NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Analysis of Fat Volume and Total Body Weight
2.3. Reagents and Antibodies
2.4. Immunohistochemistry and Immunofluorescence Analysis
2.5. Cystatin C and Cytokine Analysis
2.6. Statistical Analysis
3. Results
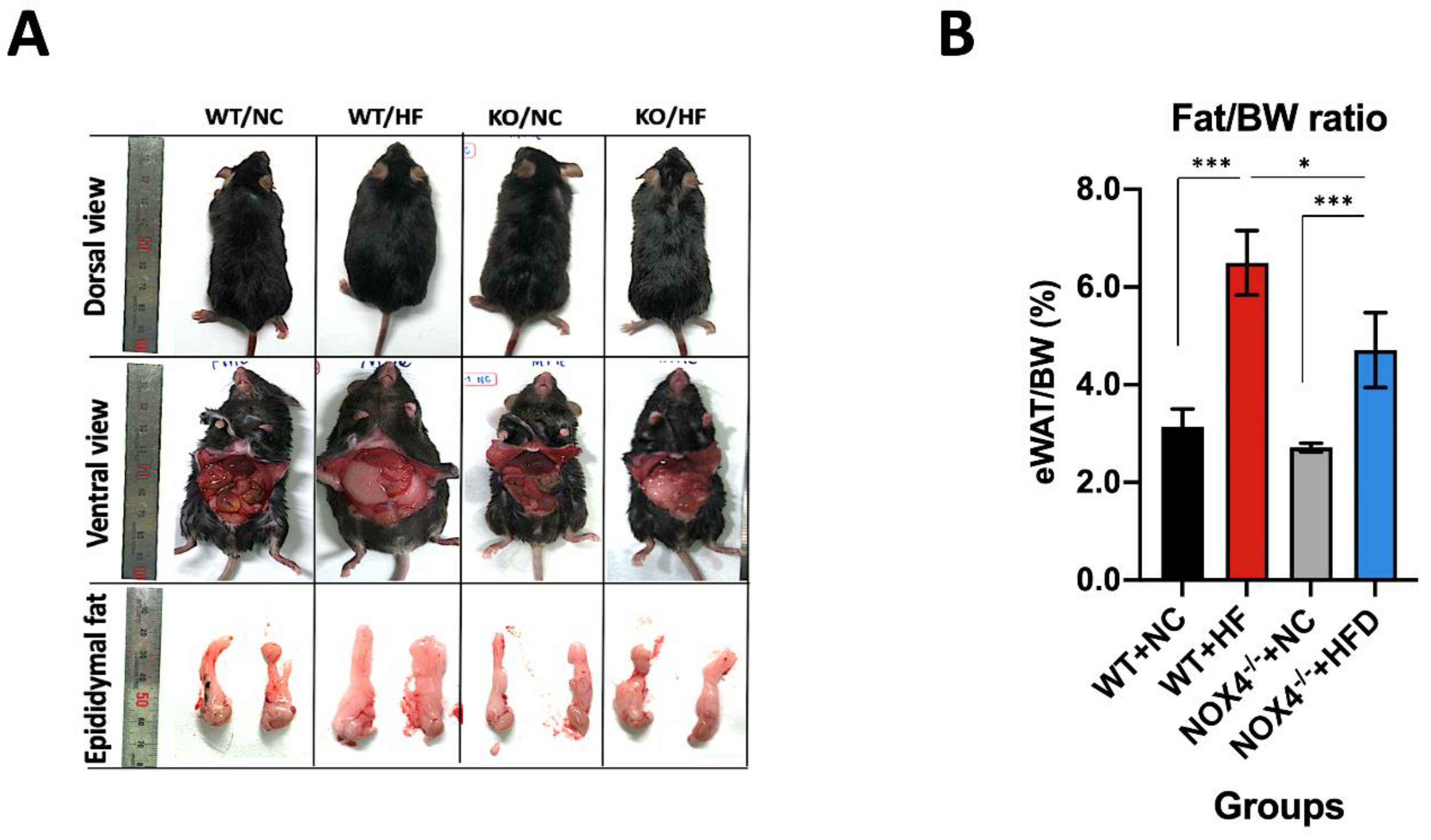
3.1. NOX4 Deficiency Decreases Fat Accumulation in Epidermal White Adipose Tissue during Chronic HFD
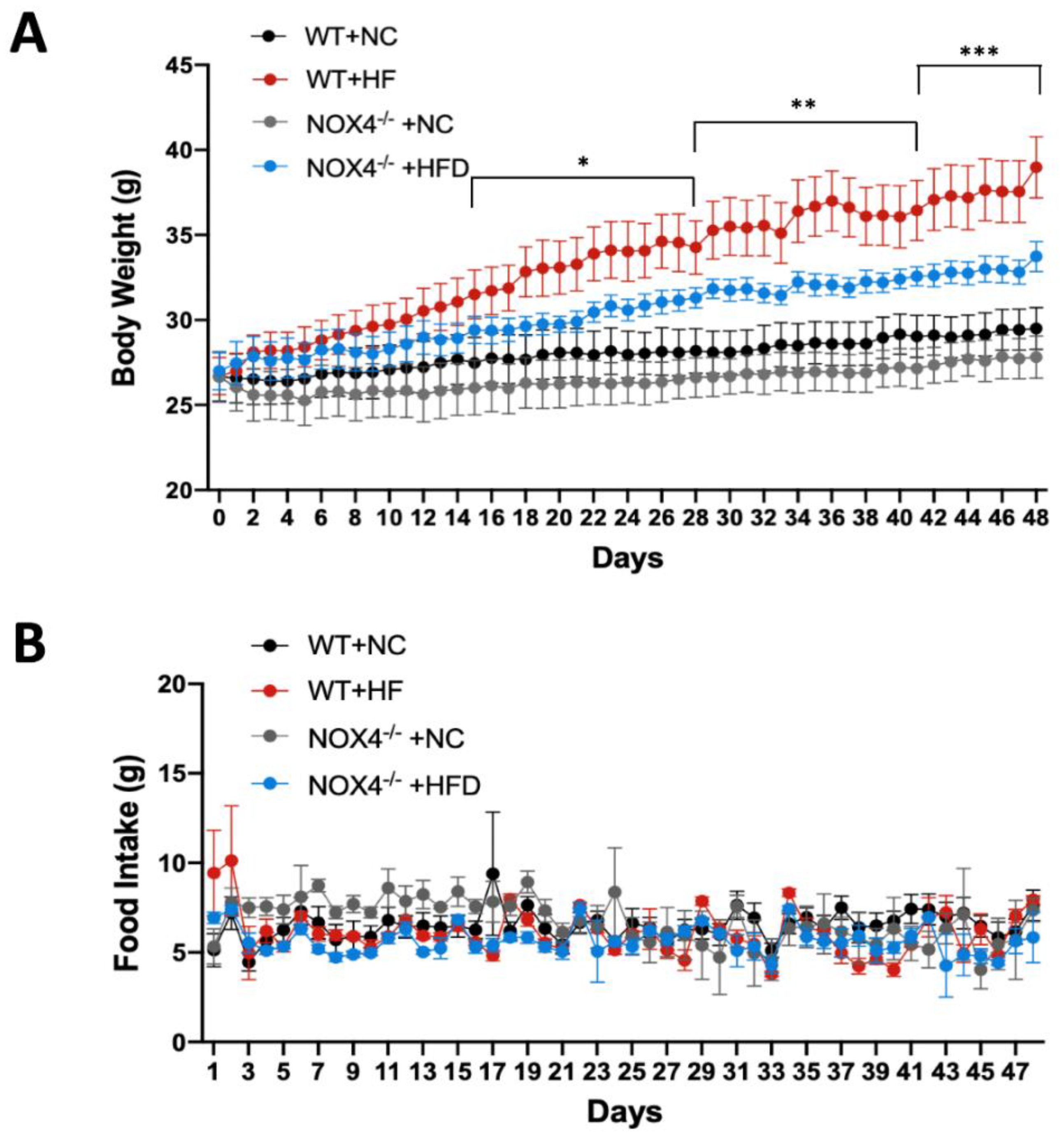
3.2. NOX4 Deficiency Affects Weight Gain during Chronic HFD
