CHIR99021 and Brdu Are Critical in Chicken iPSC Reprogramming via Small-Molecule Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Approval
2.2. Isolation and Culture of CEFs
2.3. CCK-8 Cell Proliferation Assay
2.4. iPS Induction
2.5. Quantitative Real-Time Polymerase Chain Reaction
2.6. Immunofluorescence Staining
2.7. Flow Cytometric Analysis
2.8. Alkaline Phosphatase
2.9. EdU Cell Proliferation Assay
2.10. Statistical Analysis
3. Results
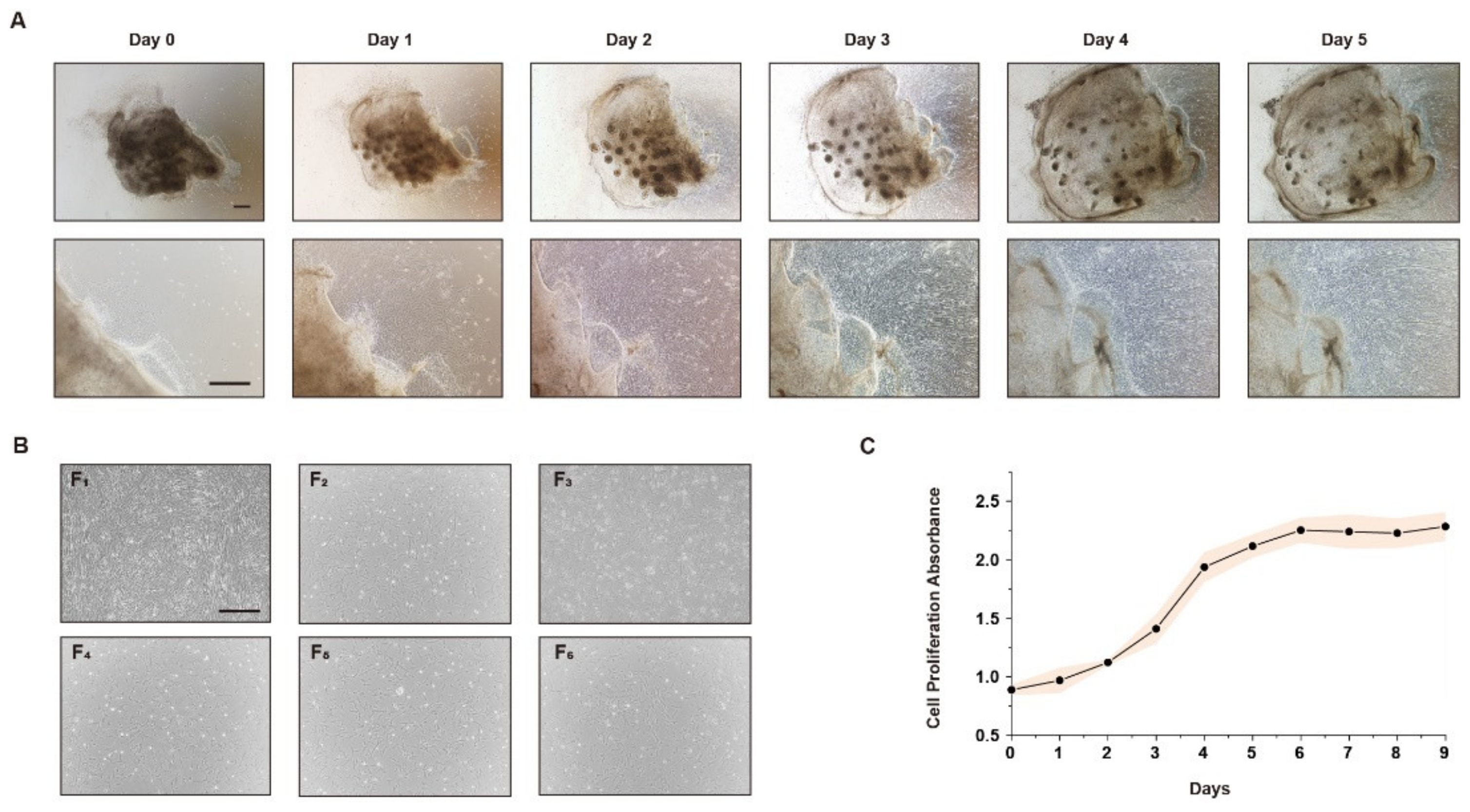
3.1. Stably Produced CEFs Can Be Obtained by the Tissue Block Culture Method
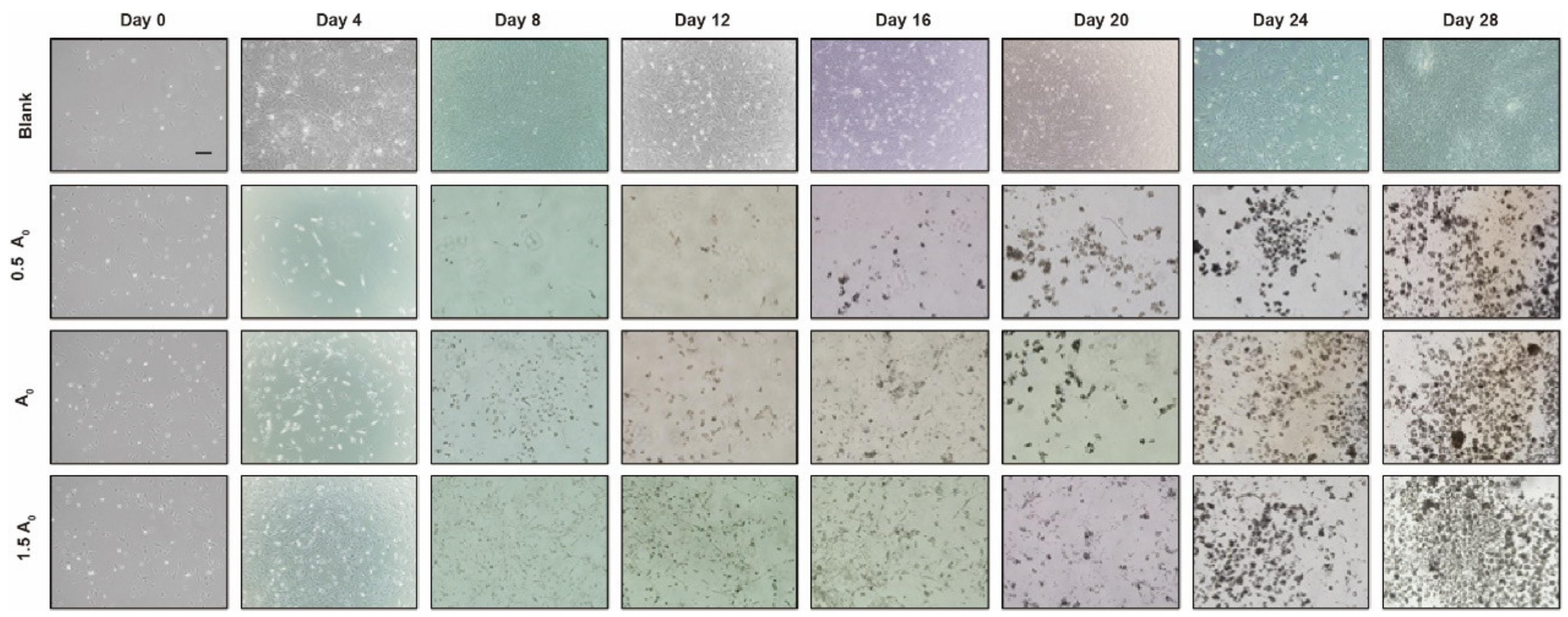
3.2. Establishment of Chicken iPSC Formation Induced by Small-Molecule Compounds
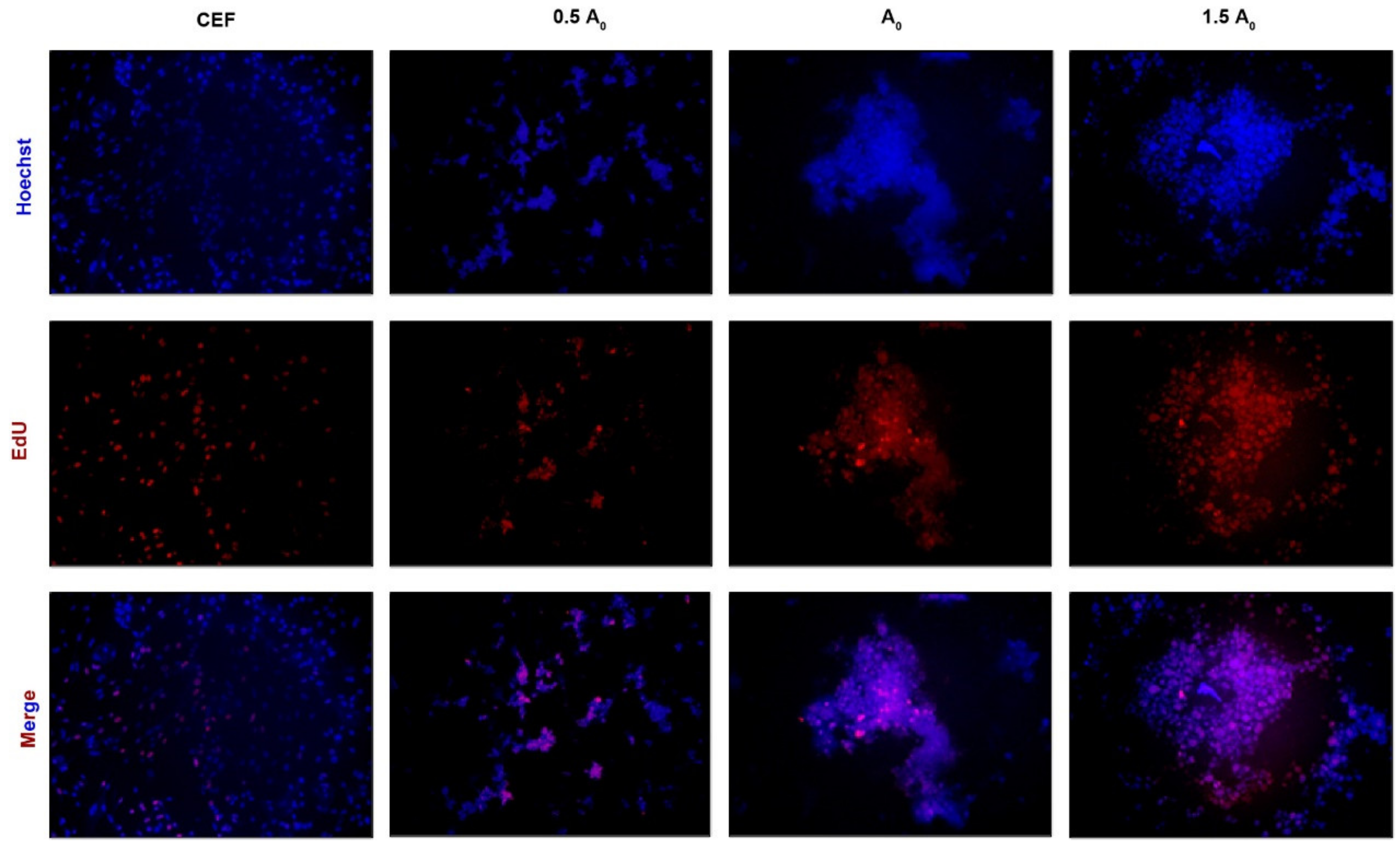
3.3. iPSCs Induced by Small-Molecule Compounds Show Typical Biological Characteristics of ESCs
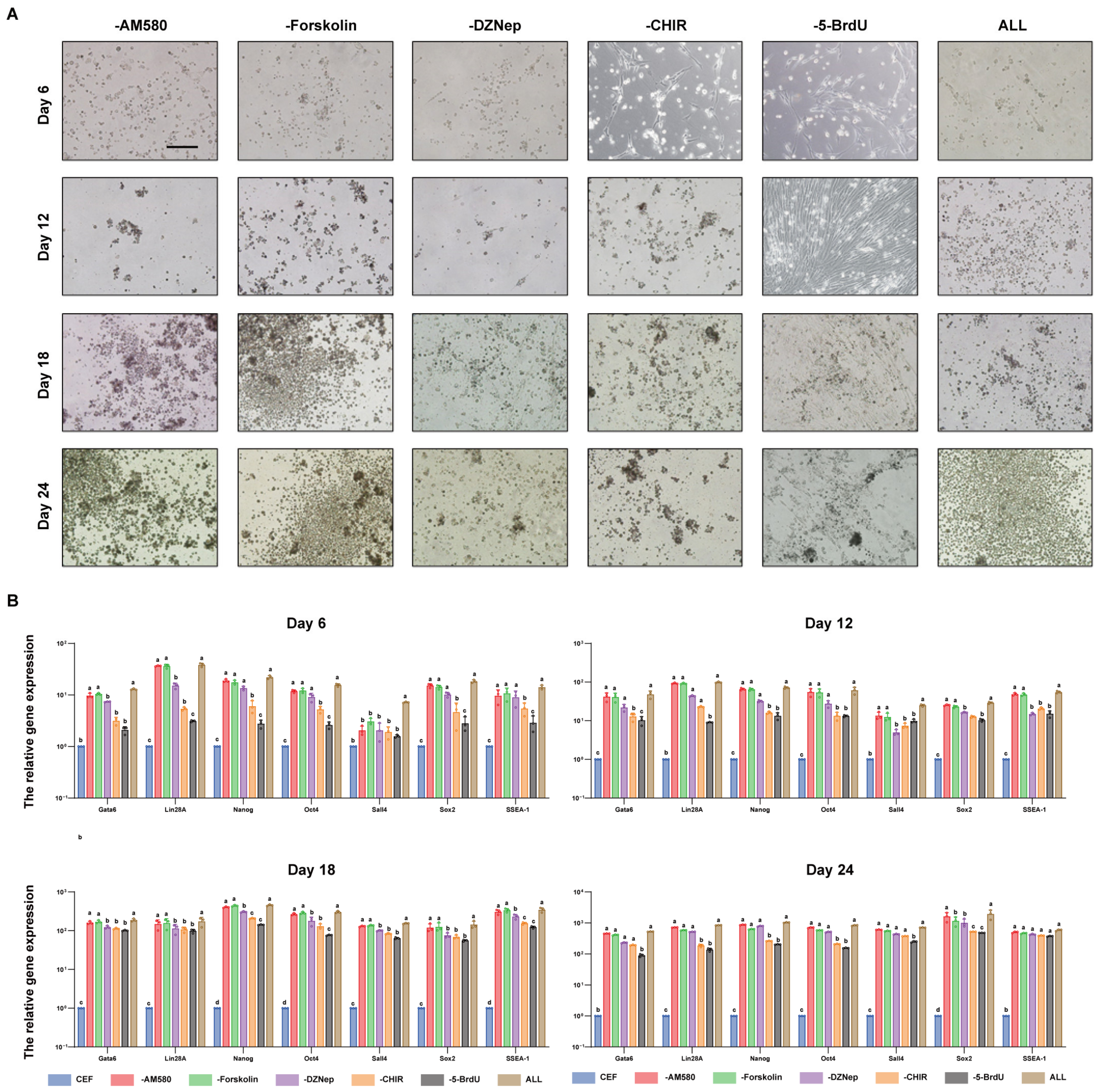
3.4. CHIR99021 and BrdU Are Small-Molecule Compounds Necessary for the In Vitro Induction of Reprogramming in iPGCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, A.; Hutchins, R. Embryonic Stem Cells. Stem Cells Dev. 2007, 16, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ye, S.; Zhou, X.; Liu, D.; Ying, Q.-L. Molecular Basis of Embryonic Stem Cell Self-Renewal: From Signaling Pathways to Pluripotency Network. Cell Mol. Life Sci. 2015, 72, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Cheng, Z.; Tian, M.; Zhang, H. In Vivo Imaging of Embryonic Stem Cell Therapy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Damdimopoulou, P.; Rodin, S.; Stenfelt, S.; Antonsson, L.; Tryggvason, K.; Hovatta, O. Human Embryonic Stem Cells. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Cherry, A.B.C.; Daley, G.Q. Reprogrammed Cells for Disease Modeling and Regenerative Medicine. Annu. Rev. Med. 2013, 64, 277–290. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of Germline-Competent Induced Pluripotent Stem Cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef]
- Le Magnen, C.; Bubendorf, L.; Ruiz, C.; Zlobec, I.; Bachmann, A.; Heberer, M.; Spagnoli, G.C.; Wyler, S.; Mengus, C. Klf4 Transcription Factor Is Expressed in the Cytoplasm of Prostate Cancer Cells. Eur. J. Cancer 2013, 49, 955–963. [Google Scholar] [CrossRef]
- Hawley, R.G. Does Retroviral Insertional Mutagenesis Play a Role in the Generation of Induced Pluripotent Stem Cells? Mol. Ther. 2008, 16, 1354–1355. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A More Efficient Method to Generate Integration-Free Human iPS Cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced Pluripotent Stem Cells Generated without Viral Integration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef]
- Lin, S.-L.; Chang, D.C.; Chang-Lin, S.; Lin, C.-H.; Wu, D.T.S.; Chen, D.T.; Ying, S.-Y. Mir-302 Reprograms Human Skin Cancer Cells into a Pluripotent ES-Cell-like State. RNA 2008, 14, 2115–2124. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ishii, H.; Nagano, H.; Haraguchi, N.; Dewi, D.L.; Kano, Y.; Nishikawa, S.; Tanemura, M.; Mimori, K.; Tanaka, F.; et al. Reprogramming of Mouse and Human Cells to Pluripotency Using Mature microRNAs. Cell Stem Cell 2011, 8, 633–638. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Ding, S. A Chemical Approach to Stem-Cell Biology and Regenerative Medicine. Nature 2008, 453, 338–344. [Google Scholar] [CrossRef]
