The Immune Response in Two Models of Traumatic Injury of the Immature Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Controlled Cortical Impact (CCI)
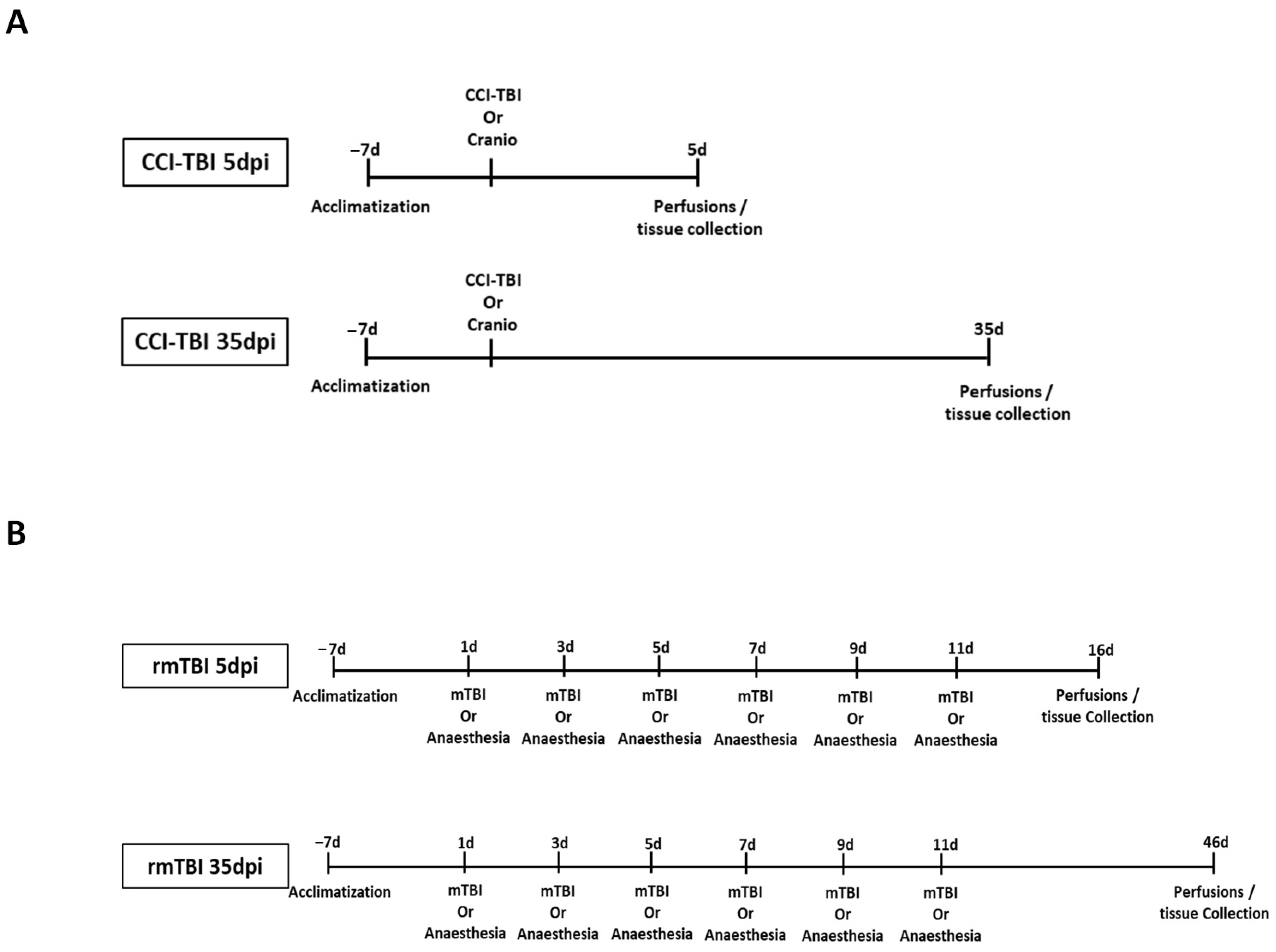
2.3. Repetitive Mild Traumatic Brain Injury (rmTBI)
2.4. Tissue Sample Collection
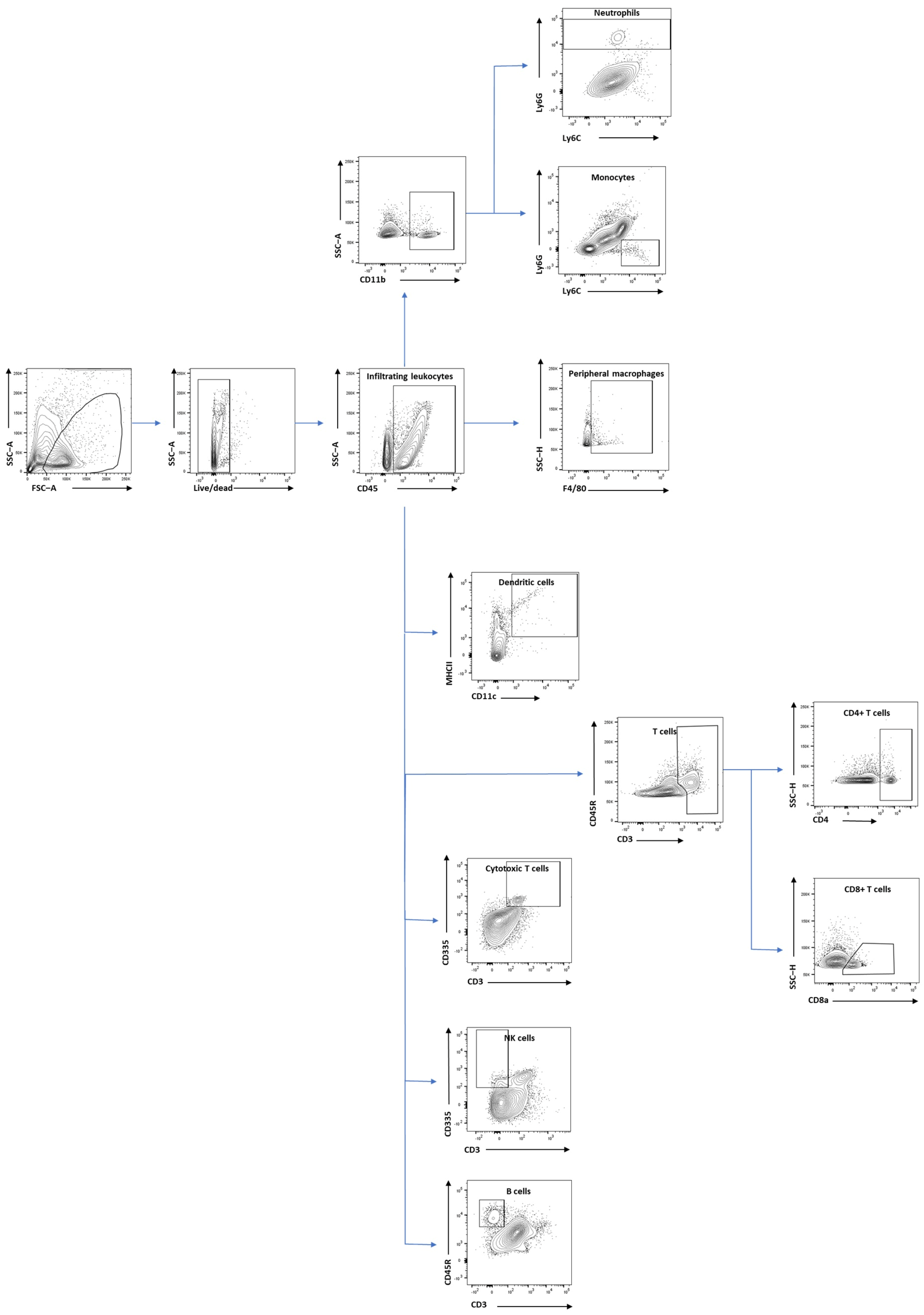
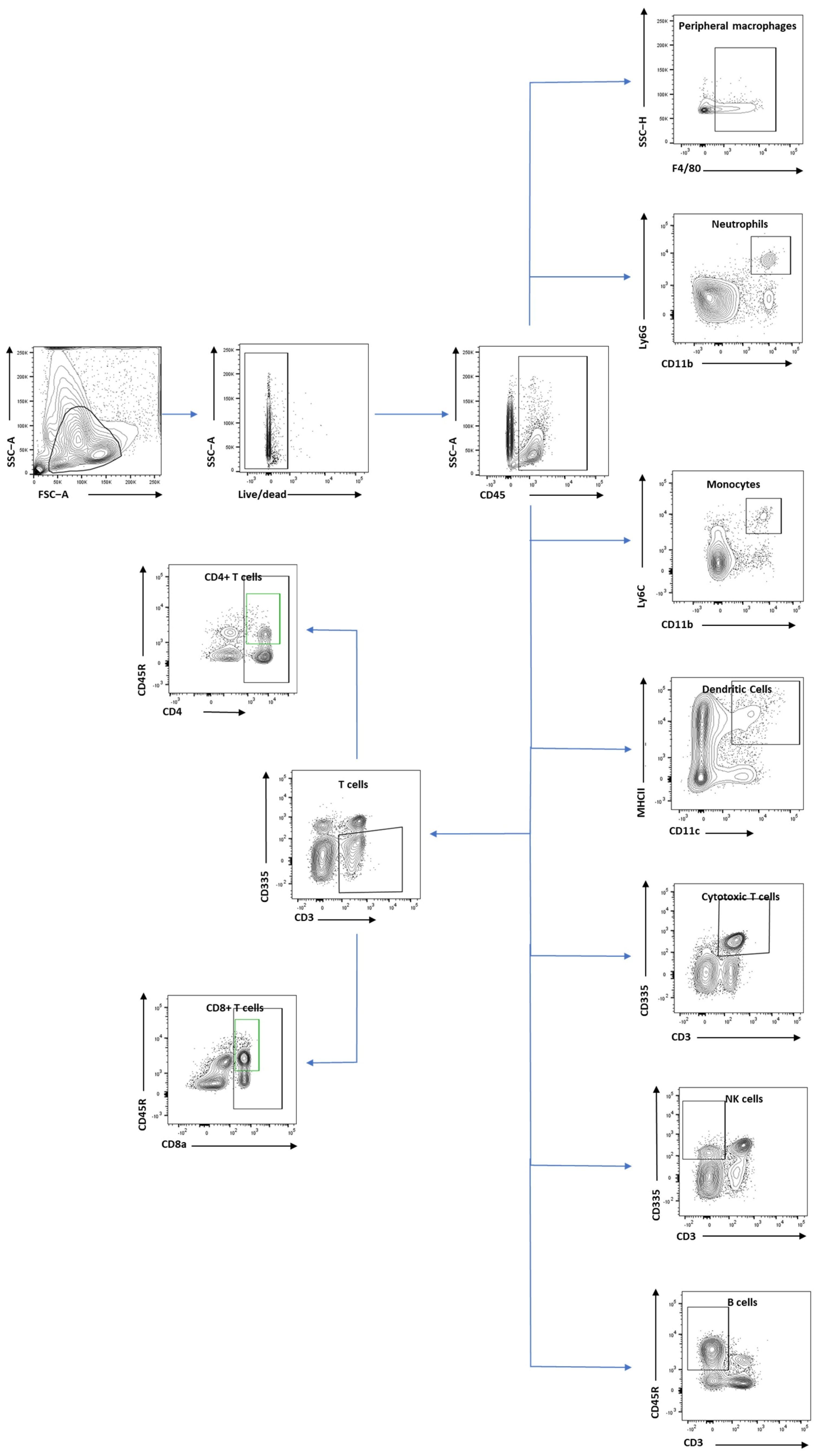
2.5. Flow Cytometry Analysis
2.6. Cytokine Array
2.7. Statistical Analysis
3. Results
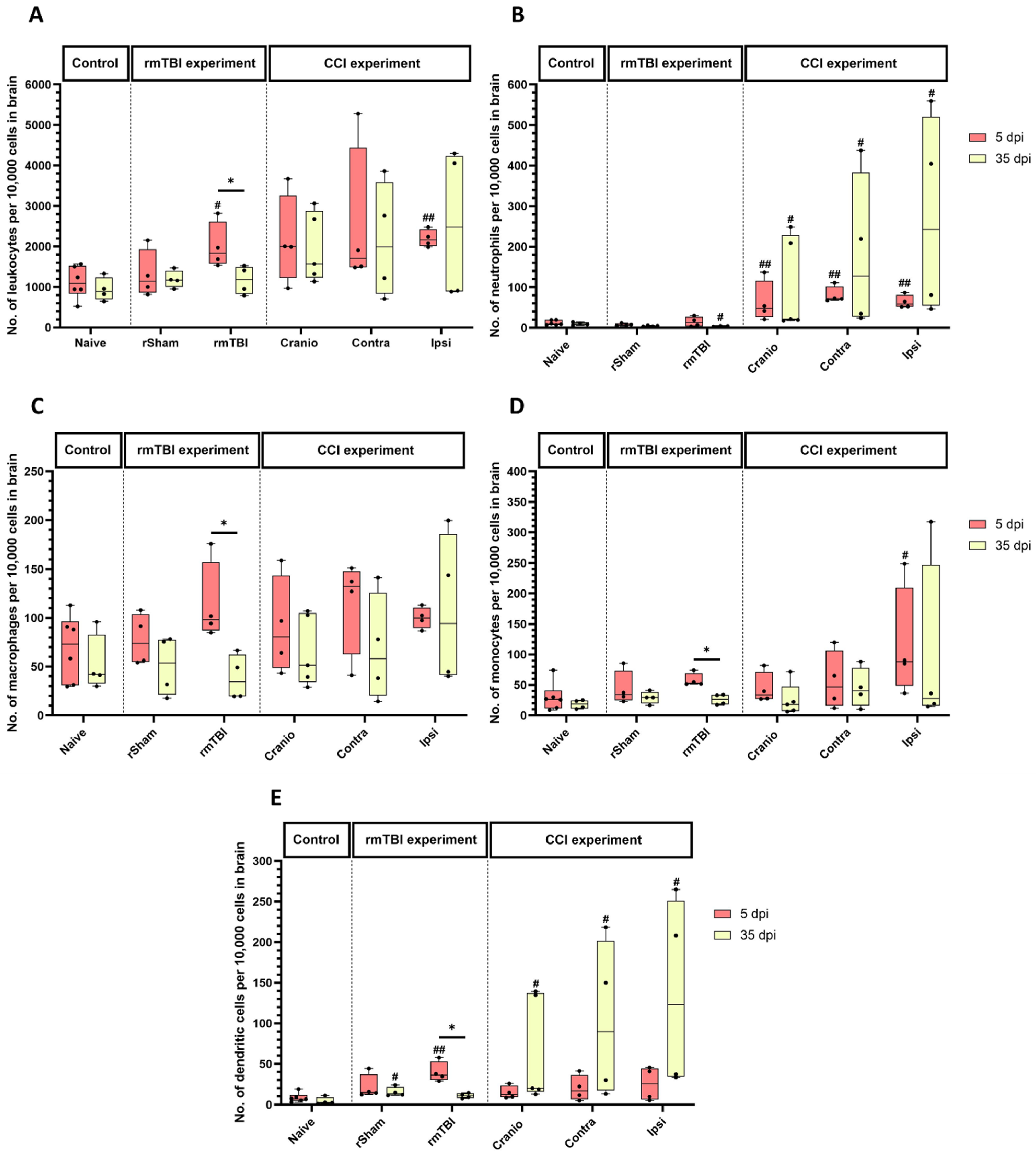
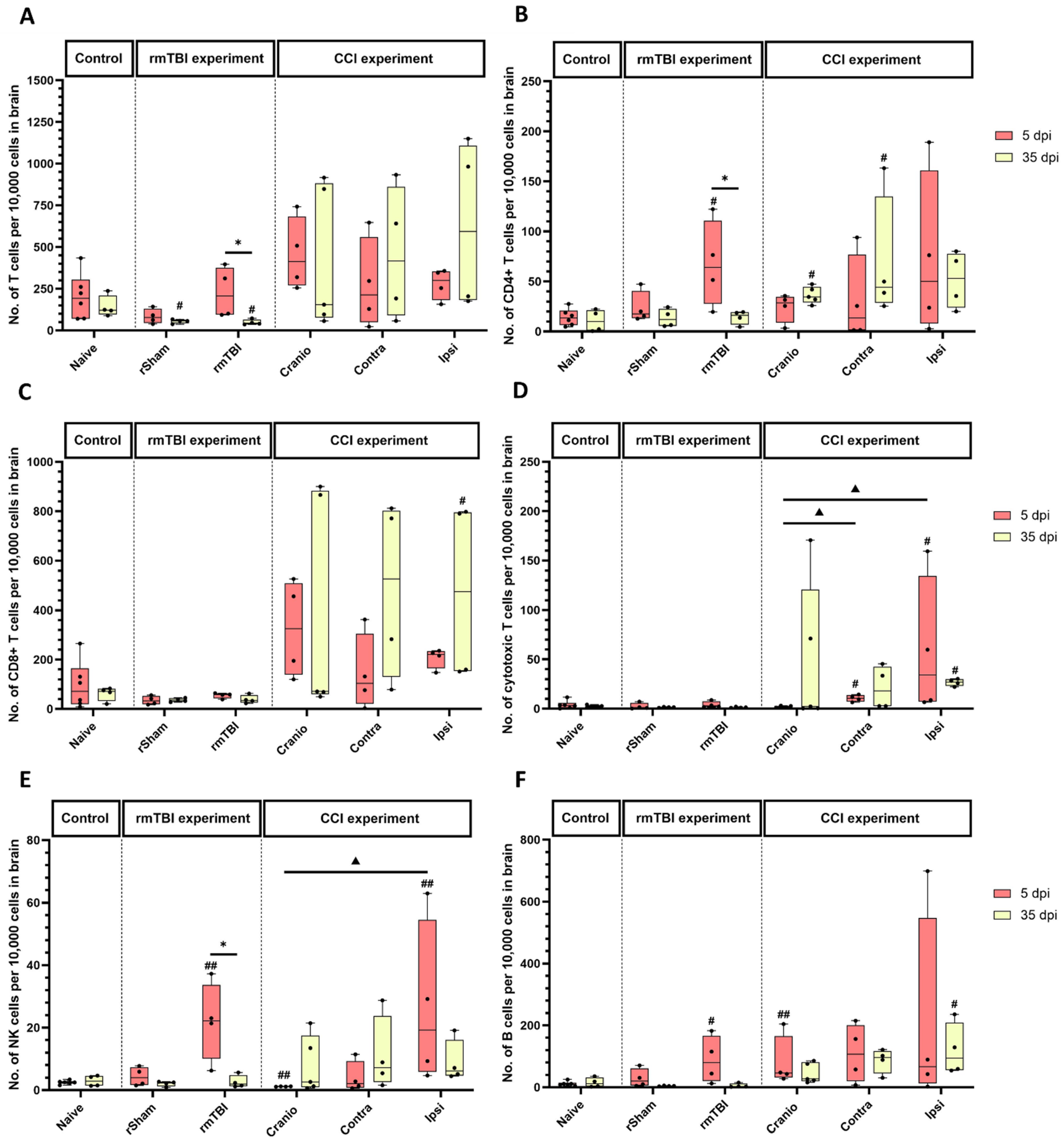
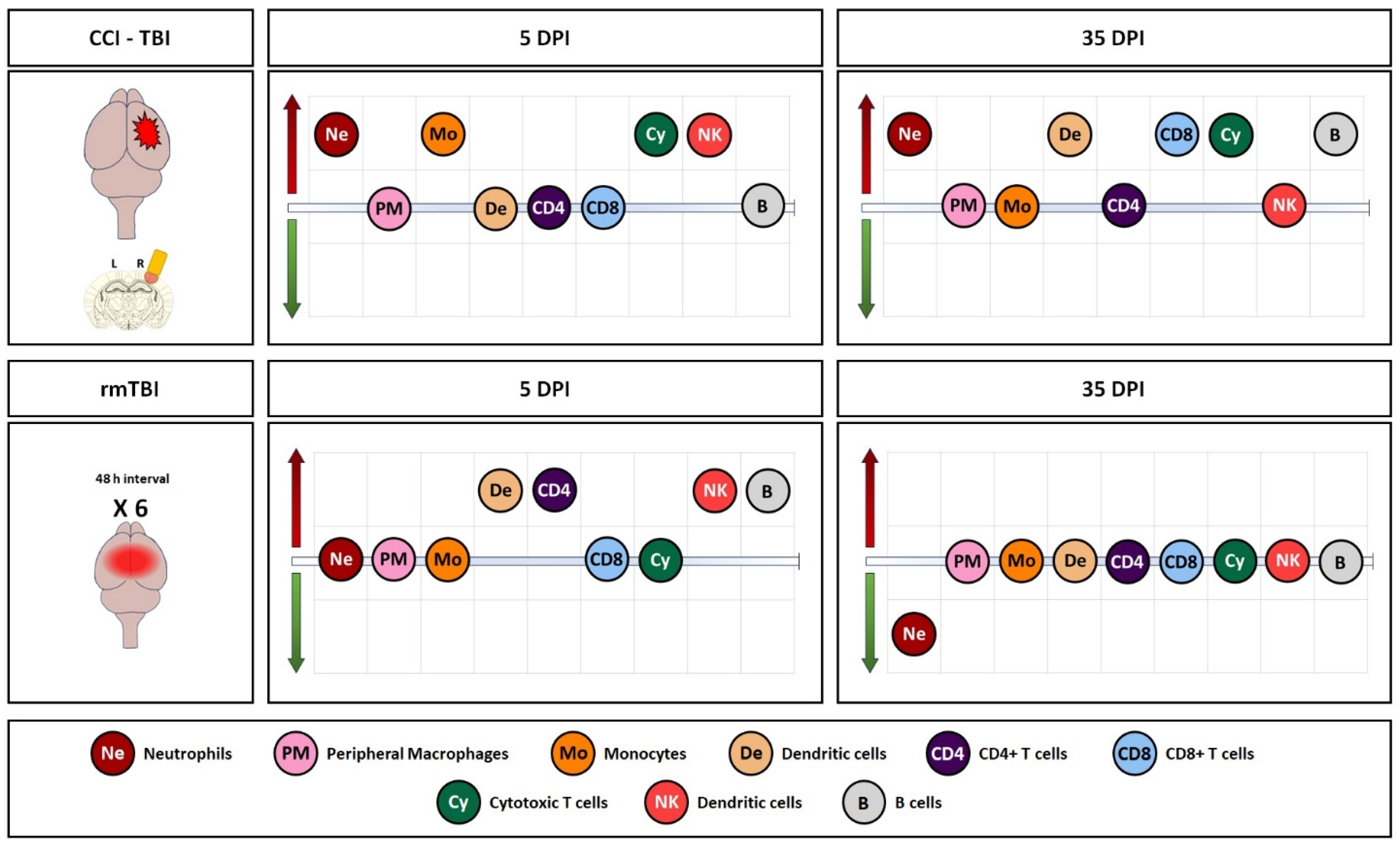
3.1. Immune System Response in Brain Tissue Following Single Invasive and Repetitive Mild Injury
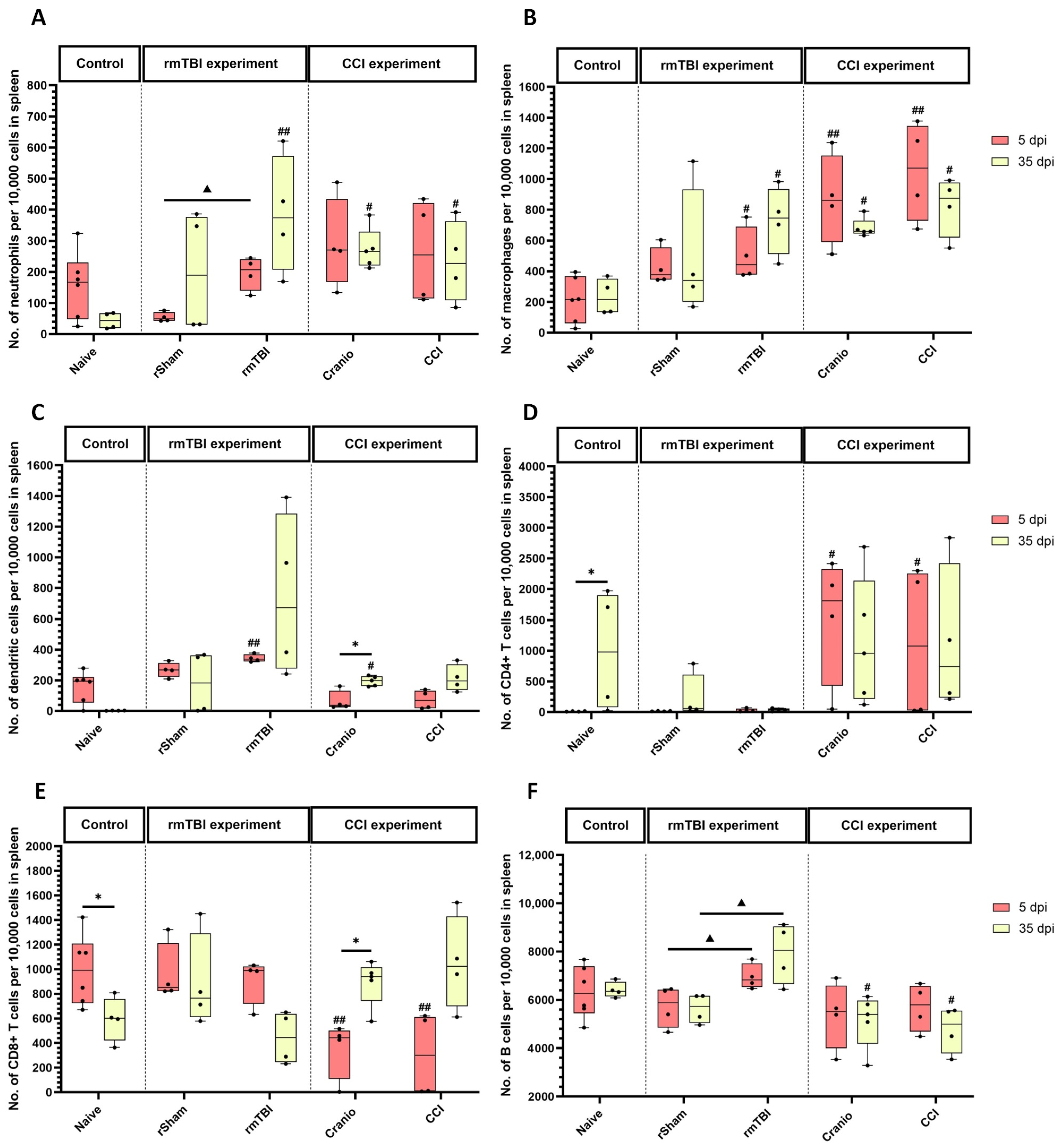
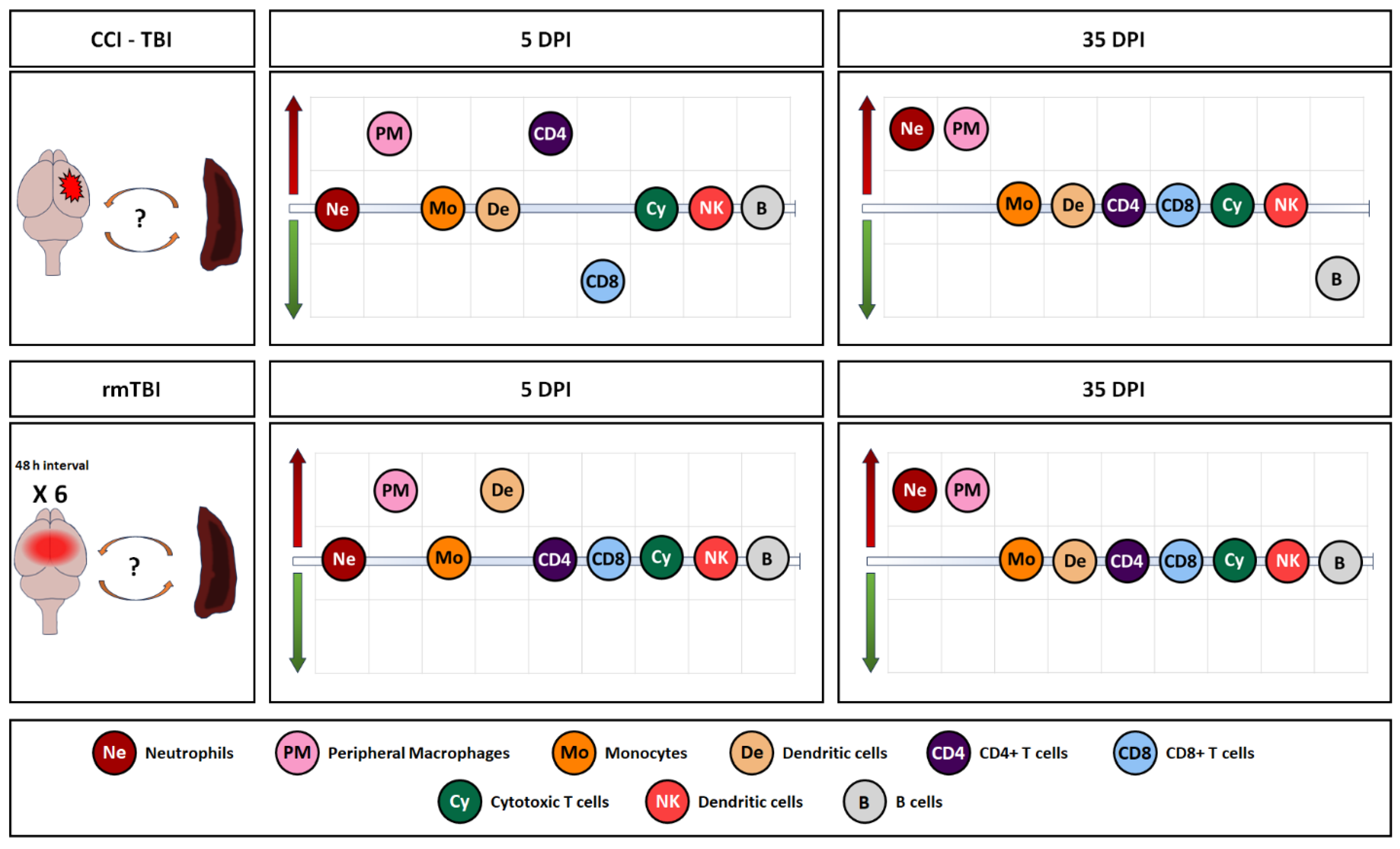
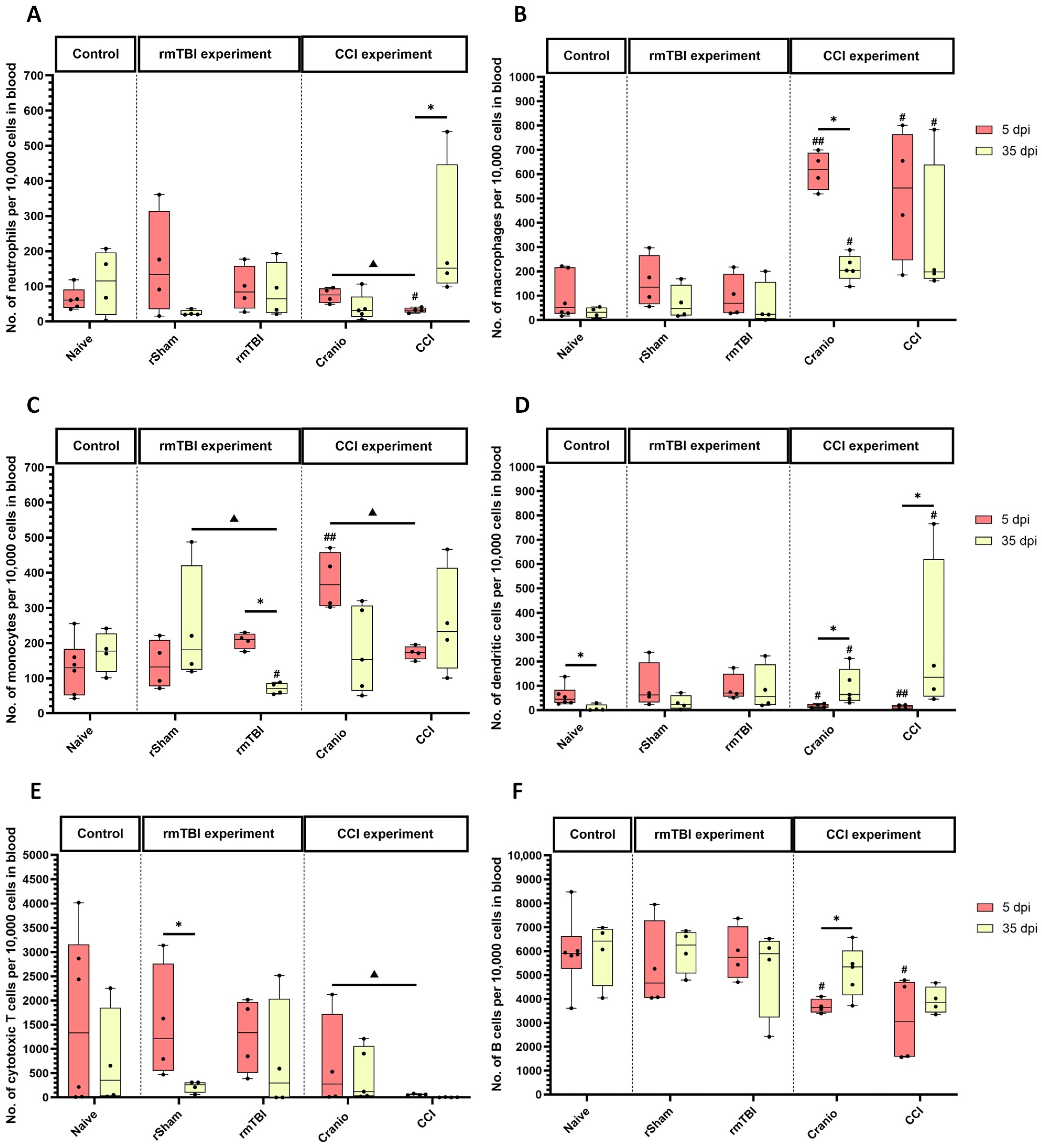
3.2. Immune System Response in Spleen, Blood, and Lymph Nodes Following Single Invasive and Repetitive Mild Injury
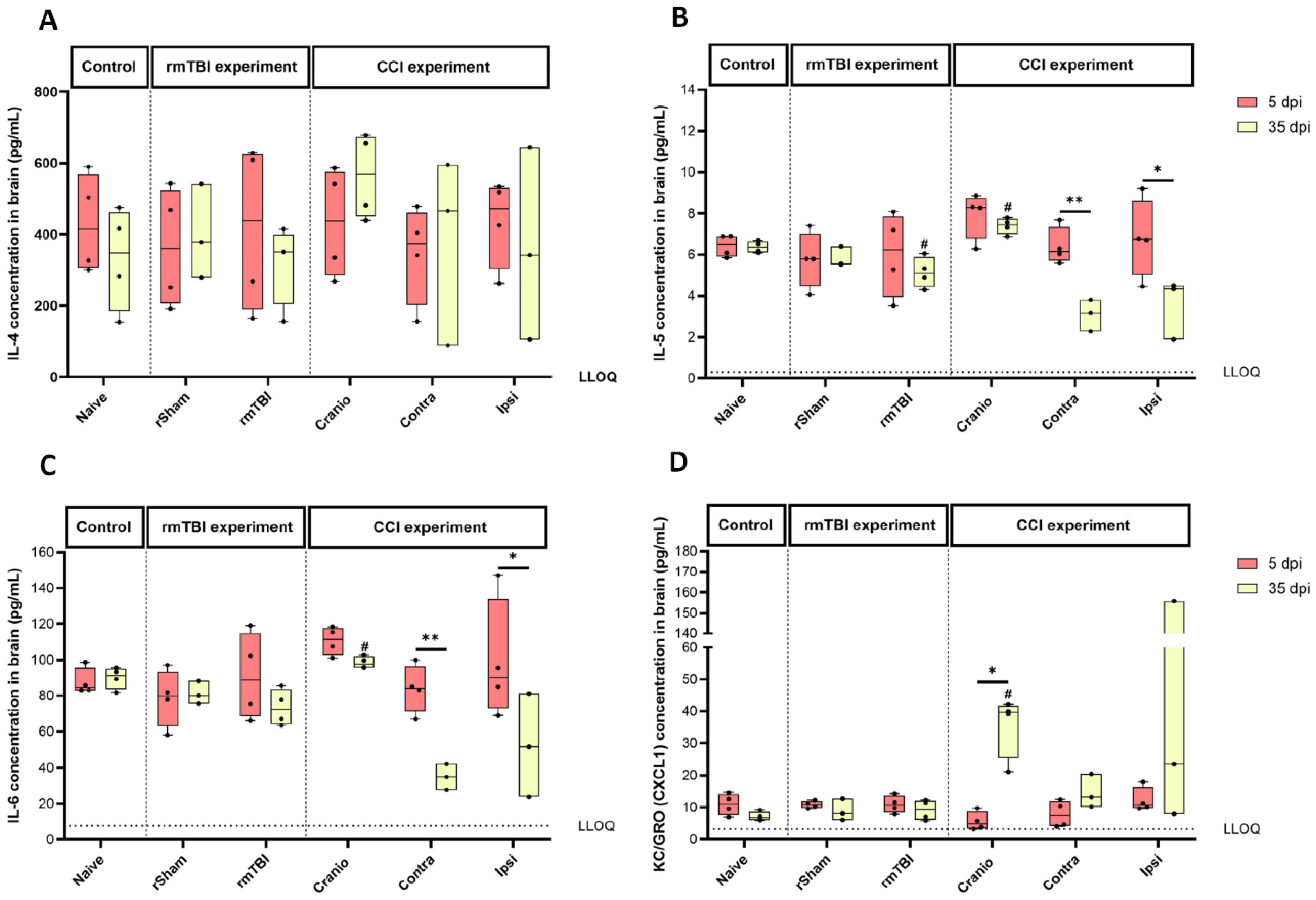
3.3. Cytokine Levels in Brain Tissue Following Single Invasive or Repetitive Mild Injury
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ULN | upper lymph nodes |
| LLN | lower lymph nodes |
| IL | interleukin |
| CNS | central nervous system |
| ROS | reactive oxygen species |
References
- Brown, A.W.; Elovic, E.P.; Kothari, S.; Flanagan, S.R.; Kwasnica, C. Congenital and acquired brain injury. 1. Epidemiology, pathophysiology, prognostication, innovative treatments, and prevention. Arch. Phys. Med. Rehabil. 2008, 89 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Algattas, H.; Huang, J.H. Traumatic Brain Injury pathophysiology and treatments: Early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 2013, 15, 309–341. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head. Trauma. Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med.-Chir. 2017, 57, 82–93. [Google Scholar] [CrossRef]
- Cattelani, R.; Lombardi, F.; Brianti, R.; Mazzucchi, A. Traumatic brain injury in childhood: Intellectual, behavioural and social outcome into adulthood. Brain Inj. 1998, 12, 283–296. [Google Scholar] [CrossRef]
- Dennis, M.; Spiegler, B.J.; Simic, N.; Sinopoli, K.J.; Wilkinson, A.; Yeates, K.O.; Taylor, H.G.; Bigler, E.D.; Fletcher, J.M. Functional plasticity in childhood brain disorders: When, what, how, and whom to assess. Neuropsychol. Rev. 2014, 24, 389–408. [Google Scholar] [CrossRef]
- Yeates, K.O.; Swift, E.; Taylor, H.G.; Wade, S.L.; Drotar, D.; Stancin, T.; Minich, N. Short- and long-term social outcomes following pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 2004, 10, 412–426. [Google Scholar] [CrossRef]