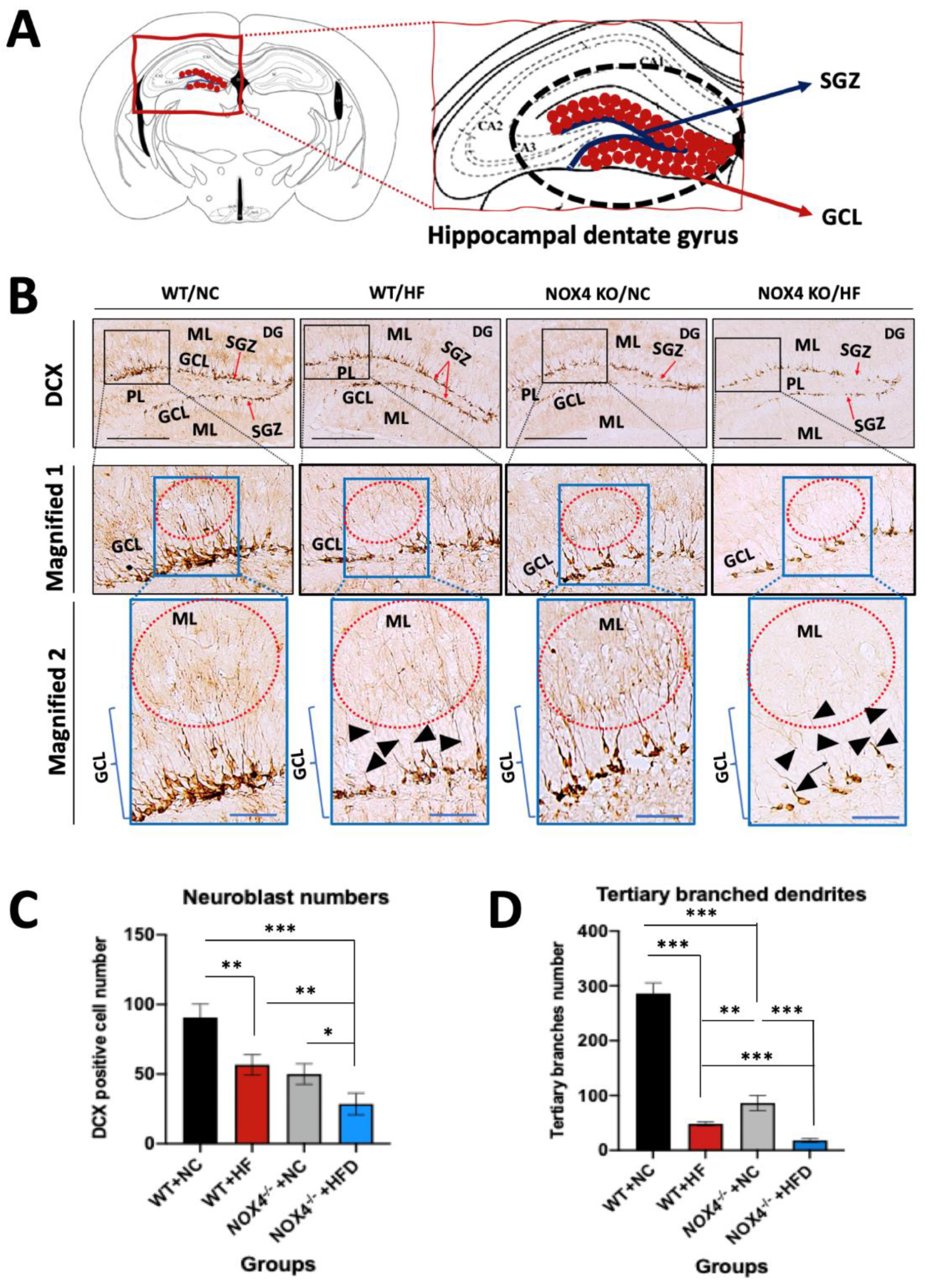
3.3. NOX4 Deficiency Exacerbates the Impairment of Hippocampal Neurogenesis by Chronic HFD
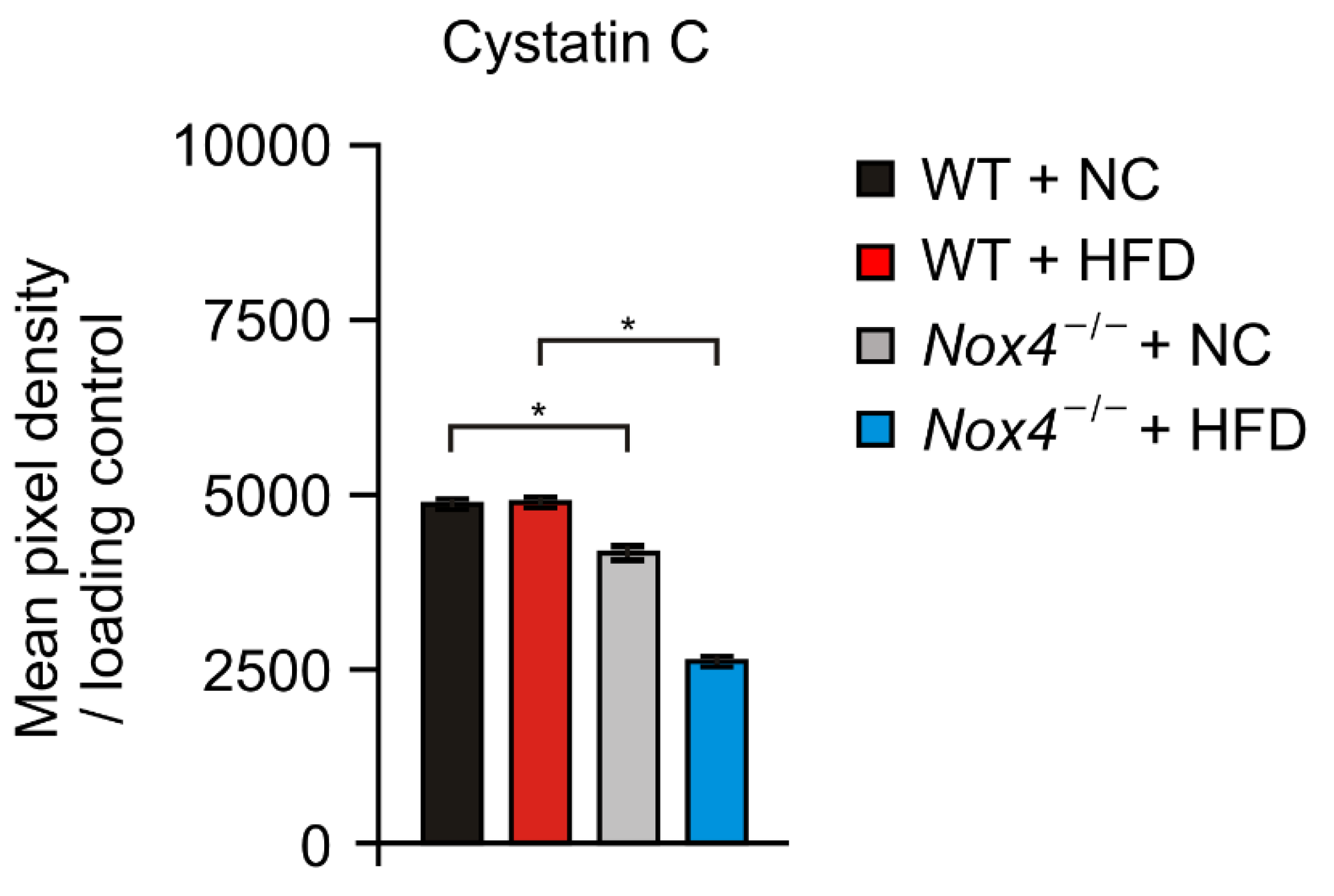
3.4. NOX4 Deficiency Suppresses the Production of Cystatin C in Hippocampus during Chronic HFD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Globalization, Diets and Noncommunicable Diseases. Available online: https://apps.who.int/iris/handle/10665/42609 (accessed on 17 November 2012).