- Schugar, R.C.; Robbins, P.D.; Deasy, B.M. Small Molecules in Stem Cell Self-Renewal and Differentiation. Gene Ther. 2008, 15, 126–135. [Google Scholar] [CrossRef]
- Ichida, J.K.; Blanchard, J.; Lam, K.; Son, E.Y.; Chung, J.E.; Egli, D.; Loh, K.M.; Carter, A.C.; Di Giorgio, F.P.; Koszka, K.; et al. A Small-Molecule Inhibitor of Tgf-β Signaling Replaces Sox2 in Reprogramming by Inducing Nanog. Cell Stem Cell 2009, 5, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, M.; Gu, H.; Xie, X. Bromodeoxyuridine Promotes Full-Chemical Induction of Mouse Pluripotent Stem Cells. Cell Res. 2015, 25, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yu, S.; Li, D.; Ye, J.; Yang, X.; Li, C.; Wang, X.; Mai, Y.; Qin, Y.; Wu, J.; et al. Chromatin Accessibility Dynamics during Chemical Induction of Pluripotency. Cell Stem Cell 2018, 22, 529–542.e5. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Cui, C.; Chen, S.; Ren, J.; Chen, J.; Gao, Y.; Li, H.; Jia, N.; Cheng, L.; Xiao, H. Cell Stem Cell Brief Report Generation of Induced Pluripotent Stem Cell Lines from Adult Rat Cells. Cell Stem Cell 2009, 11, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, F.; Yong, J.; Zhang, P.; Hou, P.; Li, H.; Jiang, W.; Cai, J.; Liu, M.; Cui, K. Generation of Induced Pluripotent Stem Cells from Adult Rhesus Monkey Fibroblasts. Cell Stem Cell 2008, 3, 587–590. [Google Scholar] [CrossRef]
