Periphytic Ciliate Communities in Lake Ecosystem of Temperate Riverine Floodplain: Variability in Taxonomic and Functional Composition and Diversity with Seasons and Hydrological Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
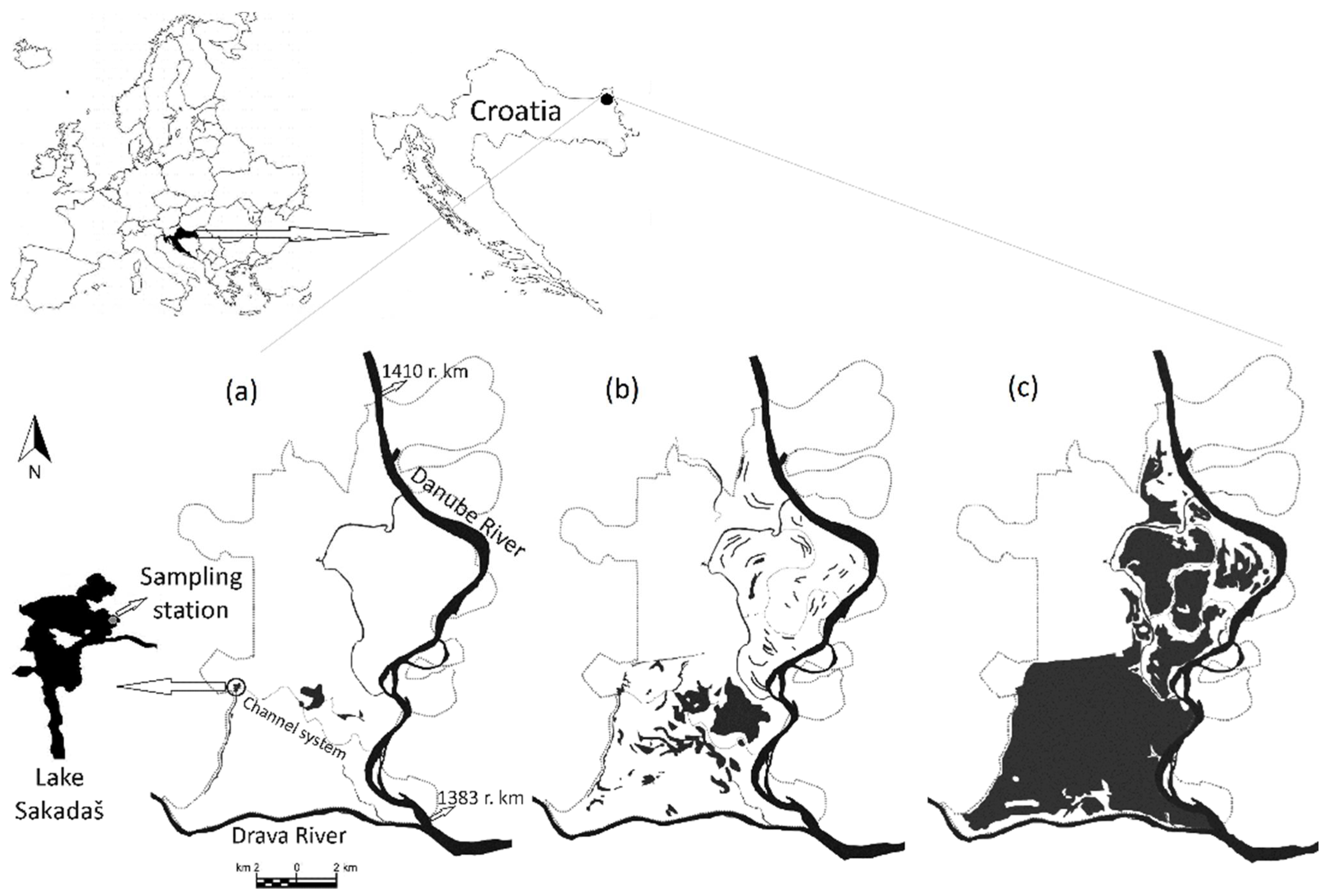
2.2. Experimental Design and Sampling Strategy
2.3. Laboratory Analyses
2.4. Statistical Analysis
3. Results
3.1. Environmental Parameters
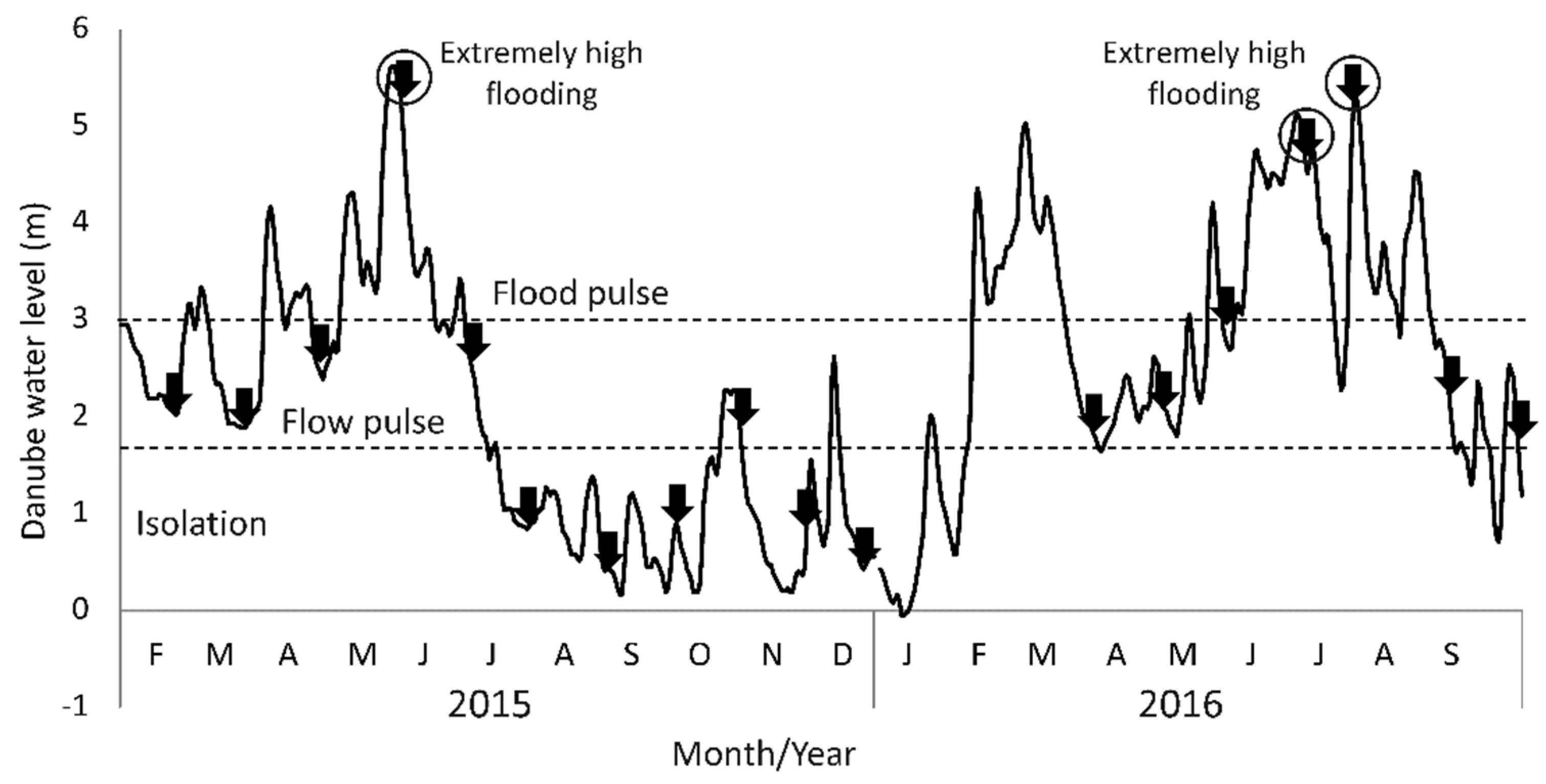
3.1.1. Hydrological Conditions
3.1.2. Abiotic and Biotic Water Parameters
3.2. Periphytic Biomass and Chlorophyll Concentrations
3.3. Periphytic Ciliate Communities
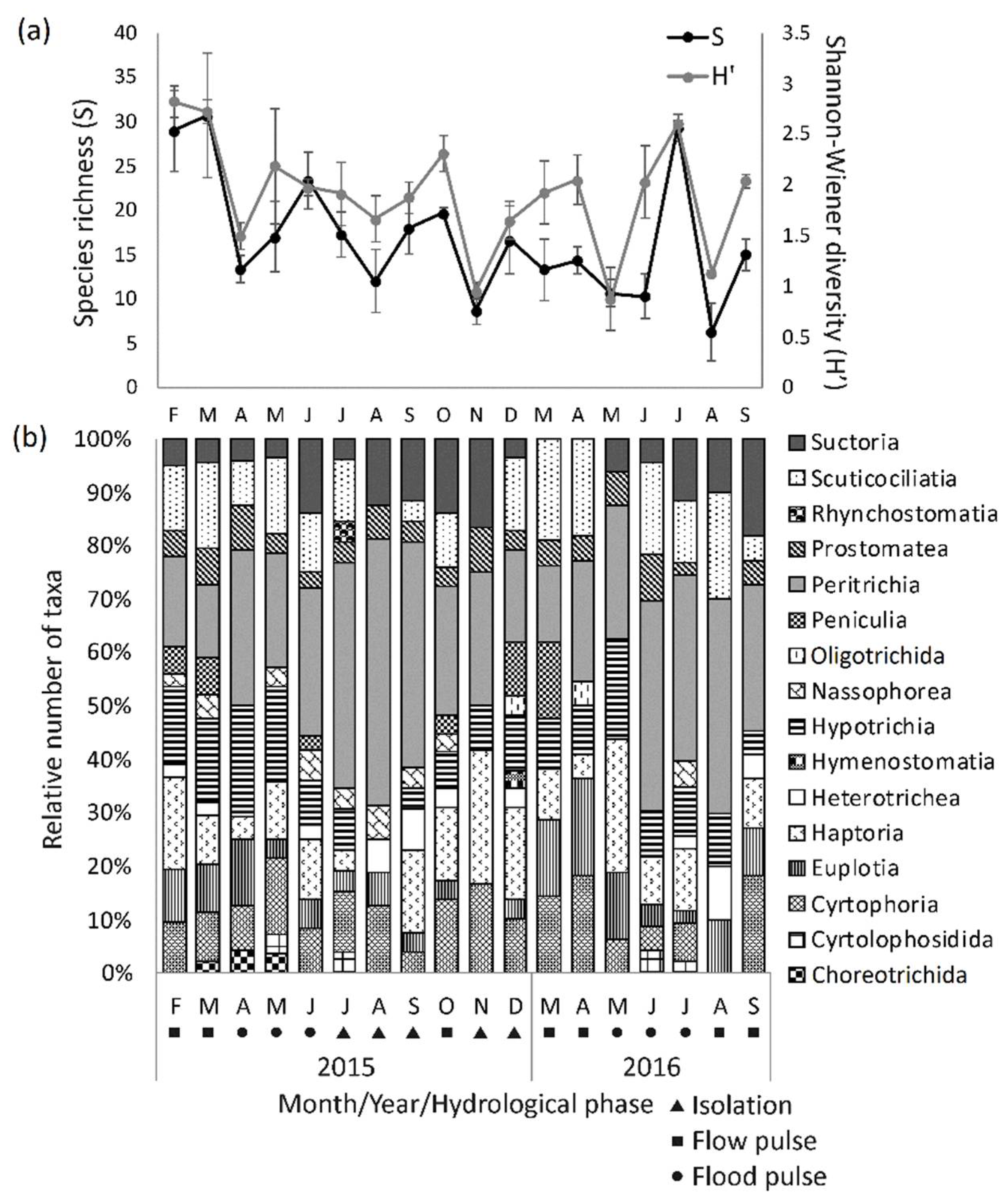
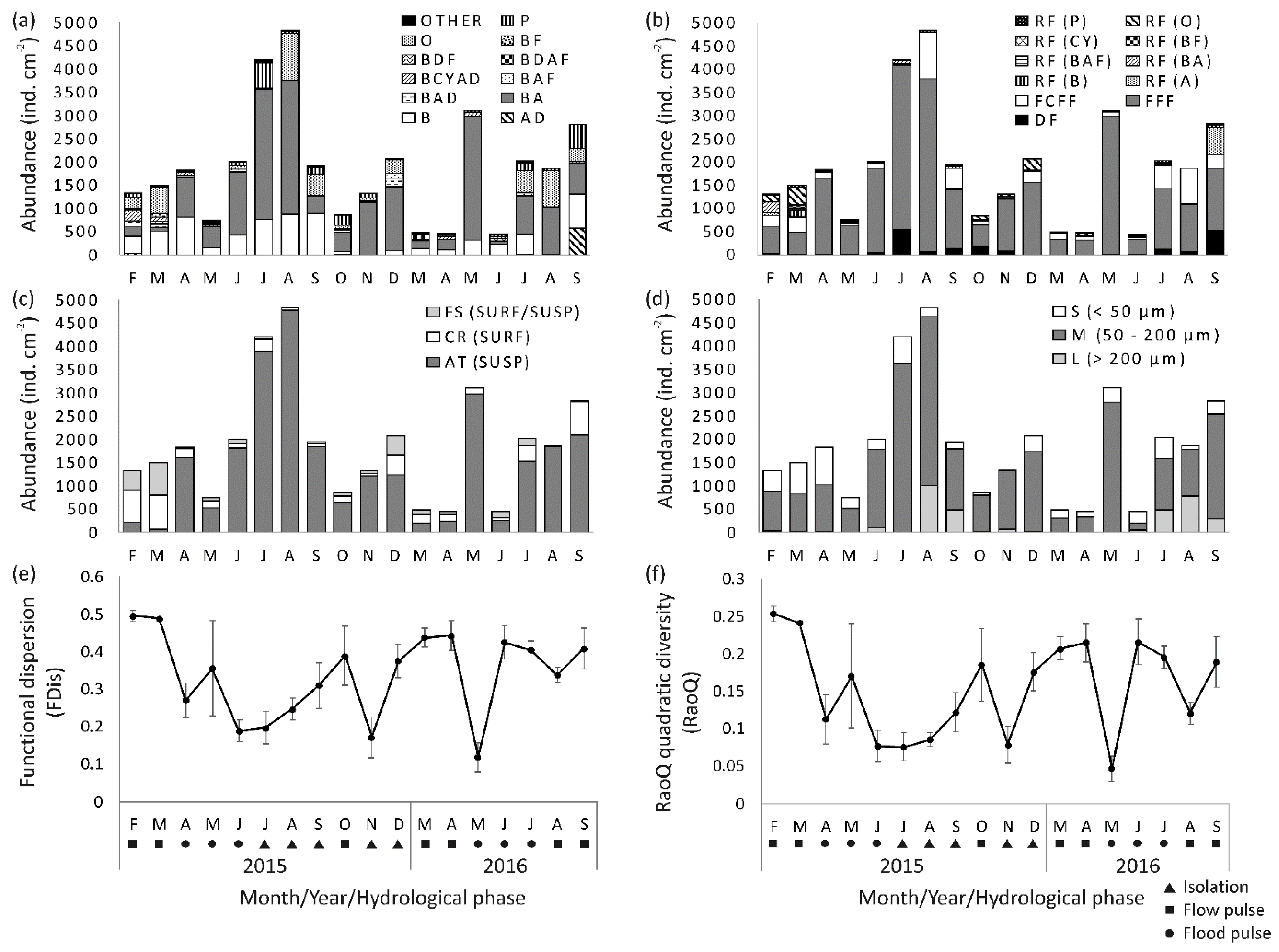
3.3.1. Taxonomic Composition, Diversity and Abundance
3.3.2. Functional Trait Composition and Functional Diversity
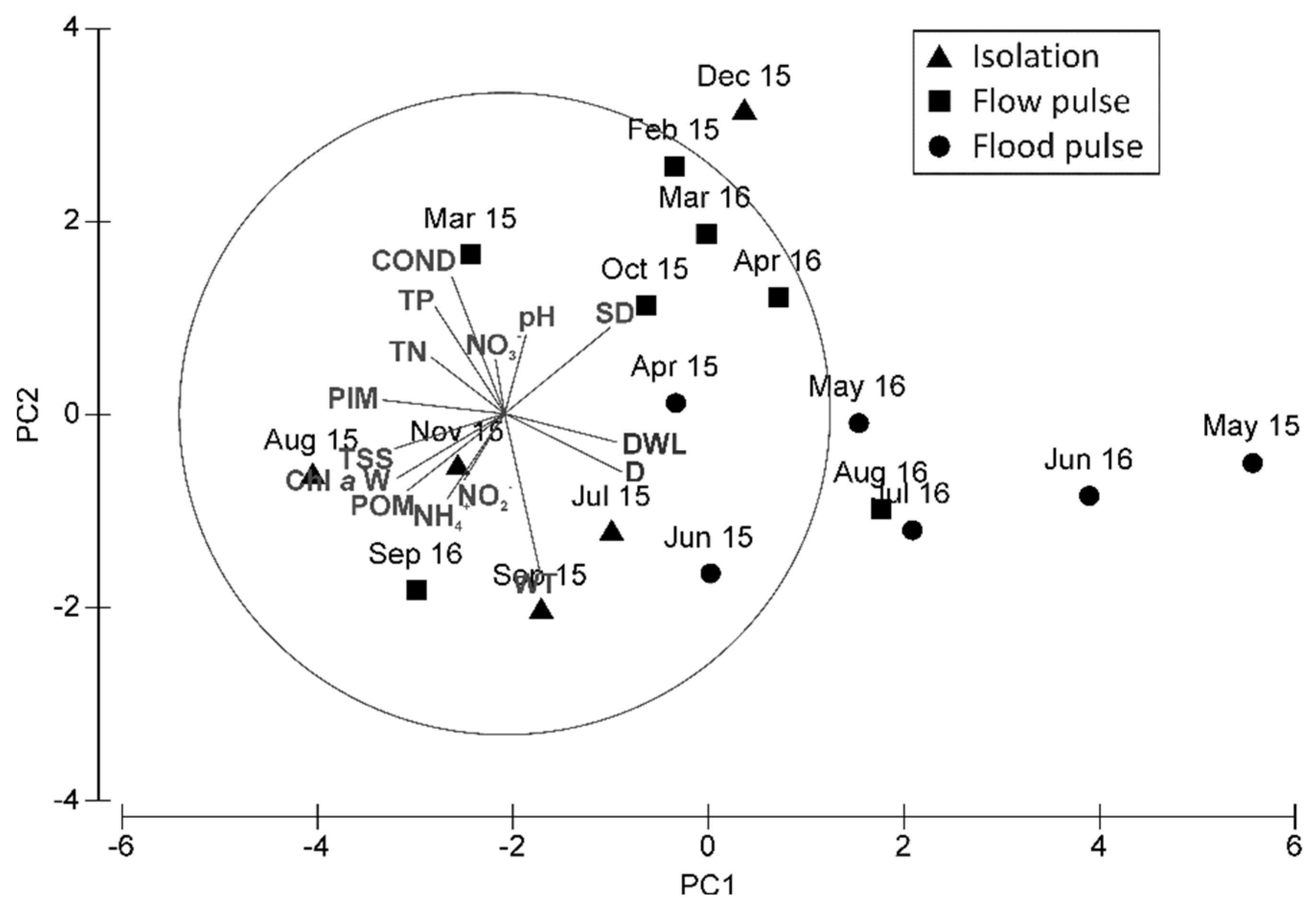
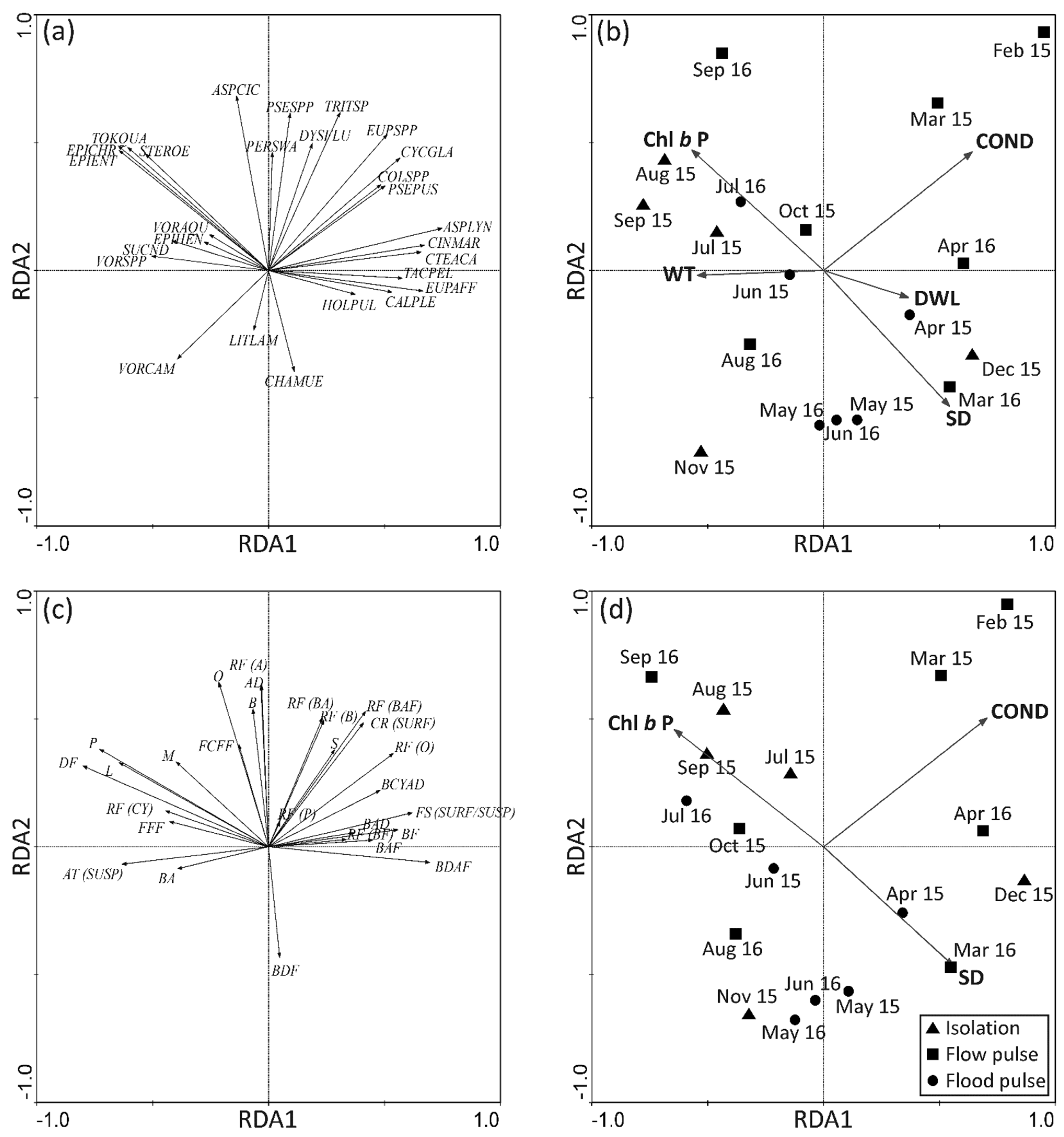
3.3.3. Seasonal Patterns of Taxonomic Composition Related to Environmental Conditions
3.3.4. Seasonal Patterns of Functional Trait Composition Related to Environmental Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azim, M.E.; Beveridge, M.C.M.; van Dam, A.A.; Verdegem, M.C.J. Periphyton and aquatic production: An introduction. In Periphyton: Ecology, Exploitation and Management; Azim, M.E., Verdegem, M.C.J., van Dam, A.A., Beveridge, M.C.M., Eds.; CABI Publishing; CAB International: Wallingford, UK, 2005; pp. 1–13. [Google Scholar]
- Mora-Gómez, J.; Freixa, A.; Perujo, N.; Barral-Fraga, L. Limits of the biofilm concept and types of aquatic biofilms. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Romani, A.M., Guasch, H., Dolors Balaguer, M., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 3–27. [Google Scholar]
- Wu, Y. Periphyton: Functions and Application in Environmental Remediation; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2017; p. 434. [Google Scholar]
- Arndt, H.; Schmidt-Denter, K.; Auer, B.; Weitere, M. Protozoans and biofilms. In Fossil and Recent Biofilms; Krumbein, W.E., Paterson, D.M., Zavarzin, G.A., Eds.; Kluwer Academic Publ: Dordrecht, The Netherlands, 2003; pp. 161–179. [Google Scholar] [CrossRef]
- Ackermann, B.; Esser, M.; Scherwass, A.; Arndt, H. Long-term dynamics of microbial biofilm communities of the River Rhine with special references to ciliates. Int. Rev. Hydrobiol. 2011, 96, 1–19. [Google Scholar] [CrossRef]
