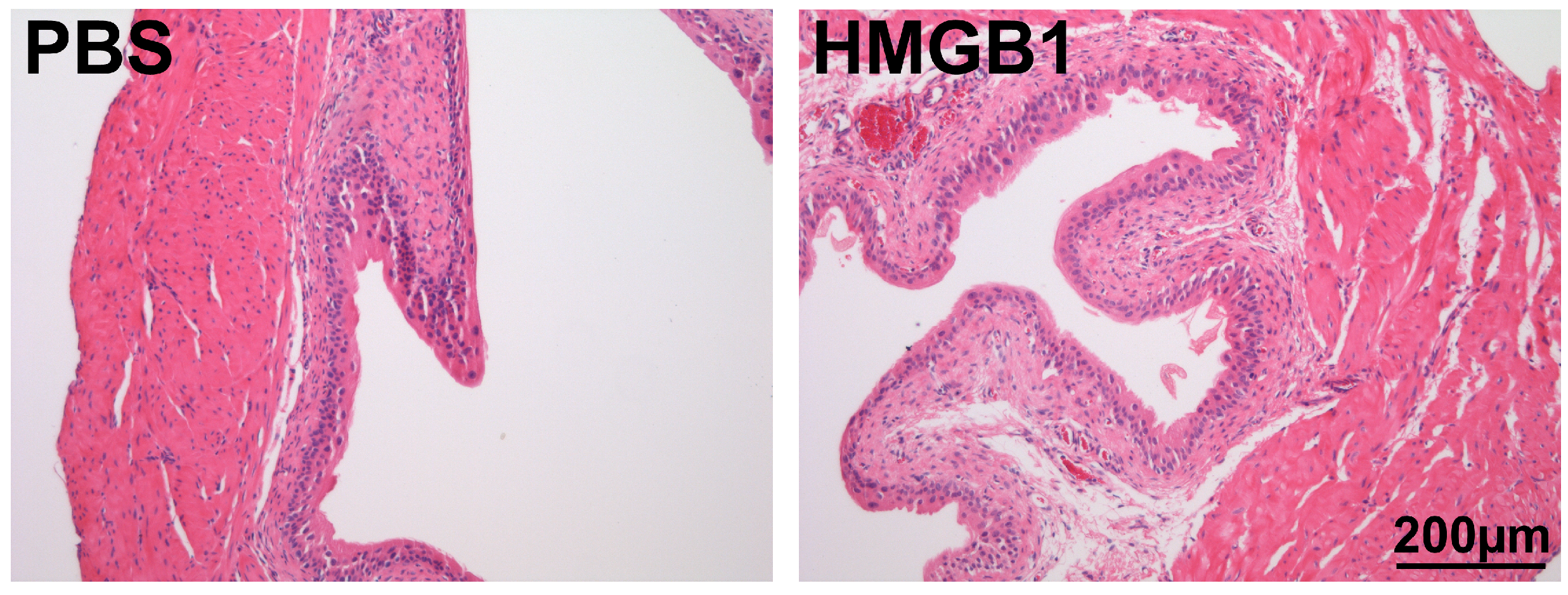
2.2.3. Histological Measurements
2.3. Reagents
2.4. Statistical Analysis
3. Results
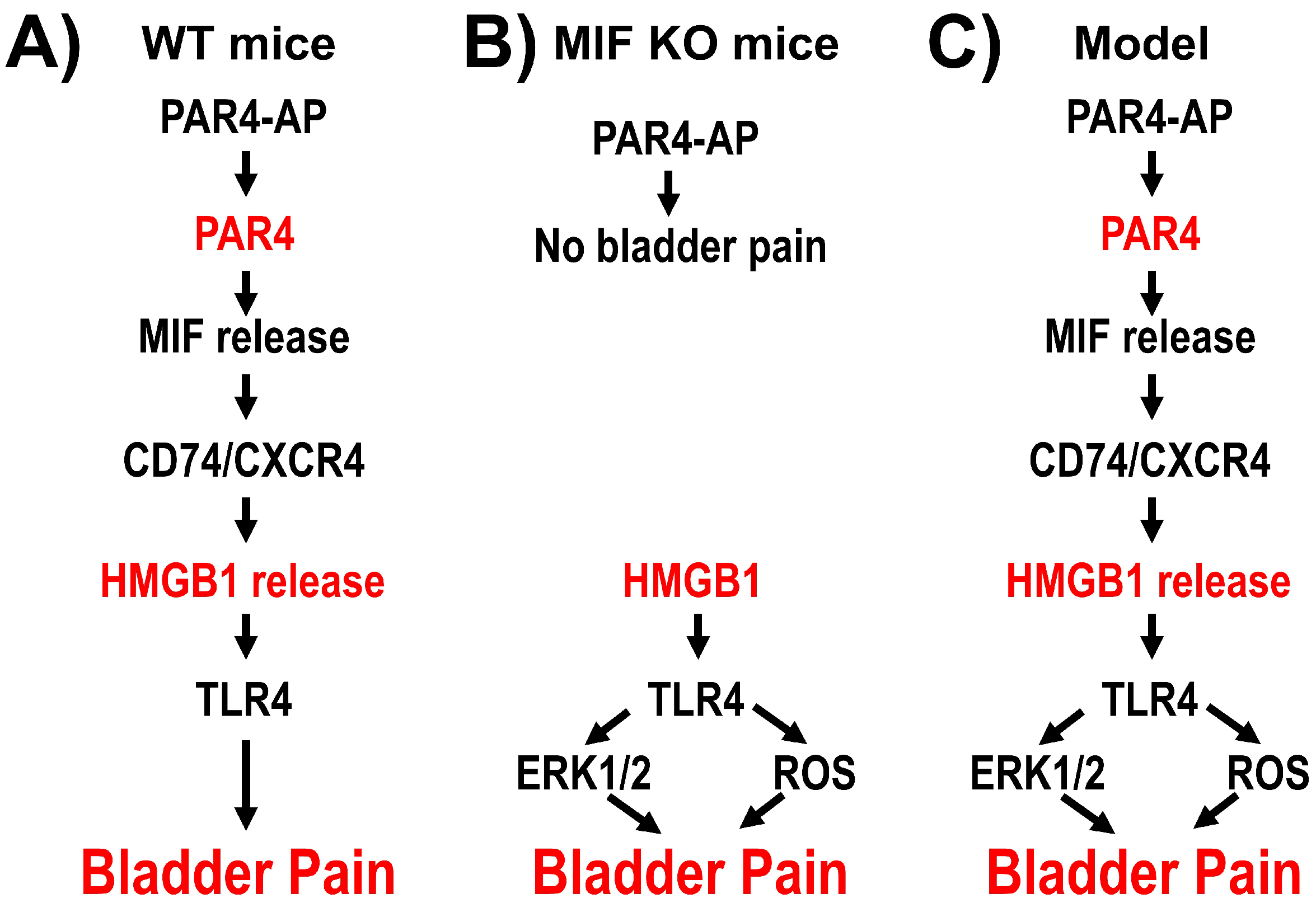
3.1. Experiment 1: Changes in Bladder Oxidative Stress and ERK Phosphorylation by Intravesical HMGB1 in MIF KO Mice
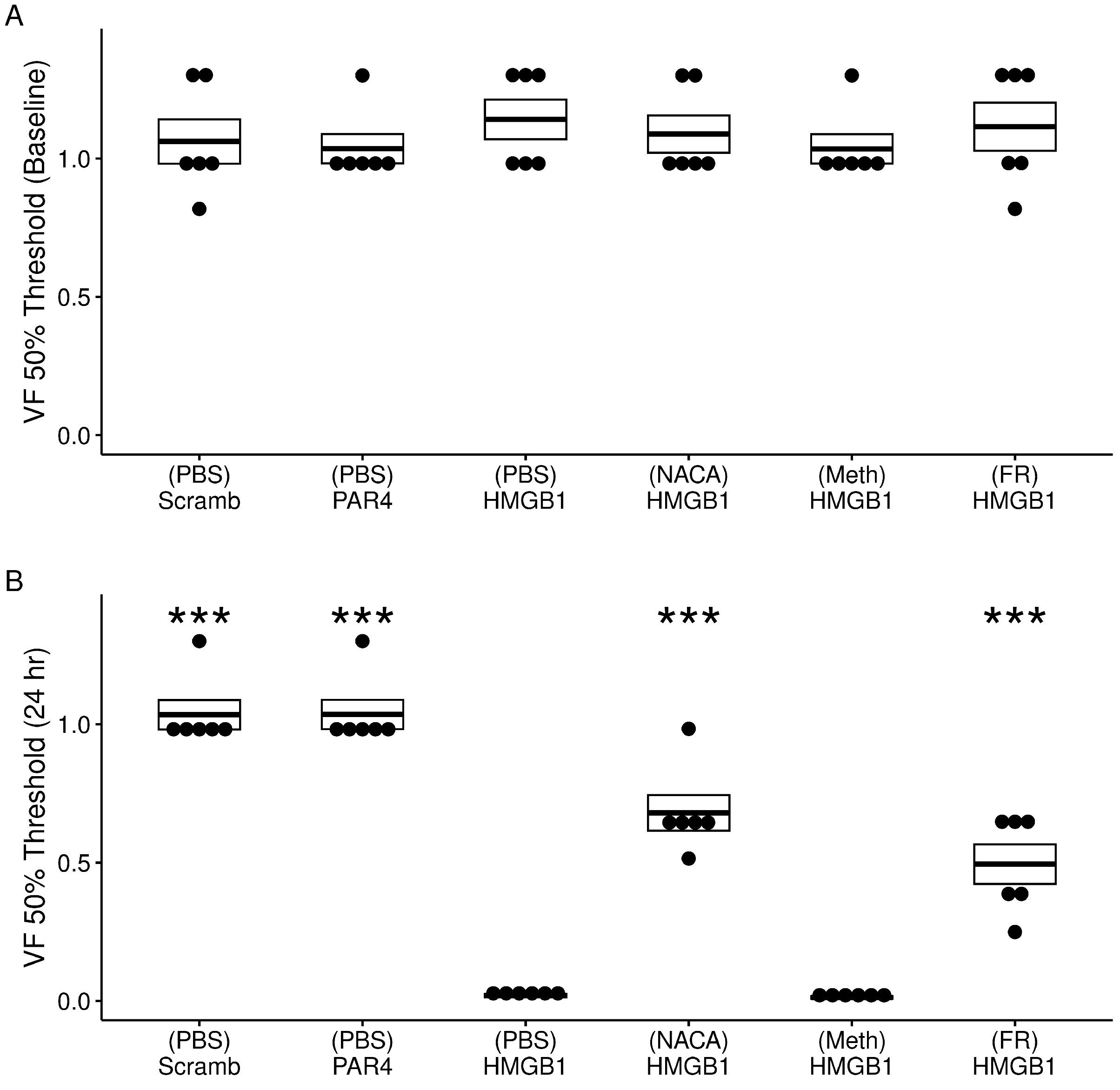
3.2. Experiment 2: Bladder Oxidative Stress and ERK Phosphorylation Antagonism on HMGB1-Induced BHA
3.2.1. Effect of Treatments on Awake Micturition Parameters
3.2.2. Effect of Treatment on Histological Evidence of Bladder Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, S.; Ma, F.; Mahmood, D.F.D.; Meyer-Siegler, K.L.; Menard, R.E.; Hunt, D.E.; Leng, L.; Bucala, R.; Vera, P.L. Intravesical CD74 and CXCR4, macrophage migration inhibitory factor (MIF) receptors, mediate bladder pain. PLoS ONE 2021, 16, e0255975. [Google Scholar] [CrossRef] [PubMed]
- Kapurniotu, A.; Gokce, O.; Bernhagen, J. The Multitasking Potential of Alarmins and Atypical Chemokines. Front. Med. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Kouzoukas, D.E.; Ma, F.; Meyer-Siegler, K.L.; Westlund, K.N.; Hunt, D.E.; Vera, P.L. Protease-Activated Receptor 4 Induces Bladder Pain through High Mobility Group Box-1. PloS ONE 2016, 11, e0152055. [Google Scholar] [CrossRef] [PubMed]
- Agalave, N.M.; Svensson, C.I. Extracellular high-mobility group box 1 protein (HMGB1) as a mediator of persistent pain. Mol. Med. 2014, 20, 569–578. [Google Scholar] [CrossRef]
- Tsujita, R.; Tsubota, M.; Sekiguchi, F.; Kawabata, A. Role of high-mobility group box 1 and its modulation by thrombomodulin/thrombin axis in neuropathic and inflammatory pain. Br. J. Pharmacol. 2020, 178, 798–812. [Google Scholar] [CrossRef]
- Tanaka, J.; Yamaguchi, K.; Ishikura, H.; Tsubota, M.; Sekiguchi, F.; Seki, Y.; Tsujiuchi, T.; Murai, A.; Umemura, T.; Kawabata, A. Bladder pain relief by HMGB1 neutralization and soluble thrombomodulin in mice with cyclophosphamide-induced cystitis. Neuropharmacology 2014, 79C, 112–118. [Google Scholar] [CrossRef]