- Ezashi, T.; Telugu, B.P.V.L.; Alexenko, A.P.; Sachdev, S.; Sinha, S.; Roberts, R.M. Derivation of Induced Pluripotent Stem Cells from Pig Somatic Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 10993–10998. [Google Scholar] [CrossRef]
- Ren, J.; Pak, Y.; He, L.; Qian, L.; Gu, Y.; Li, H.; Rao, L.; Liao, J.; Cui, C.; Xu, X. Generation of Hircine-Induced Pluripotent Stem Cells by Somatic Cell Reprogramming. Cell Res. 2011, 21, 849–853. [Google Scholar] [CrossRef]
- Han, X.; Han, J.; Ding, F.; Cao, S.; Lim, S.S.; Dai, Y.; Zhang, R.; Zhang, Y.; Lim, B.; Li, N. Generation of Induced Pluripotent Stem Cells from Bovine Embryonic Fibroblast Cells. Cell Res. 2011, 21, 1509–1512. [Google Scholar] [CrossRef]
- Nagy, K.; Sung, H.K.; Zhang, P.; Laflamme, S.; Vincent, P.; Agha-Mohammadi, S.; Woltjen, K.; Monetti, C.; Michael, I.P.; Smith, L.C.; et al. Induced Pluripotent Stem Cell Lines Derived from Equine Fibroblasts. Stem Cell Rev. Rep. 2011, 7, 693–702. [Google Scholar] [CrossRef]
- Lu, Y.; West, F.D.; Jordan, B.J.; Mumaw, J.L.; Jordan, E.T.; Gallegos-Cardenas, A.; Beckstead, R.B.; Stice, S.L. Avian-Induced Pluripotent Stem Cells Derived Using Human Reprogramming Factors. Stem Cells Dev. 2012, 21, 394–403. [Google Scholar] [CrossRef]
- Kim, Y.M.; Park, Y.H.; Lim, J.M.; Jung, H.; Han, J.Y. Technical Note: Induction of Pluripotent Stem Cell-like Cells from Chicken Feather Follicle Cells1. J. Anim. Sci. 2017, 95, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Gore, A. Somatic Coding Mutations in Human Induced Pluripotent Stem Cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Junfeng, J.I.; Ng, A.S.H.; Sharma, A.V.; Neculai, A.D.; Hussein, A.S. Stem cells embryonic stem cells/induced pluripotent stem cells Elevated Coding Mutation Rate During the Reprogramming of Human Somatic Cells into Induced Pluripotent Stem Cells. Stem Cells 2012, 30, 435–440. [Google Scholar]
- Yamanaka, S. Elite and Stochastic Models for Induced Pluripotent Stem Cell Generation. Nature 2009, 460, 49–52. [Google Scholar] [CrossRef]
- Shu, J.; Wu, C.; Wu, Y.; Li, Z.; Shao, S.; Zhao, W.; Tang, X.; Yang, H.; Shen, L.; Zuo, X.; et al. Shao Induction of Pluripotency in Mouse Somatic Cells with Lineage Specifiers. Cell 2015, 153, 963–975. [Google Scholar] [CrossRef]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of Pluripotent Stem Cells from Primary Human Fibroblasts with Only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, T.; Guan, J.; Zhang, X.; Fu, Y.; Ye, J.; Zhu, J.; Meng, G.; Ge, J.; Yang, S. A XEN-like State Bridges Somatic Cells to Pluripotency during Chemical Reprogramming. Cell 2015, 163, 1678–1691. [Google Scholar] [CrossRef]
- Marson, A.; Foreman, R.; Chevalier, B.; Bilodeau, S.; Kahn, M.; Young, R.A.; Jaenisch, R. Wnt Signaling Promotes Reprogramming of Somatic Cells to Pluripotency. Cell Stem Cell 2008, 3, 132–135. [Google Scholar] [CrossRef]
- Li, W.; Ding, S. Small Molecules That Modulate Embryonic Stem Cell Fate and Somatic Cell Reprogramming. Trends Pharmacol. Sci. 2010, 31, 36–45. [Google Scholar] [CrossRef]
- Wu, D.; Pan, W. GSK3: A Multifaceted Kinase in Wnt Signaling. Trends Biochem. Sci. 2010, 35, 161–168. [Google Scholar]