- Früh, D.; Norf, H.; Weitere, M. Response of biofilm-dwelling ciliate communities to enrichment with algae. Aquat. Microb. Ecol. 2011, 63, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Parry, J.D. Protozoan grazing of freshwater biofilms. In Advances in Applied Microbiology; Laskin, A.I., Bennett, J.W., Gadd, G.M., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2004; Volume 54, pp. 167–196. [Google Scholar] [CrossRef]
- Weitere, M.; Erken, M.; Majdi, N.; Arndt, H.; Norf, H.; Reinshagen, M.; Traunspurger, W.; Walterscheid, A.; Wey, J.K. The food web perspective on aquatic biofilms. Ecol. Monogr. 2018, 88, 543–559. [Google Scholar] [CrossRef]
- Kalinowska, K. Bacteria, nanoflagellates and ciliates as components of the microbial loop in three lakes of different trophic status. Pol. J. Ecol. 2004, 52, 19–34. [Google Scholar]
- Norf, H.; Arndt, H.; Weitere, M. Responses of biofilm-dwelling ciliate communities to planktonic and benthic resource enrichment. Microb. Ecol. 2009, 57, 687–700. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K. Biodiversity: Towards a unifying theme for river ecology. Freshw. Biol. 2001, 46, 807–819. [Google Scholar] [CrossRef]
- Villéger, S.; Novack-Gottshall, P.M.; Mouillot, D. The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol. Lett. 2011, 14, 561–568. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, X.; Warren, A.; Zhang, L.; Xu, H. Functional diversity of benthic ciliate communities in response to environmental gradients in a wetland of Yangtze Estuary, China. Mar. Pollut. Bull. 2018, 127, 726–732. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional traits. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–8. [Google Scholar] [CrossRef]
- Segovia, B.T.; Lansac-Tôha, F.M.; de Meira, B.R.; Cabral, A.F.; Lansac-Tôha, F.A.; Velho, L.F.M. Anthropogenic disturbances influencing ciliate functional feeding groups in impacted tropical streams. Environ. Sci. Pollut. Res. 2016, 23, 20003–20016. [Google Scholar] [CrossRef]
- Pratt, J.R.; Cairns, J., Jr. Functional groups in the protozoa: Roles in differing ecosystems. J. Protozool. 1985, 32, 415–423. [Google Scholar] [CrossRef]
- Mieczan, T. Periphytic ciliates in littoral zone of three lakes of different trophic status. Pol. J. Ecol. 2005, 53, 489–502. [Google Scholar]
- Risse-Buhl, U.; Küsel, K. Colonization dynamics of biofilm associated morphotypes at different flow velocities. Eur. J. Protistol. 2009, 45, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Madoni, P. Ciliated protozoan communities and saprobic evaluation of water quality in the hilly zone of some tributaries of the Po River (northern Italy). Hydrobiologia 2005, 541, 55–69. [Google Scholar] [CrossRef]