- McKinlay, A.; Grace, R.; Horwood, J.; Fergusson, D.; MacFarlane, M. Adolescent psychiatric symptoms following preschool childhood mild traumatic brain injury: Evidence from a birth cohort. J. Head Trauma Rehabil. 2009, 24, 221–227. [Google Scholar] [CrossRef]
- Ajao, D.O.; Pop, V.; Kamper, J.E.; Adami, A.; Rudobeck, E.; Huang, L.; Vlkolinsky, R.; Hartman, R.E.; Ashwal, S.; Obenaus, A.; et al. Traumatic brain injury in young rats leads to progressive behavioral deficits coincident with altered tissue properties in adulthood. J. Neurotrauma 2012, 29, 2060–2074. [Google Scholar] [CrossRef]
- Adelson, P.D.; Fellows-Mayle, W.; Kochanek, P.M.; Dixon, C.E. Morris water maze function and histologic characterization of two age-at-injury experimental models of controlled cortical impact in the immature rat. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2013, 29, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bruns, J., Jr.; Hauser, W.A. The epidemiology of traumatic brain injury: A review. Epilepsia 2003, 44, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, A.V.; Gorgan, M.R.; Tascu, A.; Sandu, A.M.; Rizea, R.E. Traumatic brain injury in infants and toddlers, 0–3 years old. J. Med. Life 2011, 4, 234–243. [Google Scholar] [PubMed]
- Ewing-Cobbs, L.; Prasad, M.R.; Kramer, L.; Cox, C.S.; Jr Baumgartner, J.; Fletcher, S.; Mendez, D.; Barnes, M.; Zhang, X.; Swank, P. Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J. Neurosurg. 2006, 105, 287–296. [Google Scholar] [CrossRef]
- Zamani, A.; Ryan, N.P.; Wright, D.K.; Caeyenberghs, K.; Semple, B.D. The impact of traumatic injury to the immature human brain: A scoping review with insights from advanced structural neuroimaging. J. Neurotrauma 2020, 37, 724–738. [Google Scholar] [CrossRef]
- Anderson, V.; Spencer-Smith, M.; Wood, A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain J. Neurol. 2011, 134 Pt 8, 2197–2221. [Google Scholar] [CrossRef]
- Nielsen, H.H.; Ladeby, R.; Fenger, C.; Toft-Hansen, H.; Babcock, A.A.; Owens, T.; Finsen, B. Enhanced microglial clearance of myelin debris in T cell-infiltrated central nervous system. J. Neuropathol. Exp. Neurol. 2009, 68, 845–856. [Google Scholar] [CrossRef]
- Engel, S.; Schluesener, H.; Mittelbronn, M.; Seid, K.; Adjodah, D.; Wehner, H.D.; Meyermann, R. Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol. 2000, 100, 313–322. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Clark, R.S.; Schiding, J.K.; Kaczorowski, S.L.; Marion, D.W.; Kochanek, P.M. Neutrophil accumulation after traumatic brain injury in rats: Comparison of weight drop and controlled cortical impact models. J. Neurotrauma 1994, 11, 499–506. [Google Scholar] [CrossRef]