- Roundy, L.M.; Jia, W.; Zhang, J.; Ye, X.; Prestwich, G.D.; Oottamasathien, S. LL-37 induced cystitis and the receptor for advanced glycation end-products (RAGE) pathway. Adv. Biosci. Biotechnol. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Irie, Y.; Tsubota, M.; Maeda, M.; Hiramoto, S.; Sekiguchi, F.; Ishikura, H.; Wake, H.; Nishibori, M.; Kawabata, A. HMGB1 and its membrane receptors as therapeutic targets in an intravesical substance P-induced bladder pain syndrome mouse model. J. Pharmacol. Sci. 2020, 143, 112–116. [Google Scholar] [CrossRef]
- Hiramoto, S.; Tsubota, M.; Yamaguchi, K.; Okazaki, K.; Sakaegi, A.; Toriyama, Y.; Tanaka, J.; Sekiguchi, F.; Ishikura, H.; Wake, H.; et al. Cystitis-Related Bladder Pain Involves ATP-Dependent HMGB1 Release from Macrophages and Its Downstream H2S/Cav3.2 Signaling in Mice. Cells 2020, 9, 1748. [Google Scholar] [CrossRef]
- Ye, S.; Ma, F.; Mahmood, D.F.D.; Meyer-Siegler, K.L.; Leng, L.; Bucala, R.; Vera, P.L. Bladder Oxidative Stress and HMGB1 Release Contribute to PAR4-Mediated Bladder Pain in Mice. Front. Syst. Neurosc. 2022, 16. [Google Scholar] [CrossRef]
- Ma, F.; Kouzoukas, D.E.; Meyer-Siegler, K.L.; Hunt, D.E.; Leng, L.; Bucala, R.; Vera, P.L. Macrophage migration Inhibitory factor mediates protease-activated receptor-4 induced bladder pain through urothelial high mobility group box 1. Physiol. Rep. 2017, 5, e13549. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Kouzoukas, D.E.; Meyer-Siegler, K.L.; Westlund, K.N.; Hunt, D.E.; Vera, P.L. Disulfide high mobility group box-1 causes bladder pain through bladder Toll-like receptor 4. BMC Physiol. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol. Iimmunol. 2018, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.p.; Li, L.; Li, J.; Wang, J.y.; Wang, L.y.; Jiang, W. High mobility group box-1 promotes the proliferation and migration of hepatic stellate cells via TLR4-dependent signal pathways of PI3K/Akt and JNK. PLoS ONE 2013, 8, e64373. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, J.M.; Kim, S.J.; Seo, W.; Kim, M.H.; Choi, W.M.; Yoo, W.; Lee, J.H.; Shim, Y.R.; Yi, H.S.; et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4–MD2 complex. Nat. Commun. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Li, P.; Ren, K.; Liang, Y.Y.; Liu, J.K.; Liang, Z.W.; Zhang, Y.F. Aloin promotes cell apoptosis by targeting HMGB1-TLR4-ERK axis in human melanoma cells. EXCLI J. 2020, 19, 641–651. [Google Scholar] [PubMed]
