A Study of Small Intestinal Epigenomic Changes Induced by Royal Jelly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Murine Models
2.2. Quantification of Voluntary Wheel Running
2.3. Analytical Methodologies and Tolerance Tests for Glucose and Insulin
2.4. Serum Biochemical Analysis
2.5. Histopathological Evaluation of Jejunum and Colon
2.6. Analysis of mRNA Sequencing of the Jejunum
2.7. Quantitative Real Time-PCR
2.8. Mass Spectrometry for Histone Modifications (Mod-Spec)
2.9. NON-TiE-UP Cleavage Under Targets and Tagmentation
2.10. Statistical Examination
3. Results
3.1. Effect of Royal Jelly on Body Weight and Food Intake
3.2. Royal Jelly Improves Locomotor Activity
3.3. Royal Jelly Enhances Insulin Sensitivity and Reduces Cholesterol and Triglyceride Levels
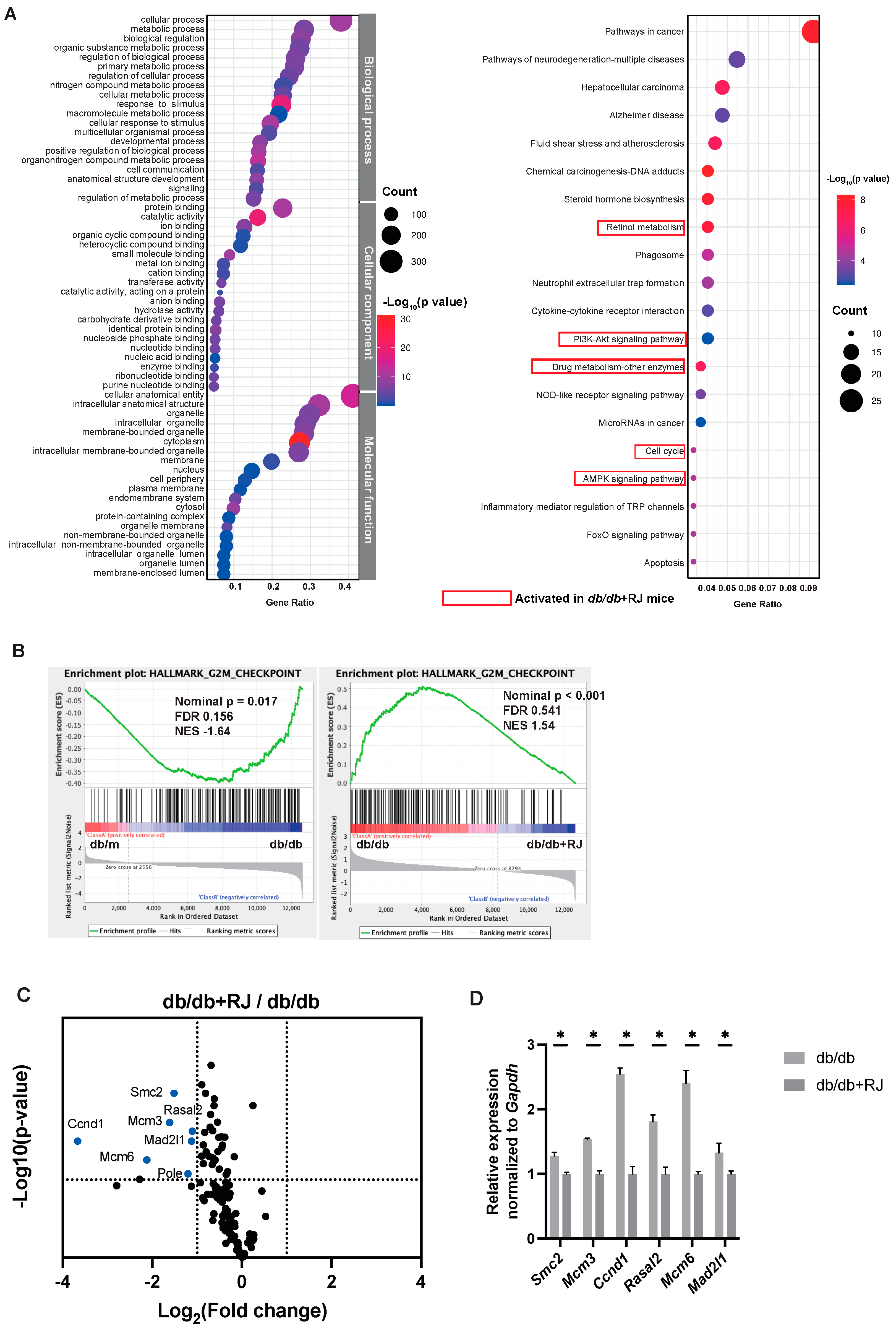
3.4. GO and KEGG Pathway Enrichment Analysis of Small Intestine mRNA
3.5. The Expression of the Gene Set Associated with the G2M Checkpoint Was Reduced by Royal Jelly
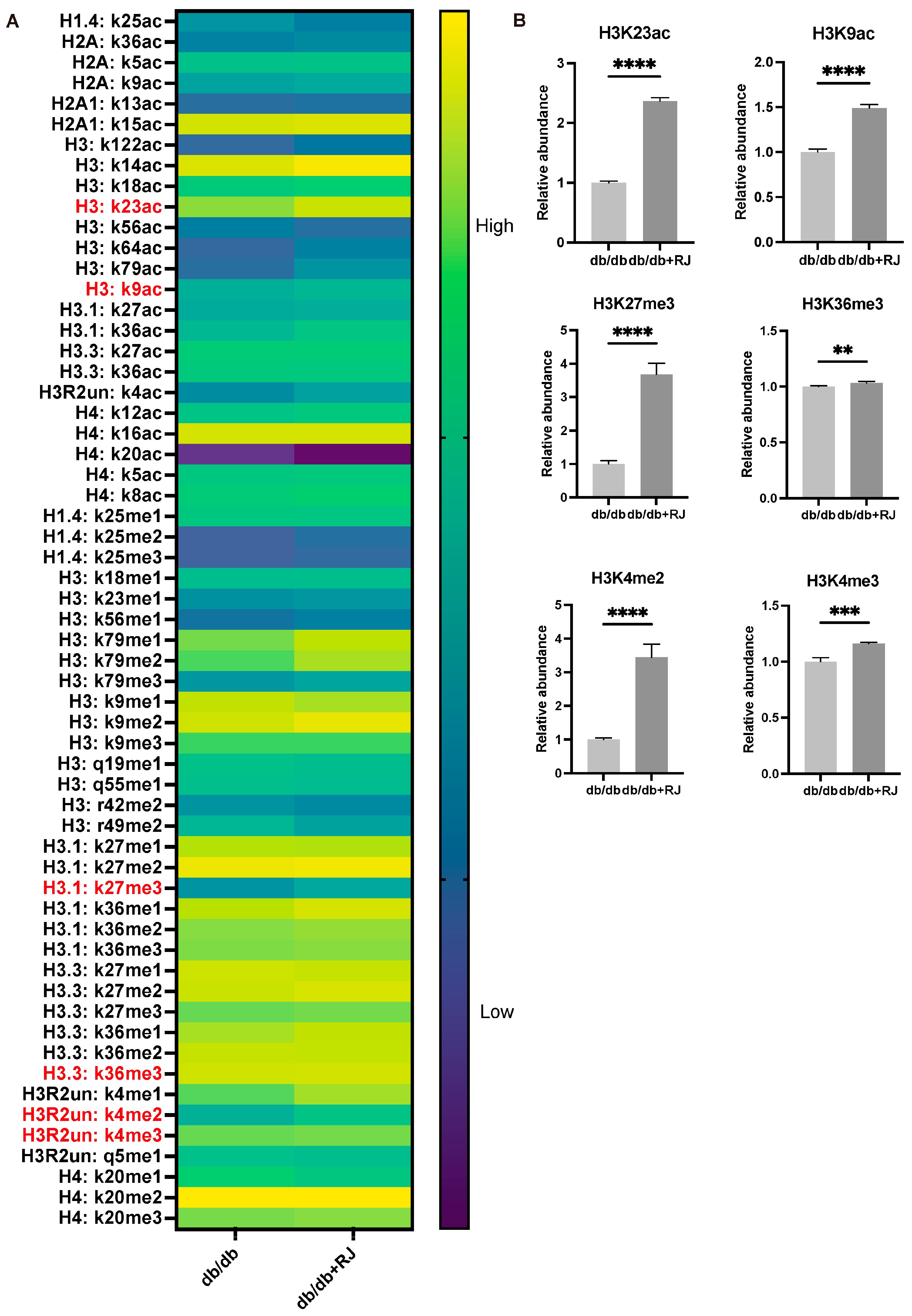
3.6. Relative Abundance of Histone Modifications in Small Intestinal Epithelial Cells
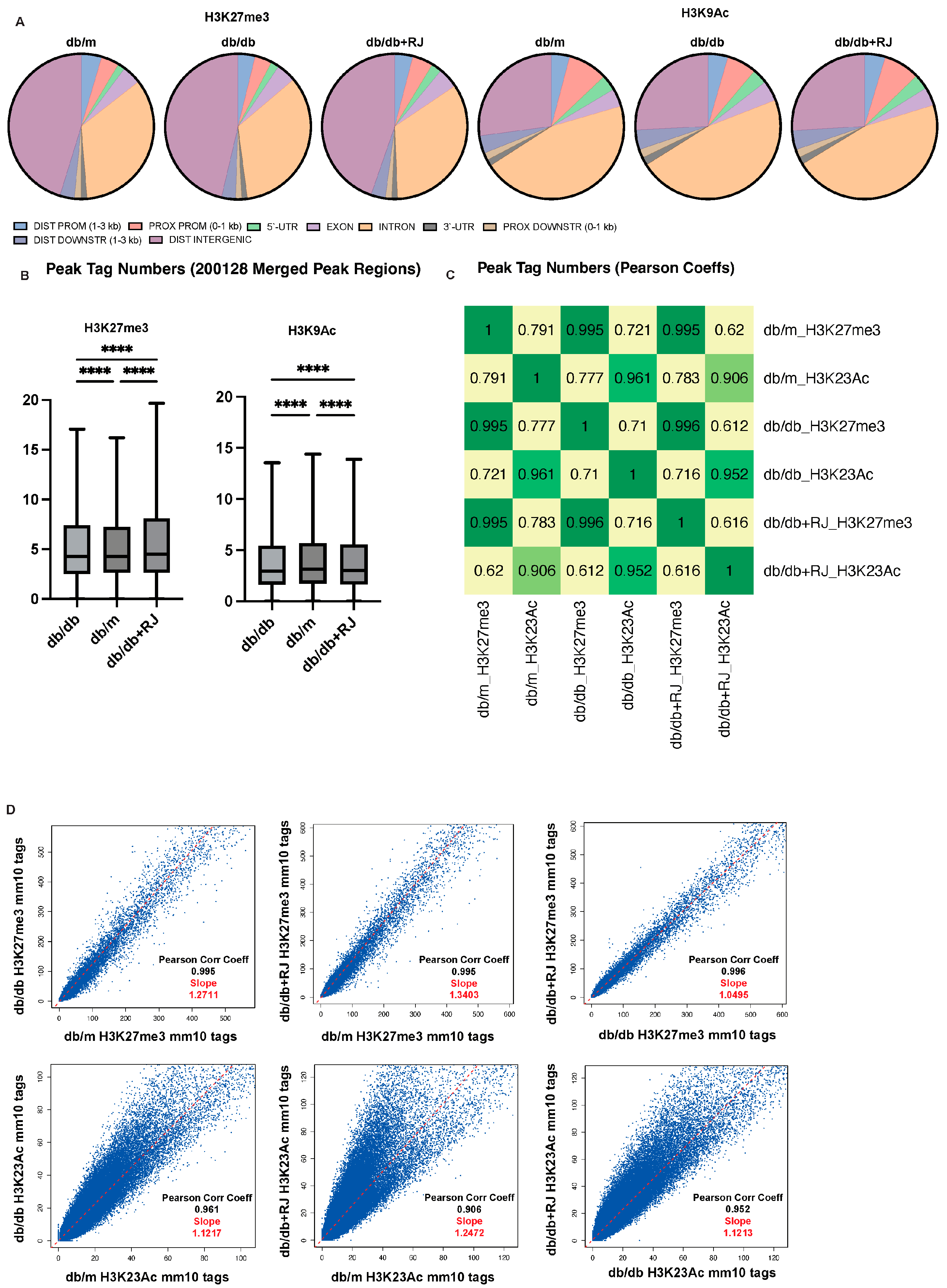
3.7. Royal Jelly Alters Distribution and Increases H3K27me3 and H3K9Ac Peak Tags
3.8. Royal Jelly Enhances Correlation and Modifies Peak Sizes of Histone Modifications
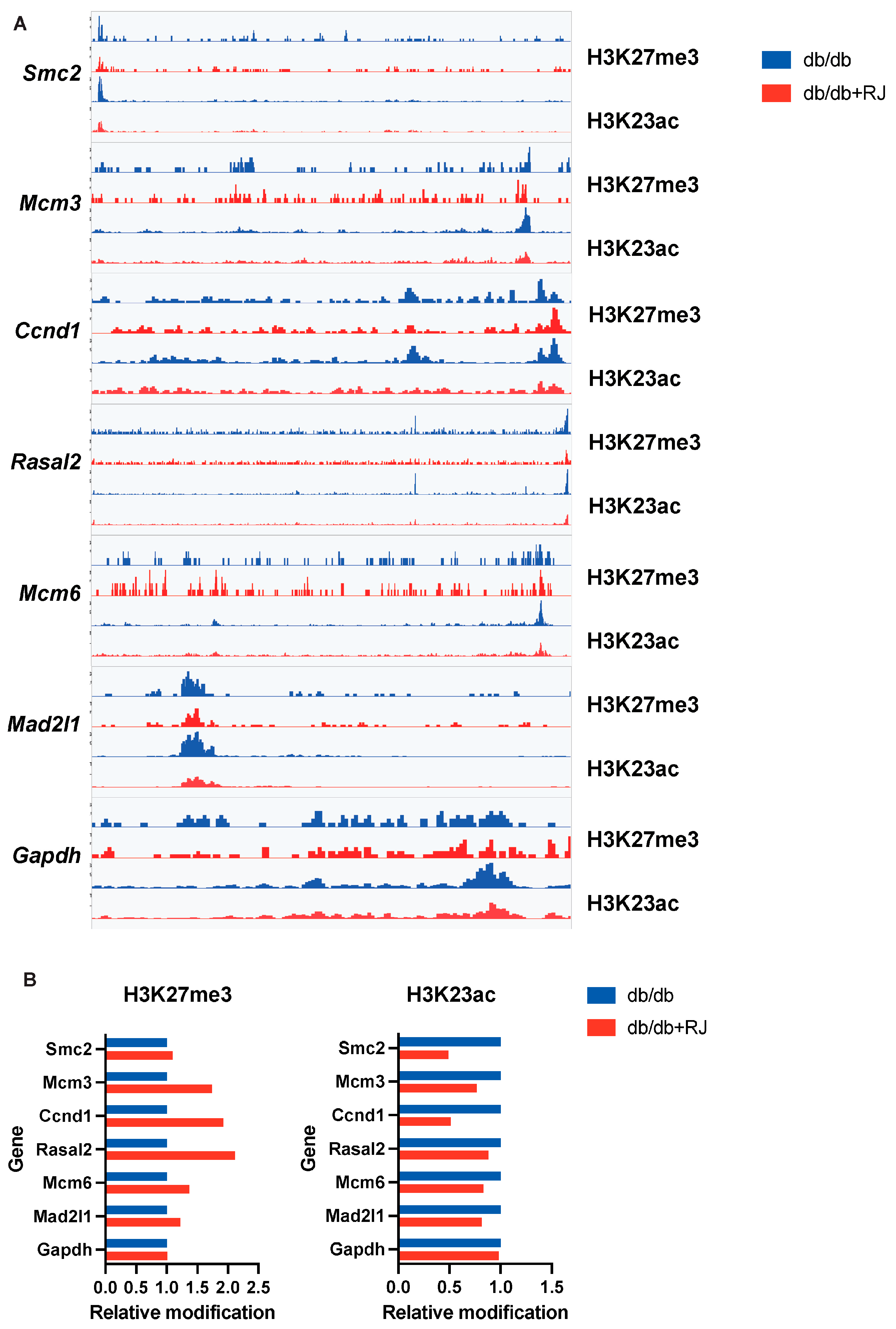