- Weisse, T. Functional diversity of aquatic ciliates. Eur. J. Protistol. 2017, 61, 331–358. [Google Scholar] [CrossRef]
- Scherwass, A.; Arndt, H. Structure, dynamics and control of the ciliate fauna in the potamoplankton of the River Rhine. Arch. Hydrobiol. 2005, 164, 287–307. [Google Scholar] [CrossRef]
- Kathol, M.; Fischer, H.; Weitere, M. Contribution of biofilm-dwelling consumers to pelagic-benthic coupling in a large river. Freshw. Biol. 2011, 56, 1160–1172. [Google Scholar] [CrossRef]
- Marcus, H.; Wey, J.K.; Norf, H.; Weitere, M. Disturbance alters the response of consumer communities towards warming: A mesocosm study with biofilm-dwelling ciliates. Ecosphere 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Böhme, A.; Risse-Buhl, U.; Küsel, K. Protists with different feeding modes change biofilm morphology. FEMS Microbiol. Ecol. 2009, 69, 158–169. [Google Scholar] [CrossRef]
- Weerman, E.J.; van der Geest, H.G.; van der Meulen, M.D.; Manders, E.M.M.; van de Koppel, J.; Herman, P.M.J.; Admiraal, W. Ciliates as engineers of phototrophic biofilms. Freshw. Biol. 2011, 56, 1358–1369. [Google Scholar] [CrossRef]
- Primc-Habdija, B.; Habdija, I.; Matoničkin, R.; Špoljar, M. Development of ciliate community on artificial substrates associated with vertical gradients of environmental conditions in a karstic lake. Arch. Hydrobiol. 2005, 164, 513–527. [Google Scholar] [CrossRef]
- Sigee, D.C. Freshwater Microbiology. Biodiversity and Dynamic Interactions of Microorganisms in the Aquatic Environment; John Wiley & Sons, Ltd.: West Sussex, UK, 2005; p. 524. [Google Scholar] [CrossRef]
- Vermaat, J.E. Periphyton dynamics and influencing factors. In Periphyton: Ecology, Exploitation and Management; Azim, M.E., Verdegem, M.C.J., van Dam, A.A., Beveridge, M.C.M., Eds.; CABI Publishing; CAB International: Wallingford, UK, 2005; pp. 35–49. [Google Scholar]
- Norf, H.; Arndt, H.; Weitere, M. Effects of resource supplements on mature ciliate biofilms: An empirical test using a new type of flow cell. Biofouling 2009, 25, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Strüder-Kypke, M. Periphyton and sphagnicolous protists of dystrophic bog lakes (Brandenburg, Germany). I. Annual cycles, distribution and comparison to other lakes. Limnologica 1999, 29, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Song, W.; Warren, A. Periphytic ciliate colonization: Annual cycle and responses to environmental conditions. Aquat. Microb. Ecol. 2005, 39, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Vlaičević, B.; Matoničkin Kepčija, R.; Gulin, V.; Turković Čakalić, I.; Kepec, M.; Čerba, D. Key drivers influencing the colonization of periphytic ciliates and their functional role in hydrologically dynamic floodplain lake ecosystem. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 33. [Google Scholar] [CrossRef]
- Wey, J.K.; Norf, H.; Arndt, H.; Weitere, M. Role of dispersal in shaping communities of ciliates and heterotrophic flagellates within riverine biofilms. Limnol. Oceanogr. 2009, 54, 1615–1626. [Google Scholar] [CrossRef]
- Vlaičević, B.; Vidaković, J.; Čerba, D. The colonization and succession patterns of the periphytic ciliate community in a temperate floodplain lake. Biologia 2017, 72, 305–318. [Google Scholar] [CrossRef]
- Neury-Ormanni, J.; Vedrenne, J.; Wagner, M.; Jan, G.; Morin, S. Micro-meiofauna morphofunctional traits linked to trophic activity. Hydrobiologia 2020, 847, 2725–2736. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process. 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Junk, W.J.; Wantzen, K.M. The flood pulse concept: New aspects, approaches and applications–an update. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries, Pnom Penh, Cambodia, 11–14 February 2003; Welcomme, R.L., Petr, T., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2004; pp. 117–149. [Google Scholar]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. In Proceedings of the International Large River Symposium, Honey Harbour, ON, Canada, 14–21 September 1986; Dodge, D.P., Ed.; Canadian Special Publication of Fisheries and Aquatic Sciences 106: Ottawa, ON, Canada, 1989; pp. 110–127. [Google Scholar]
- Mihaljević, M.; Stević, F.; Horvatić, J.; Hackenberger Kutuzović, B. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopački Rit Nature Park, Croatia). Hydrobiologia 2009, 618, 77–88. [Google Scholar] [CrossRef]
- Palijan, G. Abundance and biomass responses of microbial food web components to hydrology and environmental gradients within a floodplain of the River Danube. Microb. Ecol. 2012, 64, 39–53. [Google Scholar] [CrossRef]
- Vidaković, J.; Turković Čakalić, I.; Stević, F.; Čerba, D. The influence of different hydrological conditions on periphytic invertebrate communities in a Danubian floodplain. Fundam. Appl. Limnol. 2012, 181, 59–72. [Google Scholar] [CrossRef]