- Li, W.; Zhou, H.; Abujarour, R.; Zhu, S.; Young Joo, J.; Lin, T.; Hao, E.; Schöler, H.R.; Hayek, A.; Ding, S. Generation of Human-Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells 2009, 27, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Naujok, O.; Diekmann, U.; Lenzen, S. The Generation of Definitive Endoderm from Human Embryonic Stem Cells Is Initially Independent from Activin A but Requires Canonical Wnt-Signaling. Stem Cell Rev. Rep. 2014, 10, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chae, M.K.; Yoon, J.S.; Kim, C.Y. The Effect of CHIR 99021, a Glycogen Synthase Kinase-3β Inhibitor, on Transforming Growth Factor β-Induced Tenon Fibrosis. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Y.; Li, C.; Li, S.; Wan, X. Small Molecules That Promote Self-Renewal of Stem Cells and Somatic Cell Reprogramming. Stem Cell Rev. Rep. 2020, 16, 511–523. [Google Scholar] [CrossRef]




| Gene Name | Prime (5′ → 3′) | Size (bp) |
|---|---|---|
| Gata6 | F: GAAGATGCACAACTCGGAGATCAG R: GAGCCGTTTGGCTTCGTCA | 100 |
| Lin28A | F: GGCGTCTTCTGCATTGGC R: TGGCGACCATGTGGCTGA | 178 |
| Nanog | F: TGGTTTCAGAACCAACGAATGAAG R: TGCACTGGTCACAGCCTGAAG | 180 |
| Oct4 | F: ACCAGCATCGAGACCAACGTGA R: TTGCAGAACCAGACCCGGACAA | 137 |
| Sall4 | F: TGGTTTCAGAACCAACGAATGAAG R: TGCACTGGTCACAGCCTGAAG | 180 |
| Sox2 | F: AGGCTATGGGATGATGCAAG R: GTAGGTAGGCGATCCGTTCA | 160 |
| SSEA-1 | F: ACCAGCATCGAGACCAACGTGA R: TTGCAGAACCAGACCCGGACA | 117 |
| β-actin | F: CAGCCATCTTTCTTGGGTAT R: CTGTGATCTCCTTCTGCATCC | 169 |
| Compound | Function | Initial Concentration | Concentration Gradient |