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Laye, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. The evolution of human adiposity and obesity: Where did it all go wrong? Dis. Model. Mech. 2012, 5, 595–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, M.; Cai, W.; Konishi, M.; Kahn, C.R. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc. Natl. Acad. Sci. USA 2019, 116, 6379–6384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yon, M.A.; Mauger, S.L.; Pickavance, L.C. Relationships between dietary macronutrients and adult neurogenesis in the regulation of energy metabolism. Br. J. Nutr. 2013, 109, 1573–1589. [Google Scholar] [CrossRef] [Green Version]
- Knobloch, M.; Jessberger, S. Metabolism and neurogenesis. Curr. Opin. Neurobiol. 2017, 42, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Haring, H.U. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of Metabolic Syndrome and Dietary Intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [Green Version]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef] [Green Version]
- Cai, D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 2013, 24, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.S.; Hwang, I.K.; Yoo, K.Y.; Park, O.K.; Yu, J.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Pai, T.; Song, W.; et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem. Res. 2009, 34, 1039–1046. [Google Scholar] [CrossRef]
- Uranga, R.M.; Keller, J.N. The Complex Interactions between Obesity, Metabolism and the Brain. Front. Neurosci. 2019, 13, 513. [Google Scholar] [CrossRef] [Green Version]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef]
- Stouffer, E.M.; Warninger, E.E.; Michener, P.N. A high-fat diet impairs learning that is dependent on the dorsal hippocampus but spares other forms of learning. Hippocampus 2015, 12, 1567–1576. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 7, 1699–1707. [Google Scholar] [CrossRef]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P., Jr.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [Green Version]
- Besser, L.M.; Gill, D.P.; Monsell, S.E.; Brenowitz, W.; Meranus, D.H.; Kukull, W.; Gustafson, D.R. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2014, 28, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Cimerman, N.; Brguljan, P.M.; Krašovec, M.; Šuškovič, S.; Kos, J. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin. Chim. Acta 2000, 300, 83–95. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef]
- Koenig, W.; Twardella, D.; Brenner, H.; Rothenbacher, D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: More than simply a marker of glomerular filtration rate. Clin. Chem. 2005, 51, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Levy, E.; Lopez-Otin, C.; Ghiso, J.; Geltner, D.; Frangione, B. Stroke in Icelandic patients with hereditary amyloid angiopathy is related to a mutation in the cystatin C gene, an inhibitor of cysteine proteases. J. Exp. Med. 1989, 169, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.F.; Doust, J.; Tett, S.E.; Kirkpatrick, C.M. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—A meta-analysis. Clin. Biochem. 2007, 40, 383–391. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef]
- Levy, E.; Jaskolski, M.; Grubb, A. The role of cystatin C in cerebral amyloid angiopathy and stroke: Cell biology and animal models. Brain Pathol. 2006, 16, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, S.A.; Herzig, M.C.; Coomaraswamy, J.; Kilger, E.; Selenica, M.L.; Winkler, D.T.; Staufenbiel, M.; Levy, E.; Grubb, A.; Jucker, M. Cystatin C modulates cerebral beta-amyloidosis. Nat. Genet. 2007, 39, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Pawlik, M.; Sastre, M.; Jung, S.S.; Radvinsky, D.S.; Klein, A.M.; Sommer, J.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M. Cystatin C inhibits amyloid-β deposition in Alzheimer’s disease mouse models. Nat. Genet. 2007, 39, 1440. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef]