- Žuna Pfeiffer, T.; Mihaljević, M.; Špoljarić, D.; Stević, F.; Plenković-Moraj, A. The disturbance-driven changes of periphytic algal communities in a Danubian floodplain lake. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 2. [Google Scholar] [CrossRef] [Green Version]
- Galir, A.; Palijan, G. Change in metazooplankton abundance in response to flood dynamics and trophic relations in Danubian floodplain lake (Kopačk Rit, Croatia). Pol. J. Ecol. 2012, 60, 777–787. [Google Scholar]
- Vlaičević, B.; Matoničkin Kepčija, R.; Čerba, D. Structure and dynamics of the periphytic ciliate community under different hydrological conditions in a Danubian floodplain lake. Limnologica 2021, 87, 125847. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in tropical rain forests and coral reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [Green Version]
- Vidaković, J.; Bogut, I.; Borić, E.; Zahirović, Ž. Hydrobiological research in the Kopački Rit Nature Park in the period November 1997–October 2001. Hrvat. Vode/Croat. Wat. 2002, 10, 127–144. [Google Scholar]
- Peršić, V.; Čerba, D.; Bogut, I.; Horvatić, J. Trophic state and water quality in the Danube floodplain lake (Kopački Rit Nature Park, Croatia) in relation to hydrological connectivity. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Singh Gill, S., Lanza, G.R., Rast, W., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 109–129. [Google Scholar] [CrossRef]
- Tadić, D.; Vidaček, Ž. Klimatske, hidrološke i pedološke značajke. In Kopački Rit. Pregled Istraživanja i Bibliografija; Mihaljević, M., Getz, D., Tadić, Z., Živanović, B., Gucunski, D., Topić, J., Kalinović, I., Mikuška, J., Eds.; Hrvatska Akademija Znanosti i Umjetnosti: Zagreb, Croatia, 1999; pp. 23–28. [Google Scholar]
- Palijan, G. Determination of limit water level for flooding of Kopački rit as exemplified by the flood in October and November 2009. Hrvat. Vode/Croat. Wat. 2010, 74, 313–320. [Google Scholar]
- Mihaljević, M.; Getz, D.; Tadić, Z.; Živanović, B.; Gucunski, D.; Topić, J.; Kalinović, I.; Mikuska, J. Kopački rit. Pregled Istraživanja i Bibliografija; Hrvatska Akademija Znanosti i Umjetnosti: Zagreb, Croatia, 1999; p. 188. [Google Scholar]
- Mihaljević, M.; Stević, F. Cyanobacterial blooms in a temperate river-floodplain ecosystem: The importance of hydrological extremes. Aquat. Ecol. 2011, 45, 335–349. [Google Scholar] [CrossRef]