3.9. Quantitative Comparison of Epigenetic Modifications in Key Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collazo, N.; Carpena, M.; Nuñez-estevez, B.; Otero, P.; Simal-gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal Jelly Ameliorates Diet-Induced Obesity and Glucose Intolerance by Promoting Brown Adipose Tissue Thermogenesis in Mice. Obes. Res. Clin. Pract. 2018, 12, 127–137. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal Jelly Causes Hypotension and Vasodilation Induced by Increasing Nitric Oxide Production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Takaki-Doi, S.; Hashimoto, K.; Yamamura, M.; Kamei, C. Antihypertensive Activities of Royal Jelly Protein Hydrolysate and Its Fractions in Spontaneously Hypertensive Rats. Acta Med. Okayama 2009, 63, 57–64. [Google Scholar] [CrossRef]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal Jelly Supplementation Improves Lipoprotein Metabolism in Humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef]
- Kobayashi, G.; Okamura, T.; Majima, S.; Senmaru, T.; Okada, H.; Ushigome, E.; Nakanishi, N.; Nishimoto, Y.; Yamada, T.; Okamoto, H.; et al. Effects of Royal Jelly on Gut Dysbiosis and NAFLD in Db/Db Mice. Nutrients 2023, 15, 2580. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, H. Editorial: Functional Epigenetic Regulation in Metabolic Diseases. Front. Endocrinol. 2023, 14, 1190693. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Okur, H.S.; Wu, S.J.; Codomo, C.A.; Pledger, E.S.; Bryson, T.D.; Henikoff, J.G.; Ahmad, K.; Henikoff, S. CUT&Tag for Efficient Epigenomic Profiling of Small Samples and Single Cells. Nat. Commun. 2019, 10, 1930. [Google Scholar] [CrossRef]
- Zheng, Y.; Thomas, P.M.; Kelleher, N.L. Measurement of Acetylation Turnover at Distinct Lysines in Human Histones Identifies Long-Lived Acetylation Sites. Nat. Commun. 2013, 4, 2203. [Google Scholar] [CrossRef] [PubMed]
- Ruthenburg, A.J.; Li, H.; Patel, D.J.; David Allis, C. Multivalent Engagement of Chromatin Modifications by Linked Binding Modules. Nat. Rev. Mol. Cell Biol. 2007, 8, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M.; Ito, K. Histone Acetylation and Deacetylation: Importance in Inflammatory Lung Diseases. Eur. Respir. J. 2005, 25, 552–563. [Google Scholar] [CrossRef]
- Yasui, W.; Oue, N.; Ono, S.; Mitani, Y.; Ito, R.; Nakayama, H. Histone Acetylation and Gastrointestinal Carcinogenesis. Ann. N. Y. Acad. Sci. 2003, 983, 220–231. [Google Scholar] [CrossRef]
- Glauben, R.; Batra, A.; Fedke, I.; Zeitz, M.; Lehr, H.A.; Leoni, F.; Mascagni, P.; Fantuzzi, G.; Dinarello, C.A.; Siegmund, B. Histone Hyperacetylation Is Associated with Amelioration of Experimental Colitis in Mice. J. Immunol. 2006, 176, 5015–5022. [Google Scholar] [CrossRef]