- Kim, E.; Yang, J.; Beltran, C.D.; Cho, S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow. Metab. 2014, 34, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Carpenter, K.L.; Menon, D.K.; Pickard, J.D.; Hutchinson, P.J. The cytokine response to human traumatic brain injury: Temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2011, 31, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Sandor, M.; Suresh, M.; Fabry, Z. Traumatic injury and the presence of antigen differentially contribute to T-cell recruitment in the CNS. J. Neurosci. 2006, 26, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Chenouard, A.; Chesneau, M.; Braza, F.; Dejoie, T.; Cinotti, R.; Roquilly, A.; Brouard, S.; Asehnoune, K. Phenotype and functions of B cells in patients with acute brain injuries. Mol. Immunol. 2015, 68 Pt A, 350–356. [Google Scholar] [CrossRef]
- Alawieh, A.; Langley, E.F.; Weber, S.; Adkins, D.; Tomlinson, S. Identifying the Role of Complement in Triggering Neuroinflammation after Traumatic Brain Injury. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 2519–2532. [Google Scholar] [CrossRef] [PubMed]
- Gyoneva, S.; Ransohoff, R.M. Inflammatory reaction after traumatic brain injury: Therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 2015, 36, 471–480. [Google Scholar] [CrossRef]
- Verboon, L.N.; Patel, H.C.; Greenhalgh, A.D. The Immune System’s Role in the Consequences of Mild Traumatic Brain Injury (Concussion). Front. Immunol. 2021, 12, 620698. [Google Scholar] [CrossRef]
- Rowe, R.K.; Ellis, G.I.; Harrison, J.L.; Bachstetter, A.D.; Corder, G.F.; Van Eldik, L.J.; Taylor, B.K.; Marti, F.; Lifshitz, J. Diffuse traumatic brain injury induces prolonged immune dysregulation and potentiates hyperalgesia following a peripheral immune challenge. Mol. Pain 2016, 12, 1744806916647055. [Google Scholar] [CrossRef]
- McDonald, S.J.; Sharkey, J.M.; Sun, M.; Kaukas, L.M.; Shultz, S.R.; Turner, R.J.; Leonard, A.V.; Brady, R.D.; Corrigan, F. Beyond the brain: Peripheral interactions after traumatic brain injury. J. Neurotrauma 2020, 37, 770–781. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Nasr, I.W.; Chun, Y.; Kannan, S. Neuroimmune responses in the developing brain following traumatic brain injury. Exp. Neurol. 2019, 320, 112957. [Google Scholar] [CrossRef] [PubMed]
- Claus, C.P.; Tsuru-Aoyagi, K.; Adwanikar, H.; Walker, B.; Manvelyan, H.; Whetstone, W.; Noble-Haeusslein, L.J. Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Dev. Neurosci. 2010, 32, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, Z.F.; Boumenar, H.; Adebimpe, J.; Shekerzade, S.; Henson, S.M.; Tremoleda, J.L.; Michael-Titus, A.T. The cellular senescence response and neuroinflammation in juvenile mice following controlled cortical impact and repetitive mild traumatic brain injury. Exp. Neurol. 