- Schwarz, U. Landschaftsökologische Charakterisierung des Kopački Rit unter Besonderer Berücksichtigung von Flusslandschaftsformen sowie Deren Genese und Typologie. Ph.D. Thesis, Universität Wien, Wien, Austria, 2005. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- UNESCO. Determinations of Photosynthetic Pigments in Seawater; Report of SCOR-UNESCO Working Group 17; Monographs in Oceanographic Methodology; UNESCO: Paris, France, 1966; p. 69. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A practical handbook of sea-water analysis. Bull. Fish. Res. Bord. Can. 1968, 167, 1–310. [Google Scholar] [CrossRef]
- Luef, B.; Aspetsberger, F.; Hein, T.; Huber, F.; Peduzzi, P. Impact of hydrology on free-living and particle-associated microorganisms in a river floodplain system (Danube, Austria). Freshw. Biol. 2007, 52, 1043–1057. [Google Scholar] [CrossRef]
- Elias, C.L.; Rocha, R.J.M.; Feio, M.J.; Figueira, E.; Almeida, S.F.P. Influence of the colonizing substrate on diatom assemblages and implications for bioassessment: A mesocosm experiment. Aquat. Ecol. 2017, 51, 145–158. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H. A user-friendly guide to the ciliates (Protozoa, Ciliophora) commonly used by hydrobiologists as bioindicators in rivers, lakes, and waste waters, with notes on their ecology. Freshw. Biol. 1996, 35, 375–482. [Google Scholar] [CrossRef]
- Foissner, W.; Blatterer, H.; Berger, H.; Kohmann, F. Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems—Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1991; pp. 1–478. [Google Scholar]
- Foissner, W.; Berger, H.; Kohmann, F. Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems—Band II: Peritrichia, Heterotrichida, Odontostomatida; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1992; pp. 1–502. [Google Scholar]
- Foissner, W.; Berger, H.; Kohmann, F. Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems—Band III: Hymenostomata, Prostomatida, Nassulida; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1994; pp. 1–548. [Google Scholar]
- Foissner, W.; Berger, H.; Blatterer, H.; Kohmann, F. Taxonomische und Ökologische Revision der Ciliaten des Saprobiensystems—Band IV: Gymnostomatea, Loxodes, Suctoria; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1995; pp. 1–540. [Google Scholar]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Soininen, J. Spatial patterns of functional diversity and composition in marine benthic ciliates along the coast of China. Mar. Ecol. Prog. Ser. 2019, 627, 49–60. [Google Scholar] [CrossRef]