- Eeftens, J.M.; Katan, A.J.; Kschonsak, M.; Hassler, M.; de Wilde, L.; Dief, E.M.; Haering, C.H.; Dekker, C. Condensin Smc2-Smc4 Dimers Are Flexible and Dynamic. Cell Rep. 2016, 14, 1813–1818. [Google Scholar] [CrossRef]
- Dávalos, V.; Súarez-López, L.; Castaño, J.; Messent, A.; Abasolo, I.; Fernandez, Y.; Guerra-Moreno, A.; Espín, E.; Armengol, M.; Musulen, E.; et al. Human SMC2 Protein, a Core Subunit of Human Condensin Complex, Is a Novel Transcriptional Target of the WNT Signaling Pathway and a New Therapeutic Target. J. Biol. Chem. 2012, 287, 43472–43481. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Duan, B.; Liu, Z.; Abbruzzese, J.; Walsh, K.M.; Zhang, X.; Wei, Q. Potential Functional Variants in SMC2 and TP53 in the AURORA Pathway Genes and Risk of Pancreatic Cancer. Carcinogenesis 2019, 40, 521–528. [Google Scholar] [CrossRef]
- Diehl, J.A. Cycling to Cancer with Cyclin D1. Cancer Biol. Ther. 2002, 1, 226–231. [Google Scholar] [CrossRef]
- Shintani, M.; Okazaki, A.; Masuda, T.; Kawada, M.; Ishizuka, M.; Doki, Y.; Weinstein, I.B.; Imoto, M. Overexpression of Cyclin DI Contributes to Malignant Properties of Esophageal Tumor Cells by Increasing VEGF Production and Decreasing Fas Expression. Anticancer Res. 2002, 22, 639–647. [Google Scholar]
- Li, N.; Zhai, Y.; Zhang, Y.; Li, W.; Yang, M.; Lei, J.; Tye, B.K.; Gao, N. Structure of the Eukaryotic MCM Complex at 3.8 Å. Nature 2015, 524, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Cheng, E.; Wu, H.; Li, N.; Yung, P.Y.K.; Gao, N.; Tye, B.K. Open-Ringed