2024, 374, 114714. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Bittigau, P.; Pohl, D.; Sifringer, M.; Shimizu, H.; Ikeda, M.; Ishimaru, M.; Stadthaus, D.; Fuhr, S.; Dikranian, K.; Olney, J.W.; et al. Modeling pediatric head trauma: Mechanisms of degeneration and potential strategies for neuroprotection. Restor. Neurol. Neurosci. 1998, 13, 11–23. [Google Scholar]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nature reviews. Neurology 2017, 13, 171–191. [Google Scholar] [CrossRef]
- Bouras, M.; Asehnoune, K.; Roquilly, A. Immune modulation after traumatic brain injury. Front. Med. 2022, 9, 995044. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain J. Neurol. 2013, 136 Pt 1, 28–42. [Google Scholar] [CrossRef]
- Fenn, A.M.; Gensel, J.C.; Huang, Y.; Popovich, P.G.; Lifshitz, J.; Godbout, J.P. Immune activation promotes depression 1 month after diffuse brain injury: A role for primed microglia. Biol. Psychiatry 2014, 76, 575–584. [Google Scholar] [CrossRef]
- Morrison, H.; Young, K.; Qureshi, M.; Rowe, R.K.; Lifshitz, J. Quantitative microglia analyses reveal diverse morphologic responses in the rat cortex after diffuse brain injury. Sci. Rep. 2017, 7, 13211. [Google Scholar] [CrossRef]
- Yu, F.; Shukla, D.K.; Armstrong, R.C.; Marion, C.M.; Radomski, K.L.; Selwyn, R.G.; Dardzinski, B.J. Repetitive model of mild traumatic brain injury produces cortical abnormalities detectable by magnetic resonance diffusion imaging, histopathology, and behavior. J. Neurotrauma 2017, 34, 1364–1381. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.; Clarke, G.J.B.; Skandsen, T.; Islam, R.; Einarsen, C.E.; Vik, A.; Damås, J.K.; Mollnes, T.E.; Håberg, A.K.; Pischke, S.E. Systemic Inflammation Persists the First Year after Mild Traumatic Brain Injury: Results from the Prospective Trondheim Mild Traumatic Brain Injury Study. J. Neurotrauma 2020, 37, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G.; Rhind, S.G.; Rizoli, S.B.; Cuschieri, J.; Baker, A.J.; Shek, P.N.; Hoyt, D.B.; Bulger, E.M. Prehospital hypertonic saline resuscitation attenuates the activation and promotes apoptosis of neutrophils in patients with severe traumatic brain injury. Shock 2013, 40, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Rhind, S.G.; Crnko, N.T.; Baker, A.J.; Morrison, L.J.; Shek, P.N.; Scarpelini, S.; Rizoli, S.B. Prehospital resuscitation with hypertonic saline-dextran modulates inflammatory, coagulation and endothelial activation marker profiles in severe traumatic brain injured patients. J. Neuroinflamm. 2010, 7, 5. [Google Scholar] [CrossRef]