- Verni, F.; Gualtieri, P. Feeding behaviour in ciliated protists. Micron 1997, 28, 487–504. [Google Scholar] [CrossRef]
- Finlay, B.J.; Esteban, G.F. Freshwater protozoa: Biodiversity and ecological function. Biodivers. Conserv. 1998, 7, 1163–1186. [Google Scholar] [CrossRef]
- Foissner, W.; Berger, H.; Schaumburg, J. Identification and Ecology of Limnetic Plankton Ciliates; Informationsberichte des Bayerischen Landesamtes für Wasserwirtschaft: München, Germany, 1999; pp. 1–793. [Google Scholar]
- Macek, M.; Callieri, C.; Šimek, K.; Vázquez, A.L. Seasonal dynamics, composition and feeding patterns of ciliate assemblages in oligotrophic lakes covering a wide pH range. Arch. Hydrobiol. 2006, 166, 261–287. [Google Scholar] [CrossRef]
- Tirjaková, E.; Krajčovičová, K.; Illyová, M.; Vďačný, P. Interaction of ciliate communities with cyanobacterial water bloom in a shallow, hypertrophic reservoir. Acta Protozool. 2016, 55, 173–188. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006; p. 192. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Ltd.: Plymouth, UK, 2001; p. 172. [Google Scholar]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Cappelatti, L.; Mauffrey, A.R.L.; Griffin, J.N. Functional diversity of habitat formers declines scale-dependently across an environmental stress gradient. Oecologia 2020, 194, 135–149. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 24 January 2022).
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: New York, NY, USA, 2003; p. 269. [Google Scholar] [CrossRef]
- Tockner, K.; Pennetzdorfer, D.; Reiner, N.; Schiemer, F.; Ward, J.V. Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria). Freshw. Biol. 1999, 41, 521–535. [Google Scholar] [CrossRef]
- Galir Balkić, A.; Ternjej, I. Assessing Cladocera and Copepoda individual disturbance levels in hydrologically dynamic environment. Wetl. Ecol. Manag. 2018, 26, 733–749. [Google Scholar] [CrossRef]
- Hein, T.; Baranyi, C.; Herndl, G.J.; Wanek, W.; Schiemer, F. Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: The importance of hydrological connectivity. Freshw. Biol. 2003, 48, 220–232. [Google Scholar] [CrossRef]
- Connell, J.H.; Slatyer, R.O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Safi, L.S.L.; Fontoura, N.F.; Severo, H.J.; Utz, L.R.P. Temporal structure of the peritrich ciliate assemblage in a large Neotropical lake. Zool. Stud. 2014, 53, 17. [Google Scholar] [CrossRef] [Green Version]