- Seo, S.W.; Park, S.K.; Oh, S.J.; Shin, O.S. TLR4-mediated activation of the ERK pathway following UVA irradiation contributes to increased cytokine and MMP expression in senescent human dermal fibroblasts. PLoS ONE 2018, 13, e0202323. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Kapurniotu, A.; Fingerle-Rowson, G.; Roger, T.; Leng, L.; Thiele, M.; Calandra, T.; Bucala, R.; Bernhagen, J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal 2006, 18, 688–703. [Google Scholar] [CrossRef]
- Chen, C.A.; Chang, J.M.; Yang, Y.L.; Chang, E.E.; Chen, H.C. Macrophage migration inhibitory factor regulates integrin-β1 and cyclin D1 expression via ERK pathway in podocytes. Biomed. Pharmacother. 2020, 124, 109892. [Google Scholar] [CrossRef]
- Chen, M.Q.; Luan, J.J. HMGB1 promotes bone fracture healing through activation of ERK signaling pathway in a rat tibial fracture model. Kaohsiung J. Med. Sci. 2019, 35, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Ye, F.H.; Peng, M.; Dong, J.J.; Chai, W.W.; Deng, W.J.; Zhang, H.; Yang, L.C. Endogenous HMGB1 regulates GSDME-mediated pyroptosis via ROS/ERK1/2/caspase-3/GSDME signaling in neuroblastoma. Am. J. Cancer Res. 2023, 13, 436. [Google Scholar] [PubMed]
- Jorgačević, B.; Stanković, S.; Filipović, J.; Samardžić, J.; Vučević, D.; Radosavljević, T. Betaine Modulating MIF-Mediated Oxidative Stress, Inflammation and Fibrogenesis in Thioacetamide-Induced Nephrotoxicity. Curr. Med. Chem. 2022, 29, 5254–5267. [Google Scholar] [PubMed]
- Yang, D.; Shu, T.; Zhao, H.; Sun, Y.; Xu, W.; Tu, G. Knockdown of macrophage migration inhibitory factor (MIF), a novel target to protect neurons from parthanatos induced by simulated post-spinal cord injury oxidative stress. Biochem. Biophys. Res. Commun. 2020, 523, 719–725. [Google Scholar] [CrossRef]