- Jin, X.; Ishii, H.; Bai, Z.; Itokazu, T.; Yamashita, T. Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS ONE 2012, 7, e41892. [Google Scholar] [CrossRef]
- Carlos, T.M.; Clark, R.S.; Franicola-Higgins, D.; Schiding, J.K.; Kochanek, P.M. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 1997, 61, 279–285. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M.; Belli, A. Traumatic Brain Injury and Peripheral Immune Suppression: Primer and Prospectus. Front. Neurol. 2015, 6, 235. [Google Scholar] [CrossRef]
- Tsymbalyuk, O.; Gerzanich, V.; Simard, J.M.; Rathinam, C.V. Traumatic brain injury alters dendritic cell differentiation and distribution in lymphoid and non-lymphoid organs. J. Neuroinflamm. 2022, 19, 238. [Google Scholar] [CrossRef]
- Trahanas, D.M.; Cuda, C.M.; Perlman, H.; Schwulst, S.J. Differential Activation of Infiltrating Monocyte-Derived Cells After Mild and Severe Traumatic Brain Injury. Shock 2015, 43, 255–260. [Google Scholar] [CrossRef]
- Kong, X.D.; Bai, S.; Chen, X.; Wei, H.J.; Jin, W.N.; Li, M.S.; Yan, Y.; Shi, F.D. Alterations of natural killer cells in traumatic brain injury. Neurosci. Bull. 2014, 30, 903–912. [Google Scholar] [CrossRef]
- Lünemann, A.; Lünemann, J.D.; Roberts, S.; Messmer, B.; Barreira da Silva, R.; Raine, C.S.; Münz, C. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J. Immunol. 2008, 181, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Liu, R.; Piao, W.; Zhou, Q.; Vollmer, T.L.; Campagnolo, D.I.; Xiang, R.; La Cava, A.; Van Kaer, L.; Shi, F.D. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 2010, 207, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Armant, M.; Delespesse, G.; Sarfati, M. IL-2 and IL-7 but not IL-12 protect natural killer cells from death by apoptosis and up-regulate bcl-2 expression. Immunology 1995, 85, 331–337. [Google Scholar] [PubMed]
- Srivastava, A.; Chandra, A.; Yadav, A.; Ojha, B.K.; Srivastava, C.; Verma, R.; Ali, W. Dynamic change in cortisol levels associated with severity, progression, and survival of patients with traumatic brain injury. Clin. Neurol. Neurosurg. 2022, 222, 107419. [Google Scholar] [CrossRef]
- Daglas, M.; Draxler, D.F.; Ho, H.; McCutcheon, F.; Galle, A.; Au, A.E.; Larsson, P.; Gregory, J.; Alderuccio, F.; Sashindranath, M.; et al. Activated CD8+ T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell Rep. 2019, 29, 1178–1191.e6. [Google Scholar] [CrossRef]
- Needham, E.J.; Stoevesandt, O.; Thelin, E.P.; Zetterberg, H.; Zanier, E.R.; Al Nimer, F.; Ashton, N.J.; Outtrim, J.G.; Newcombe, V.F.J.; Mousa, H.S.; et al. Complex autoantibody responses occur following moderate to severe traumatic brain injury. J. Immunol. 2021, 207, 90–100. [Google Scholar] [CrossRef]