- Chuo, L.-J.; Sheu, W.H.; Pai, M.-C.; Kuo, Y.-M. Genotype and plasma concentration of cystatin C in patients with late-onset Alzheimer disease. Dement. Geriatr. Cogn. Disord. 2007, 23, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [Green Version]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-dependent neuronal autotoxicity and BBB breakdown explain the superior sensitivity of the brain to ischemic damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Chen, F.; Haigh, S.; Barman, S.; Fulton, D.J. From form to function: The role of Nox4 in the cardiovascular system. Front. Physiol. 2012, 3, 412. [Google Scholar] [CrossRef] [Green Version]
- Bodai, B.I.; Nakata, T.E.; Wong, W.T.; Clark, D.R.; Lawenda, S.; Tsou, C.; Liu, R.; Shiue, L.; Cooper, N.; Rehbein, M.; et al. Lifestyle Medicine: A Brief Review of Its Dramatic Impact on Health and Survival. Perm. J. 2018, 22, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Bruce-Keller, A.J.; White, C.L.; Gupta, S.; Knight, A.G.; Pistell, P.J.; Ingram, D.K.; Morrison, C.D.; Keller, J.N. NOX activity in brain aging: Exacerbation by high fat diet. Free Radic. Biol. Med. 2010, 49, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Topchiy, E.; Panzhinskiy, E.; Griffin, W.S.; Barger, S.W.; Das, M.; Zawada, W.M. Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation. Dev. Neurosci. 2013, 35, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, Y.; Ago, T.; Kuroda, J.; Wakisaka, Y.; Tachibana, M.; Komori, M.; Shibahara, T.; Nakashima, H.; Nakashima, K.; Kitazono, T. Nox4 Promotes Neural Stem/Precursor Cell Proliferation and Neurogenesis in the Hippocampus and Restores Memory Function Following Trimethyltin-Induced Injury. Neuroscience 2019, 398, 193–205. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosas-Molist, E.; Bertran, E.; Rodriguez-Hernandez, I.; Herraiz, C.; Cantelli, G.; Fabra, A.; Sanz-Moreno, V.; Fabregat, I. The NADPH oxidase NOX4 represses epithelial to amoeboid transition and efficient tumour dissemination. Oncogene 2017, 36, 3002–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.S.; Nakahira, K.; Chung, K.P.; DeNicola, G.M.; Koo, M.J.; Pabon, M.A.; Rooney, K.T.; Yoon, J.H.; Ryter, S.W.; Stout-Delgado, H.; et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016, 22, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.C.; et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrouki, B.; Pillon, N.J.; Kalbacher, E.; Soula, H.A.; Nia N’Jomen, G.; Grand, L.; Chambert, S.; Geloen, A.; Soulage, C.O. Cirsimarin, a potent antilipogenic flavonoid, decreases fat deposition in mice intra-abdominal adipose tissue. Int. J. Obes. (Lond.) 2010, 34, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Mulder, P.; Morrison, M.C.; Wielinga, P.Y.; van Duyvenvoorde, W.; Kooistra, T.; Kleemann, R. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int. J. Obes. (Lond.) 2016, 40, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar]
- Mann, A.; Thompson, A.; Robbins, N.; Blomkalns, A.L. Localization, identification, and excision of murine adipose depots. J. Vis. Exp. 2014, 52174. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Kim, G.H.; Lim, K.; Yang, H.S.; Lee, J.K.; Kim, Y.; Park, S.K.; Kim, S.H.; Park, S.; Kim, T.H.; Moon, J.S.; et al. Improvement in neurogenesis and memory function by administration of Passiflora incarnata L. extract applied to sleep disorder in rodent models. J. Chem. Neuroanat. 2019, 98, 27–40. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yi, S.S.; Yoo, K.Y.; Park, O.K.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Song, W.; Moon, S.M.; Won, M.H.; et al. Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: Correlation with the neuroblasts. Brain Res. 2010, 1341, 84–92. [Google Scholar] [CrossRef]