|---|---|---|---|
| RepSox | TGFβR-1/ALK5 Inhibitor | 5 μM | 0–7.5 μM |
| CHIR99021 | Selective Activator of Glycogen Synthase Kinase 3β | 3 μM | 0–4.5 μM |
| DZNep | EZH2 Inhibitor | 0.05 μM | 0–0.075 μM |
| Forskolin | Adenylyl Cyclase Activator | 10 μM | 0–15 μM |
| VPA | HDAC1 Inhibitor | 0.1 mM | 0–0.15 mM |
| Vitamin C | Histone Demethylase | 50 μg/mL | 0–75 μg/mL |
| 5-BrdU | DOT1L Inhibitor | 10 μM | 0–15 μM |
| SGC0946 | DOT1L Inhibitor | 5 μM | 0–7.5 μM |
| BMP4 | BMP4 Signaling Activator | 10 ng/mL | 0–15 ng/mL |
| bFGF | Cell Proliferation Promoter | 10 ng/mL | 0–15 ng/mL |
| AM580 | RARα-Selective Agonist | 0.05 μM | 0–0.075 μM |
| EPZ-5676 | DOT1L Inhibitor | 5 μM | 0–7.5 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, K.; Zhou, J.; Wu, G.; Li, Z.; Zhu, X.; Liang, Y.; Li, T.; Chen, G.; Zuo, Q.; Niu, Y.; et al. CHIR99021 and Brdu Are Critical in Chicken iPSC Reprogramming via Small-Molecule Screening. Genes 2024, 15, 1206. https://doi.org/10.3390/genes15091206
Jin K, Zhou J, Wu G, Li Z, Zhu X, Liang Y, Li T, Chen G, Zuo Q, Niu Y, et al. CHIR99021 and Brdu Are Critical in Chicken iPSC Reprogramming via Small-Molecule Screening. Genes. 2024; 15(9):1206. https://doi.org/10.3390/genes15091206
Chicago/Turabian StyleJin, Kai, Jing Zhou, Gaoyuan Wu, Zeyu Li, Xilin Zhu, Youchen Liang, Tingting Li, Guohong Chen, Qisheng Zuo, Yingjie Niu, and et al. 2024. "CHIR99021 and Brdu Are Critical in Chicken iPSC Reprogramming via Small-Molecule Screening" Genes 15, no. 9: 1206. https://doi.org/10.3390/genes15091206