- Mrakovcic-Sutic, I.; Tokmadzic, V.S.; Laskarin, G.; Mahmutefendic, H.; Lucin, P.; Zupan, Z.; Sustic, A. Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand. J. Immunol. 2010, 72, 57–65. [Google Scholar] [CrossRef]
- Wu, L.; Chung, J.Y.; Saith, S.; Tozzi, L.; Buckley, E.M.; Sanders, B.; Franceschini, M.A.; Lule, S.; Izzy, S.; Lok, J.; et al. Repetitive head injury in adolescent mice: A role for vascular inflammation. J. Cereb. Blood Flow. Metab. 2019, 39, 2196–2209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zoltewicz, J.S.; Mondello, S.; Newsom, K.J.; Yang, Z.; Yang, B.; Kobeissy, F.; Guingab, J.; Glushakova, O.; Robicsek, S.; et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS ONE 2014, 9, e92698. [Google Scholar] [CrossRef]
- Yilmaz, G.; Arumugam, T.V.; Stokes, K.Y.; Granger, D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006, 113, 2105–2112. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Lucin, K.M.; Sanders, V.M.; McGaughy, V.M.; Popovich, P.G. Spinal cord injury triggers systemic autoimmunity: Evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006, 99, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, D.P.; Guan, Z.; Popovich, P.G. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J. Clin. Investig. 2009, 119, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Matic, D.; Djogo, N.; Szpotowicz, E.; Schachner, M.; Jakovcevski, I. Improved regeneration after spinal cord injury in mice lacking functional T- and B-lymphocytes. Exp. Neurol. 2012, 237, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ahn, B.J.; Shi, J.; Nakamura, Y.; Park, J.H.; Mandeville, E.T.; Yu, Z.; Chan, S.J.; Desai, R.; Hayakawa, A.; et al. Brain-to-cervical lymph node signaling after stroke. Nat. Commun. 2019, 10, 5306. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Frugier, T.; Morganti-Kossmann, M.C.; O’Reilly, D.; McLean, C.A. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J. Neurotrauma 2010, 27, 497–507. [Google Scholar] [CrossRef]
- Ryan, E.; Kelly, L.; Stacey, C.; Huggard, D.; Duff, E.; McCollum, D.; Leonard, A.; Boran, G.; Doherty, D.R.; Bolger, T.; et al. Mild-to-severe traumatic brain injury in children: Altered cytokines reflect severity. J. Neuroinflamm. 2022, 19, 36. [Google Scholar] [CrossRef]
- Algamal, M.; Saltiel, N.; Pearson, A.J.; Ager, B.; Burca, I.; Mouzon, B.; Diamond, D.M.; Mullan, M.; Ojo, J.O.; Crawford, F. Impact of Repetitive Mild Traumatic Brain Injury on Behavioral and Hippocampal Deficits in a Mouse Model of Chronic Stress. J. Neurotrauma 2019, 36, 2590–2607. [Google Scholar] [CrossRef]
