Study on the Dynamic Process of the Attachment of a Single Bubble to Rough Surfaces with Different Hydrophobicity
Abstract
:1. Introduction
2. Experimental Materials and Methods
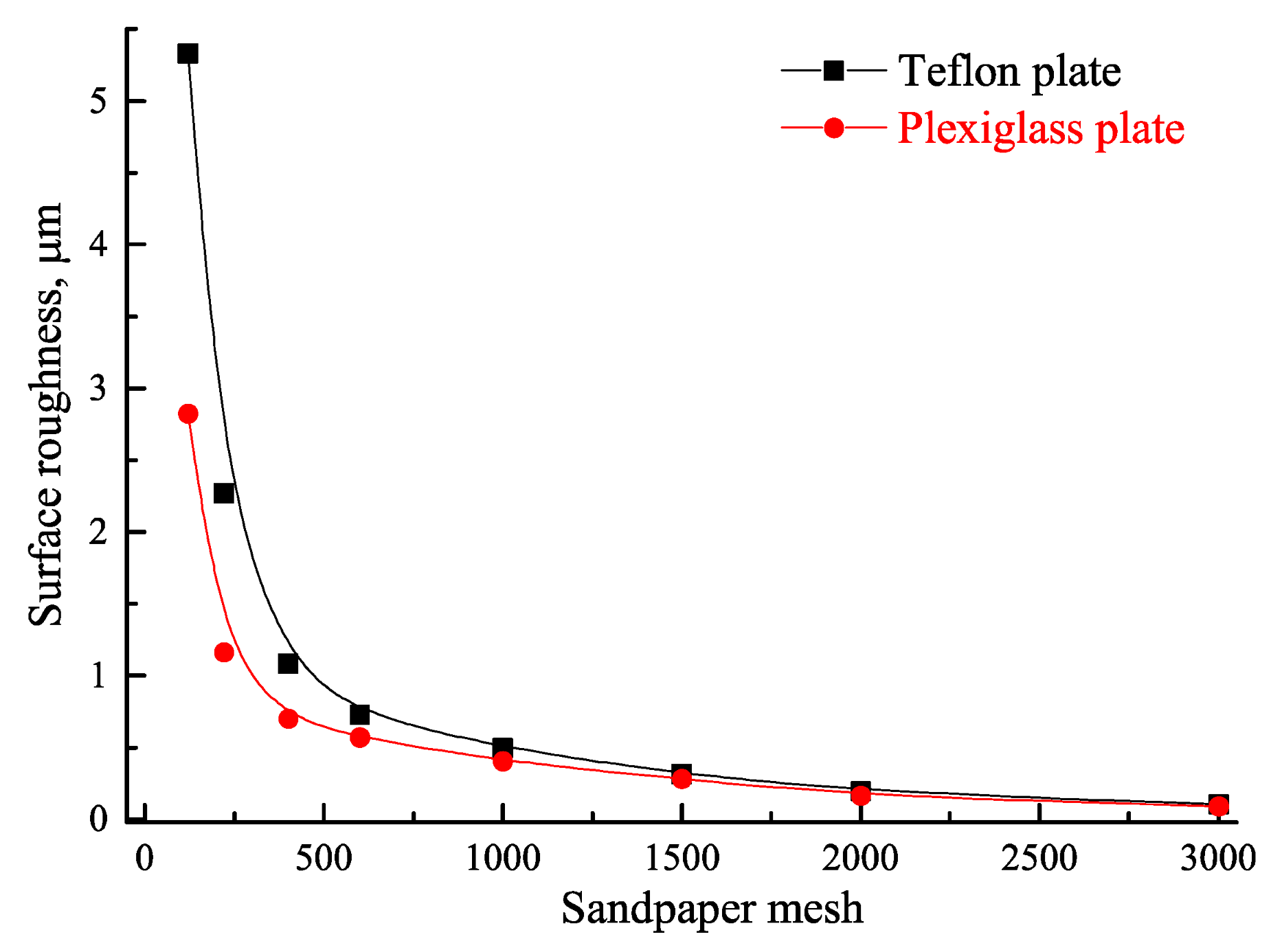
2.1. Materials
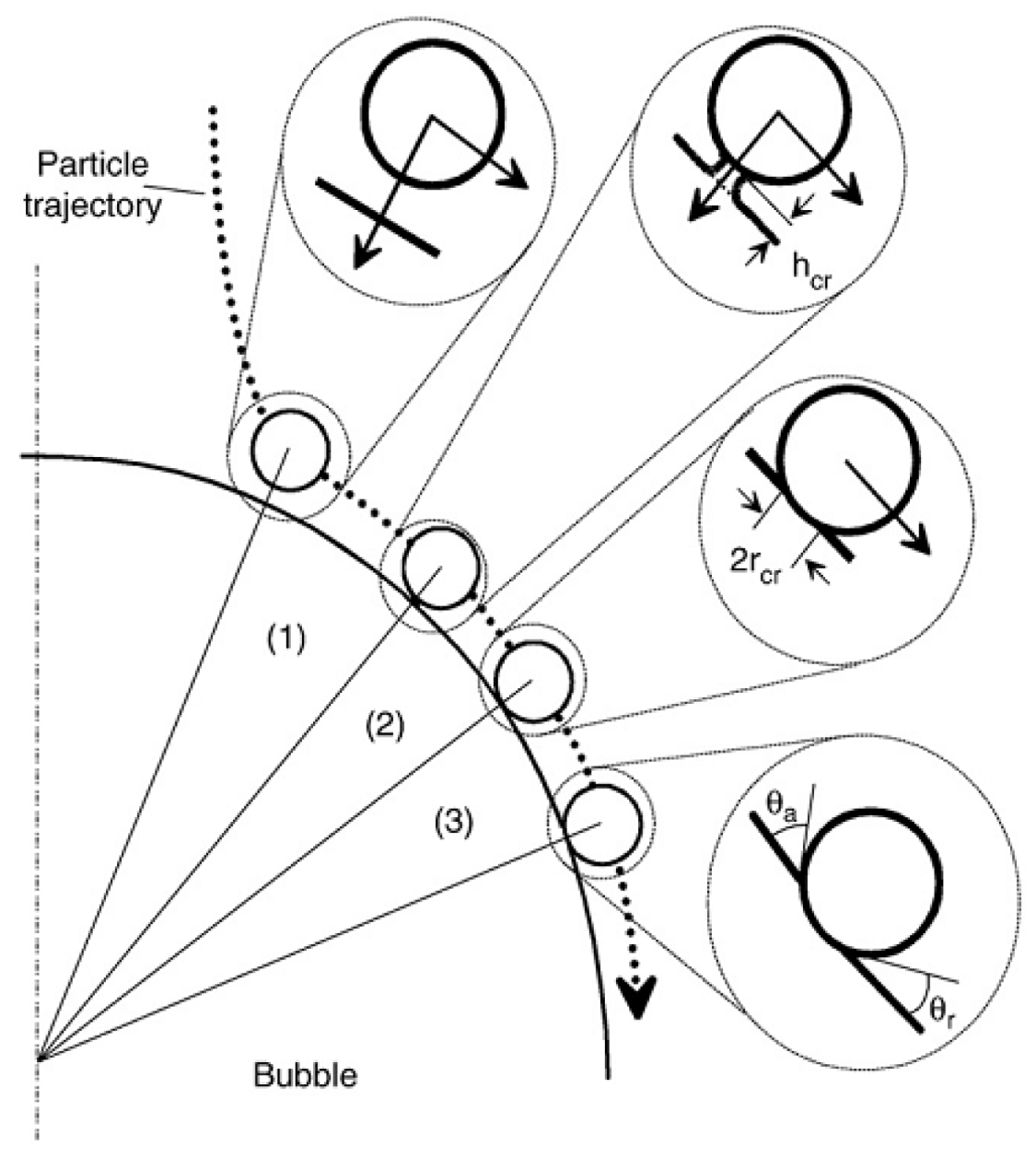