- Fang, P.; Dou, B.; Liang, J.; Hou, W.; Ma, C.; Zhang, Q. Quercetin Reduces Oxidative Stress and Apoptosis by Inhibiting HMGB1 and Its Translocation, Thereby Alleviating Liver Injury in ACLF Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Shih, C.P.; Kuo, C.Y.; Lin, Y.Y.; Lin, Y.C.; Chen, H.K.; Wang, H.; Chen, H.C.; Wang, C.H. Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss. Cells 2021, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Fingerle-Rowson, G.; Petrenko, O.; Metz, C.N.; Forsthuber, T.G.; Mitchell, R.; Huss, R.; Moll, U.; Muller, W.; Bucala, R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. USA 2003, 100, 9354–9359. [Google Scholar] [CrossRef] [PubMed]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol.-Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Kouzoukas, D.E.; Meyer-Siegler, K.L.; Ma, F.; Westlund, K.N.; Hunt, D.E.; Vera, P.L. Macrophage Migration Inhibitory Factor Mediates PAR-Induced Bladder Pain. PLoS ONE 2015, 10, e0127628. [Google Scholar] [CrossRef]
- Grinberg, L.; Fibach, E.; Amer, J.; Atlas, D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic. Biol. Med. 2005, 38, 136–145. [Google Scholar] [CrossRef]
- Pandya, J.D.; Readnower, R.D.; Patel, S.P.; Yonutas, H.M.; Pauly, J.R.; Goldstein, G.A.; Rabchevsky, A.G.; Sullivan, P.G. N-acetylcysteine amide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Exp. Neurol. 2014, 257, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ohori, M.; Takeuchi, M.; Maruki, R.; Nakajima, H.; Miyake, H. FR180204, a novel and selective inhibitor of extracellular signal-regulated kinase, ameliorates collagen-induced arthritis in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 374, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Hunt, D.E.; Leng, L.; Bucala, R.; Meyer-Siegler, K.L.; Vera, P.L. Protease activated-receptor 4 activation as a model of persistent bladder pain: Essential role of macrophage migration inhibitory factor and high mobility group box 1. Int. J. Urol. 2018, 25, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Meyer-Siegler, K.L.; Leng, L.; Bucala, R.; Vera, P.L. Spinal macrophage migration inhibitory factor and high mobility group box 1 mediate persistent bladder pain. Neurosci. Lett. 2019, 699, 54–58. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sugino, Y.; Kanematsu, A.; Hayashi, Y.; Haga, H.; Yoshimura, N.; Yoshimura, K.; Ogawa, O. Voided stain on paper method for analysis of mouse urination. Neurourol. Urodynam. 2008, 27, 548–552. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Morikawa, Y.; Kato, H.; Kashiwagi, H.; Nishiura, N.; Akuta, K.; Honda, S.; Kanakura, Y.; Tomiyama, Y. Protease-activated receptor-4 (PAR4) variant influences on platelet reactivity induced by PAR4-activating peptide through altered Ca2+ mobilization and ERK phosphorylation in healthy Japanese subjects. Thromb. Res. 2018, 162, 44–52. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell. Signal. 2019, 57, 76–88. [Google Scholar] [CrossRef]
- Karpova, T.; de Oliveira, A.A.; Naas, H.; Priviero, F.; Nunes, K.P. Blockade of Toll-like receptor 4 (TLR4) reduces oxidative stress and restores phospho-ERK1/2 levels in Leydig cells exposed to high glucose. Life Sci. 2020, 245, 117365. [Google Scholar] [CrossRef]
- Xie, X.; Liang, J.; Huang, R.; Luo, C.; Yang, J.; Xing, H.; Zhou, L.; Qiao, H.; Ergu, E.; Chen, H. Molecular pathways underlying tissue injuries in the bladder with ketamine cystitis. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Corrow, K.A.; Vizzard, M.A. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am. J. Physiol. Regul. Integr. 2007, 293, R125–R134. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Kobayashi, T.; Negoro, H.; Sengiku, A.; Hiratsuka, T.; Kamioka, Y.; Liou, L.S.; Ogawa, O.; Matsuda, M. Intravital imaging of mouse urothelium reveals activation of extracellular signal-regulated kinase by stretch-induced intravesical release of ATP. Physiol. Rep. 2016, 4, e13033. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.T.; Duara, J.L.; Bushnell, D.S.; Nouraie, M.; Holden, J.; Pfister, K.; Lucas, P.C.; Sims-Lucas, S.; Bates, C.M. Role of ERK signaling in bladder urothelium in response to cyclophosphamide injury. Physiol. Rep. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.R.; Jiang, Y.H.; Jhang, J.F.; Chang, W.C.; Kuo, H.C. Treatment Outcomes of Intravesical Botulinum Toxin A Injections on Patients with Interstitial Cystitis/Bladder Pain Syndrome. Toxins 2022, 14, 871. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Mullins, C.; Ackerman, A.L.; Bavendam, T.; van Bokhoven, A.; Ellingson, B.M.; Harte, S.E.; Kutch, J.J.; Lai, H.H.; Martucci, K.T.; et al. Urologic chronic pelvic pain syndrome: Insights from the MAPP Research Network. Nat. Rev. Urol. 2019, 16, 187–200. [Google Scholar] [CrossRef]
- Killinger, K.A.; Boura, J.A.; Peters, K.M. Pain in interstitial cystitis/bladder pain syndrome: Do characteristics differ in ulcerative and non-ulcerative subtypes? Int. Urogynecol. J. 2013, 24, 1295–1301. [Google Scholar] [CrossRef]
- Kim, H.J. Update on the Pathology and Diagnosis of Interstitial Cystitis/Bladder Pain Syndrome: A Review. Int. Neurourol. J. 2016, 20, 13–17. [Google Scholar] [CrossRef]
- Akiyama, Y.; Hanno, P. Phenotyping of interstitial cystitis/bladder pain syndrome. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2019, 26 (Suppl. 1), 17–19. [Google Scholar] [CrossRef]
- Homma, Y.; Akiyama, Y.; Tomoe, H.; Furuta, A.; Ueda, T.; Maeda, D.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Oh, S.J.; et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020, 27, 578–589. [Google Scholar] [CrossRef]
- D’Andrea, M.R.; Saban, M.R.; Nguyen, N.B.; Andrade-Gordon, P.; Saban, R. Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am. J. Pathol. 2003, 162, 907–923. [Google Scholar] [CrossRef]
- Wang, W.; Bo, Q.; Du, J.; Yu, X.; Zhu, K.; Cui, J.; Zhao, H.; Wang, Y.; Shi, B.; Zhu, Y. Endogenous H2S sensitizes PAR4-induced bladder pain. Am. J. Physiol.-Renal Physiol. 2018, 314, F1077–F1086. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jing, X.; Lutgendorf, S.K.; Bradley, C.S.; Schrepf, A.; Erickson, B.A.; Magnotta, V.A.; Ness, T.J.; Kreder, K.J.; O’Donnell, M.A.; et al. Cystitis-induced bladder pain is Toll-like receptor 4 dependent in a transgenic autoimmune cystitis murine model: A MAPP Research Network Animal Study. Am. J. Physiol.-Renal Physiol. 2019, 317, F90–F98. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Siegler, K.L.; Xia, S.L.; Vera, P.L. Substance P Increases Cell-Surface Expression of CD74 (Receptor for Macrophage Migration Inhibitory Factor): In Vivo Biotinylation of Urothelial Cell-Surface Proteins. Mediat. Inflamm 2009, 2009, 535348. [Google Scholar] [CrossRef] [PubMed]
- Doiron, R.C.; Tolls, V.; Irvine-Bird, K.; Kelly, K.L.; Nickel, J.C. Clinical Phenotyping Does Not Differentiate Hunner Lesion Subtype of Interstitial Cystitis/Bladder Pain Syndrome: A Relook at the Role of Cystoscopy. J. Urol. 2016, 196, 1136–1140. [Google Scholar] [CrossRef] [PubMed]


| Intravesical Pretreat. | Intravesical Treat. | Volume (L) | Frequency (Voids/3 h) |
|---|---|---|---|
| PBS | Scramb peptide (control) | 348 ± 37.1 | 2.0 ± 0.5 |
| PBS | PAR4 | 421 ± 32.3 | 1.5 ± 0.2 |
| PBS | HMGB1 | 345 ± 45.5 | 2.0 ± 0.3 |
| NACA | HMGB1 | 286 ± 45.4 | 2.2 ± 0.4 |
| Methcell | HMGB1 | 292 ± 67.7 | 1.7 ± 0.3 |
| FR | HMGB1 | 365 ± 49.0 | 1.3 ± 0.2 |
| Intravesical Pretreat. | Intravesical Treat. | Edema | Inflammation |
|---|---|---|---|
| PBS | PAR4 | 0.5 ± 0.1 | 0.3 ± 0.2 |
| PBS | HMGB1 | 0.3 ± 0.2 | 0.5 ± 0.3 |
| NACA | HMGB1 | 0.7 ± 0.3 | 0.9 ± 0.2 |
| Methcell | HMGB1 | 0.7 ± 0.5 | 0.5 ± 0.2 |
| FR | HMGB1 | 1.5 ± 0.3 | 1.3 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Mahmood, D.F.D.; Ma, F.; Leng, L.; Bucala, R.; Vera, P.L. Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain. Cells 2023, 12, 1440. https://doi.org/10.3390/cells12101440
Ye S, Mahmood DFD, Ma F, Leng L, Bucala R, Vera PL. Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain. Cells. 2023; 12(10):1440. https://doi.org/10.3390/cells12101440
Chicago/Turabian StyleYe, Shaojing, Dlovan F. D. Mahmood, Fei Ma, Lin Leng, Richard Bucala, and Pedro L. Vera. 2023. "Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain" Cells 12, no. 10: 1440. https://doi.org/10.3390/cells12101440
APA StyleYe, S., Mahmood, D. F. D., Ma, F., Leng, L., Bucala, R., & Vera, P. L. (2023). Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain. Cells, 12(10), 1440. https://doi.org/10.3390/cells12101440