- Bai, R.; Gao, H.; Han, Z.; Ge, X.; Huang, S.; Chen, F.; Lei, P. Long-Term Kinetics of Immunologic Components and Neurological Deficits in Rats Following Repetitive Mild Traumatic Brain Injury. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 1707–1718. [Google Scholar] [CrossRef]
- Corrigan, F.; Arulsamy, A.; Collins-Praino, L.E.; Holmes, J.L.; Vink, R. Toll like receptor 4 activation can be either detrimental or beneficial following mild repetitive traumatic brain injury depending on timing of activation. Brain Behav. Immun. 2017, 64, 124–139. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Popiolek-Barczyk, K.; Pawlik, K.; Ciapała, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. Changes in macrophage inflammatory protein-1 (MIP-1) family members expression induced by traumatic brain injury in mice. Immunobiology 2020, 225, 151911. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Katki, K.; Arisi, G.M.; Foresti, M.L.; Shapiro, L.A. Early TBI-induced cytokine alterations are similarly detected by two distinct methods of multiplex assay. Front. Mol. Neurosci. 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Newell, E.A.; Todd, B.P.; Luo, Z.; Evans, L.P.; Ferguson, P.J.; Bassuk, A.G. A Mouse Model for Juvenile, Lateral Fluid Percussion Brain Injury Reveals Sex-Dependent Differences in Neuroinflammation and Functional Recovery. J. Neurotrauma 2020, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Shein, S.L.; Shellington, D.K.; Exo, J.L.; Jackson, T.C.; Wisniewski, S.R.; Jackson, E.K.; Vagni, V.A.; Bayır, H.; Clark, R.S.; Dixon, C.E.; et al. Hemorrhagic shock shifts the serum cytokine profile from pro- to anti-inflammatory after experimental traumatic brain injury in mice. J. Neurotrauma 2014, 31, 1386–1395. [Google Scholar] [CrossRef]
- Sifringer, M.; Stefovska, V.; Endesfelder, S.; Stahel, P.F.; Genz, K.; Dzietko, M.; Ikonomidou, C.; Felderhoff-Mueser, U. Activation of caspase-1 dependent interleukins in developmental brain trauma. Neurobiol. Dis. 2007, 25, 614–622. [Google Scholar] [CrossRef]
- Xu, X.; Gao, W.; Cheng, S.; Yin, D.; Li, F.; Wu, Y.; Sun, D.; Zhou, S.; Wang, D.; Zhang, Y.; et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J. Neuroinflamm. 2017, 14, 167. [Google Scholar] [CrossRef]
- Maier, B.; Schwerdtfeger, K.; Mautes, A.; Holanda, M.; Müller, M.; Steudel, W.I.; Marzi, I. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock 2001, 15, 421–426. [Google Scholar] [CrossRef]
- Ross, S.A.; Halliday, M.I.; Campbell, G.C.; Byrnes, D.P.; Rowlands, B.J. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br. J. Neurosurg. 1994, 8, 419–425. [Google Scholar] [CrossRef]
- Csuka, E.; Morganti-Kossmann, M.C.; Lenzlinger, P.M.; Joller, H.; Trentz, O.; Kossmann, T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J. Neuroimmunol. 1999, 101, 211–221. [Google Scholar] [CrossRef]