- Weitere, M.; Schmidt-Denter, K.; Arndt, H. Laboratory experiments on the impact of biofilms on the plankton of a large river. Freshw. Biol. 2003, 48, 1983–1992. [Google Scholar] [CrossRef]
- Haddad, N.M.; Holyoak, M.; Mata, T.M.; Davies, K.F.; Melbourne, B.A.; Preston, K. Species’ traits predict the effects of disturbance and productivity on diversity. Ecol. Lett. 2008, 11, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Tockner, K.; Schiemer, F.; Ward, J.V. Conservation by restoration: The management concept for a river-floodplain system on the Danube River in Austria. Aquat. Conserv. 1998, 8, 71–86. [Google Scholar] [CrossRef]
- Tockner, K.; Schiemer, F.; Baumgartner, C.; Kum, G.; Weigand, E.; Zweimüller, I.; Ward, J.V. The Danube restoration project: Species diversity patterns across connectivity gradients in the floodplain system. Regul. Rivers 1999, 15, 245–258. [Google Scholar] [CrossRef]
- Dunck, B.; Bortolini, J.C.; Rodrigues, L.; Rodrigues, L.C.; Jati, S.; Train, S. Functional diversity and adaptative strategies of planktonic and periphytic algae in isolated tropical floodplain lake. Braz. J. Bot. 2013, 36, 257–266. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Laureto, L.M.O.; Cianciaruso, M.V.; Samia, D.S.M. Functional diversity: An overview of its history and applicability. Nat. Conserv. 2015, 13, 112–116. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaičević, B.; Gulin, V.; Matoničkin Kepčija, R.; Turković Čakalić, I. Periphytic Ciliate Communities in Lake Ecosystem of Temperate Riverine Floodplain: Variability in Taxonomic and Functional Composition and Diversity with Seasons and Hydrological Changes. Water 2022, 14, 551. https://doi.org/10.3390/w14040551
Vlaičević B, Gulin V, Matoničkin Kepčija R, Turković Čakalić I. Periphytic Ciliate Communities in Lake Ecosystem of Temperate Riverine Floodplain: Variability in Taxonomic and Functional Composition and Diversity with Seasons and Hydrological Changes. Water. 2022; 14(4):551. https://doi.org/10.3390/w14040551
Chicago/Turabian StyleVlaičević, Barbara, Vesna Gulin, Renata Matoničkin Kepčija, and Ivana Turković Čakalić. 2022. "Periphytic Ciliate Communities in Lake Ecosystem of Temperate Riverine Floodplain: Variability in Taxonomic and Functional Composition and Diversity with Seasons and Hydrological Changes" Water 14, no. 4: 551. https://doi.org/10.3390/w14040551