- Macpherson, H.; Teo, W.P.; Schneider, L.A.; Smith, A.E. A Life-Long Approach to Physical Activity for Brain Health. Front. Aging Neurosci. 2017, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-Y.; Kim, M.S. Inhibition of Proliferation and Neurogenesis of Mouse Subventricular Zone Neural Stem Cells by a Mitochondrial Inhibitor Rotenone. 생명과학회지 2018, 28, 1397–1405. [Google Scholar]
- Park, K.-Y.; Oh, H.-C.; Lee, J.-Y.; Kim, M.S. Inhibition of Neurogenesis of Subventricular Zone Neural Stem Cells by 5-ethynyl-2-deoxyuridine (EdU). 생명과학회지 2017, 27, 623–631. [Google Scholar]
- Watt, J.; Alund, A.W.; Pulliam, C.F.; Mercer, K.E.; Suva, L.J.; Chen, J.R.; Ronis, M.J.J. NOX4 Deletion in Male Mice Exacerbates the Effect of Ethanol on Trabecular Bone and Osteoblastogenesis. J. Pharmacol. Exp. Ther. 2018, 366, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Chen, Z.; Wei, X.; Chen, Z.; Fu, Y.; Yang, X.; Chen, D.; Wang, R.; Jenner, P.; Lu, J.H.; et al. Cystatin C as a potential therapeutic mediator against Parkinson’s disease via VEGF-induced angiogenesis and enhanced neuronal autophagy in neurovascular units. Cell Death Dis. 2017, 8, e2854. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, J.; Wang, Z.; Yu, Z.; Chen, G. Attenuation of early brain injury and learning deficits following experimental subarachnoid hemorrhage secondary to Cystatin C: Possible involvement of the autophagy pathway. Mol. Neurobiol. 2014, 49, 1043–1054. [Google Scholar] [CrossRef]
- Watanabe, S.; Hayakawa, T.; Wakasugi, K.; Yamanaka, K. Cystatin C protects neuronal cells against mutant copper-zinc superoxide dismutase-mediated toxicity. Cell Death Dis. 2014, 5, e1497. [Google Scholar] [CrossRef] [Green Version]
- Pirttila, T.J.; Lukasiuk, K.; Hakansson, K.; Grubb, A.; Abrahamson, M.; Pitkanen, A. Cystatin C modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiol. Dis. 2005, 20, 241–253. [Google Scholar] [CrossRef]
- Lukasiuk, K.; Pirttila, T.J.; Pitkanen, A. Upregulation of cystatin C expression in the rat hippocampus during epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Epilepsia 2002, 43 (Suppl. S5), 137–145. [Google Scholar] [CrossRef] [Green Version]
- Aronica, E.; van Vliet, E.A.; Hendriksen, E.; Troost, D.; Lopes da Silva, F.H.; Gorter, J.A. Cystatin C, a cysteine protease inhibitor, is persistently up-regulated in neurons and glia in a rat model for mesial temporal lobe epilepsy. Eur. J. Neurosci. 2001, 14, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Heike, T.; Okawa, K.; Haruyama, M.; Shiraishi, K.; Yoshimoto, M.; Nagato, M.; Shibata, M.; Kumada, T.; Yamanaka, Y. A neurosphere-derived factor, cystatin C, supports differentiation of ES cells into neural stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 6019–6024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Ding, Y.; Li, X.; Wu, X. Cystatin C is a disease-associated protein subject to multiple regulation. Immunol. Cell Biol. 2015, 93, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lindemann, P.; Vega-Ramos, J.; Zhang, J.G.; Villadangos, J.A. Developmental regulation of synthesis and dimerization of the amyloidogenic protease inhibitor cystatin C in the hematopoietic system. J. Biol. Chem. 2014, 289, 9730–9740. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiranugrom, P.; Yoo, I.D.; Park, M.W.; Ryu, J.H.; Moon, J.-S.; Yi, S.S. NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet. Genes 2020, 11, 567. https://doi.org/10.3390/genes11050567
Jiranugrom P, Yoo ID, Park MW, Ryu JH, Moon J-S, Yi SS. NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet. Genes. 2020; 11(5):567. https://doi.org/10.3390/genes11050567
Chicago/Turabian StyleJiranugrom, Piyanart, Ik Dong Yoo, Min Woo Park, Ji Hwan Ryu, Jong-Seok Moon, and Sun Shin Yi. 2020. "NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet" Genes 11, no. 5: 567. https://doi.org/10.3390/genes11050567
APA StyleJiranugrom, P., Yoo, I. D., Park, M. W., Ryu, J. H., Moon, J. -S., & Yi, S. S. (2020). NOX4 Deficiency Exacerbates the Impairment of Cystatin C-Dependent Hippocampal Neurogenesis by a Chronic High Fat Diet. Genes, 11(5), 567. https://doi.org/10.3390/genes11050567