- Hillman, J.; Aneman, O.; Persson, M.; Andersson, C.; Dabrosin, C.; Mellergård, P. Variations in the response of interleukins in neurosurgical intensive care patients monitored using intracerebral microdialysis. J. Neurosurg. 2007, 106, 820–825. [Google Scholar] [CrossRef]
- Winter, C.D.; Pringle, A.K.; Clough, G.F.; Church, M.K. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain 2004, 127 Pt 2, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.; Morganti-Kossmann, M.C. The role of markers of inflammation in traumatic brain injury. Front. Neurol. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Stollings, L.M.; Jia, L.J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiology 2016, 125, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Luan, T.; Li, Y.; Sun, L.; Xu, S.; Wang, H.; Wang, J.; Li, C. Systemic immune effects of anesthetics and their intracellular targets in tumors. Front. Med. 2022, 9, 810189. [Google Scholar] [CrossRef]

| Antigen | Fluorochrome | Laser | Wavelength | Manufacturer |
|---|---|---|---|---|
| CD45 | FITC | B | 530/30 | Miltenyi Biotec (Bergisch Gladbach, Germany) |
| F4/80 | APC | R | 670/14 | Biolegend |
| Live/Dead | Zombie NIR | R | 780/60 | Biolegend |
| Ly6C | VioBlue | V | 450/50 | Miltenyi Biotec |
| Ly6G | BV605 | V | 610/20 | Biolegend |
| MHC II | BV711 | V | 710/50 | Biolegend |
| CD11c | PE | YG | 582/10 | Miltenyi Biotec |
| CD11b | PE-Vio615 | YG | 610/20 | Miltenyi Biotec |
| Antigen | Fluorochrome | Laser | Wavelength | Manufacturer |
|---|---|---|---|---|
| CD45 | FITC | B | 530/30 | Miltenyi Biotec |
| CD8a | PerCP-Vio700 | B | 695/40 | Miltenyi Biotec |
| CD3 | APC | R | 670/14 | Biolegend |
| Live/Dead | Zombie NIR | R | 780/60 | Biolegend |
| CD45R/B219 | VioGreen | V | 525/49 | Miltenyi Biotec |
| CD19 | BV785 | V | 780/60 | Biolegend |
| CD335/NKp46 | PE | YG | 582/10 | Miltenyi Biotec |
| CD4 | PE-Vio615 | YG | 615/20 | Miltenyi Biotec |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khateeb, Z.F.; Henson, S.M.; Tremoleda, J.L.; Michael-Titus, A.T. The Immune Response in Two Models of Traumatic Injury of the Immature Brain. Cells 2024, 13, 1612. https://doi.org/10.3390/cells13191612
Al-Khateeb ZF, Henson SM, Tremoleda JL, Michael-Titus AT. The Immune Response in Two Models of Traumatic Injury of the Immature Brain. Cells. 2024; 13(19):1612. https://doi.org/10.3390/cells13191612
Chicago/Turabian StyleAl-Khateeb, Zahra F., Siân M. Henson, Jordi L. Tremoleda, and Adina T. Michael-Titus. 2024. "The Immune Response in Two Models of Traumatic Injury of the Immature Brain" Cells 13, no. 19: 1612. https://doi.org/10.3390/cells13191612