Structure of the Cdt1-Mcm2-7 Complex as a Precursor of the MCM Double Hexamer. Nat. Struct. Mol. Biol. 2017, 24, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.A.; Shin, S.M.; Namkoong, H.; Lee, H.; Cho, G.W.; Hur, S.Y.; Kim, T.E.; Kim, J.W. Cancer-Associated Expression of Minichromosome Maintenance 3 Gene in Several Human Cancers and Its Involvement in Tumorigenesis. Clin. Cancer Res. 2004, 10, 8386–8395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tan, Z.; Huang, Z.; Pan, Y.; Zhang, W.; Wang, J. Expression Profile and Prognostic Values of Mini-Chromosome Maintenance Families (MCMs) in Breast Cancer. Med. Sci. Monit. 2020, 26, e923673-1. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Chen, J.; Shan, Q.; Dai, H.; Xie, H.; Zhou, L.; Xu, X.; Zheng, S. MCM Family in HCC: MCM6 Indicates Adverse Tumor Features and Poor Outcomes and Promotes S/G2 Cell Cycle Progression. BMC Cancer 2018, 18, 200. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, B.S.; Jiang, Y.Y.; Cao, J.; Hao, J.J.; Zhang, Y.; Xu, X.; Cai, Y.; Wang, M.R. MCMs Expression in Lung Cancer: Implication of Prognostic Significance. J. Cancer 2017, 8, 3641. [Google Scholar] [CrossRef]
- Yu, J.; Wang, R.; Wu, J.; Dang, Z.; Zhang, Q.; Li, B. Knockdown of Minichromosome Maintenance Proteins Inhibits Foci Forming of Mediator of DNA-Damage Checkpoint 1 in Response to DNA Damage in Human Esophageal Squamous Cell Carcinoma TE-1 Cells. Biochemistry 2016, 81, 1221–1228. [Google Scholar] [CrossRef]
- Pan, Y.; Tong, J.H.M.; Lung, R.W.M.; Kang, W.; Kwan, J.S.H.; Chak, W.P.; Tin, K.Y.; Chung, L.Y.; Wu, F.; Ng, S.S.M.; et al. RASAL2 Promotes Tumor Progression through LATS2/YAP1 Axis of Hippo Signaling Pathway in Colorectal Cancer. Mol. Cancer 2018, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tong, D.; Jing, X.; Ma, P.; Li, F.; Jiang, Q.; Zhang, J.; Wen, H.; Cui, M.; Huang, C.; et al. MAD2L1 Is Transcriptionally Regulated by TEAD4 and Promotes Cell Proliferation and Migration in Colorectal Cancer. Cancer Gene Ther. 2023, 30, 727–737. [Google Scholar] [CrossRef]
- Morral, C.; Ayyaz, A.; Kuo, H.-C.; Fink, M.; Verginadis, I.I.; Daniel, A.R.; Burner, D.N.; Driver, L.M.; Satow, S.; Hasapis, S.; et al. P53 Promotes Revival Stem Cells in the Regenerating Intestine after Severe Radiation Injury. Nat. Commun. 2024, 15, 3018. [Google Scholar] [CrossRef] [PubMed]


| Sample | Number of Mapped Reads (%) |
|---|---|
| db/m_1 | 47,501,746 (97.73) |
| db/m_2 | 43,732,867 (97.08) |
| db/m_3 | 48,231,655 (96.29) |
| db/m_4 | 44,039,336 (97.39) |
| db/db_1 | 47,501,746 (97.73) |
| db/db_2 | 41,823,389 (97.62) |
| db/db_3 | 44,158,962 (96.48) |
| db/db_4 | 41,520,847 (97.9) |
| db/db+RJ_1 | 39,459,436 (95.91) |
| db/db+RJ_2 | 41,564,058 (98.07) |
| db/db+RJ_3 | 46,891,376 (97.01) |
| db/db+RJ_4 | 41,309,857 (96.86) |
| Sample | Target | Total Number of Reads | Usable Number of Alignments | Used Number of Alignments | Number of Peaks |
|---|---|---|---|---|---|
| db/m | H3K27me3 | 34,840,978 | 13,892,705 | 11,279,709 | 70,350 |
| db/m | H3K23Ac | 28,296,100 | 12,356,384 | 11,279,699 | 67,071 |
| db/db | H3K27me3 | 32,545,792 | 12,677,717 | 11,279,691 | 65,687 |
| db/db | H3K23Ac | 30,115,334 | 12,745,405 | 11,279,679 | 96,801 |
| db/db+RJ | H3K27me3 | 33,225,536 | 11,279,720 | 11,279,669 | 51,294 |
| db/db+RJ | H3K23Ac | 33,789,784 | 14,173,301 | 11,279,660 | 98,254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, G.; Ichikawa, T.; Okamura, T.; Matsuyama, T.; Hamaguchi, M.; Okamoto, H.; Okumura, N.; Fukui, M. A Study of Small Intestinal Epigenomic Changes Induced by Royal Jelly. Cells 2024, 13, 1419. https://doi.org/10.3390/cells13171419
Kobayashi G, Ichikawa T, Okamura T, Matsuyama T, Hamaguchi M, Okamoto H, Okumura N, Fukui M. A Study of Small Intestinal Epigenomic Changes Induced by Royal Jelly. Cells. 2024; 13(17):1419. https://doi.org/10.3390/cells13171419
Chicago/Turabian StyleKobayashi, Genki, Takahiro Ichikawa, Takuro Okamura, Tomoyuki Matsuyama, Masahide Hamaguchi, Hideto Okamoto, Nobuaki Okumura, and Michiaki Fukui. 2024. "A Study of Small Intestinal Epigenomic Changes Induced by Royal Jelly" Cells 13, no. 17: 1419. https://doi.org/10.3390/cells13171419