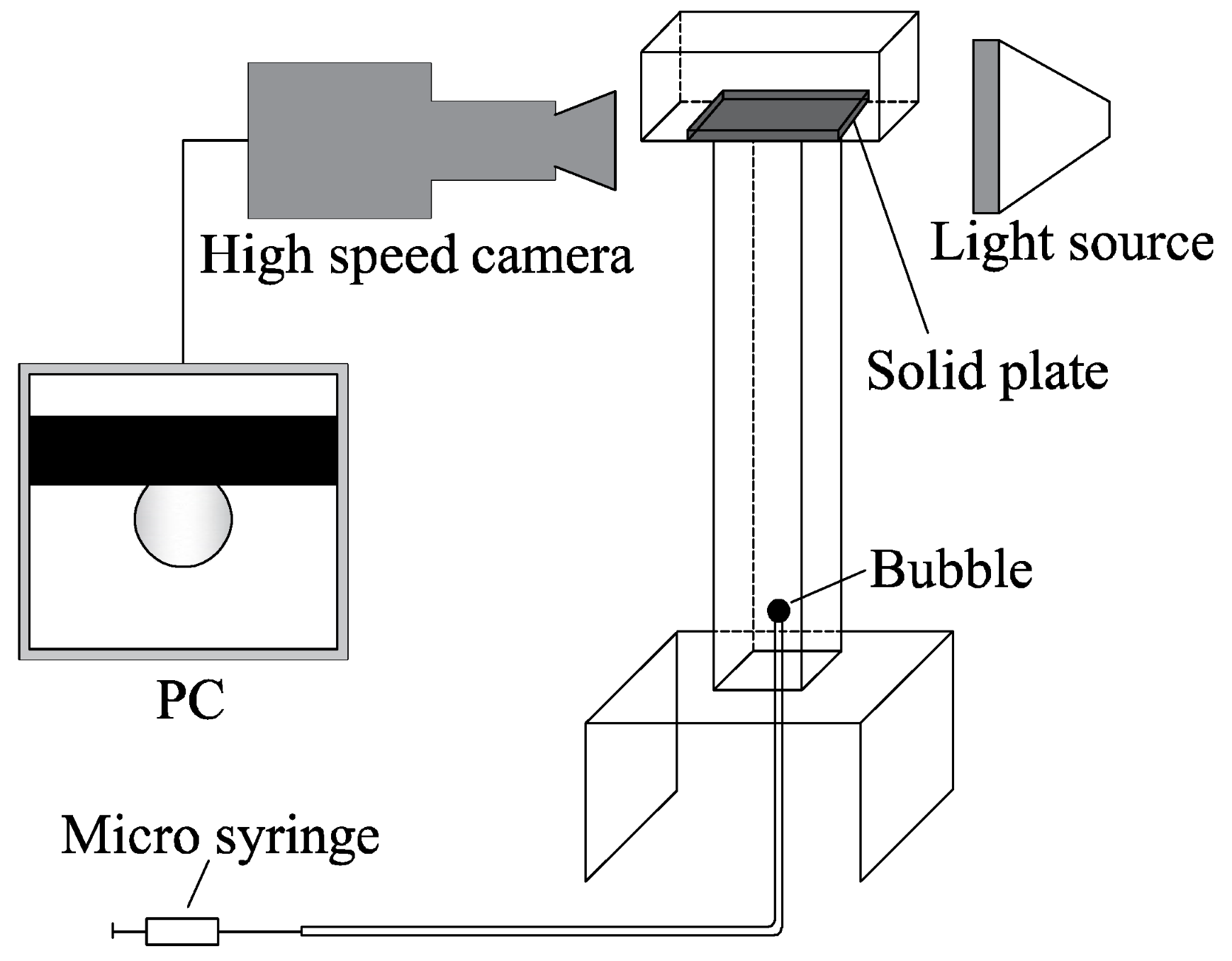
2.2. Experiment on Collision and Attachment of Air Bubbles to Solid Surfaces
3. Results and Discussion
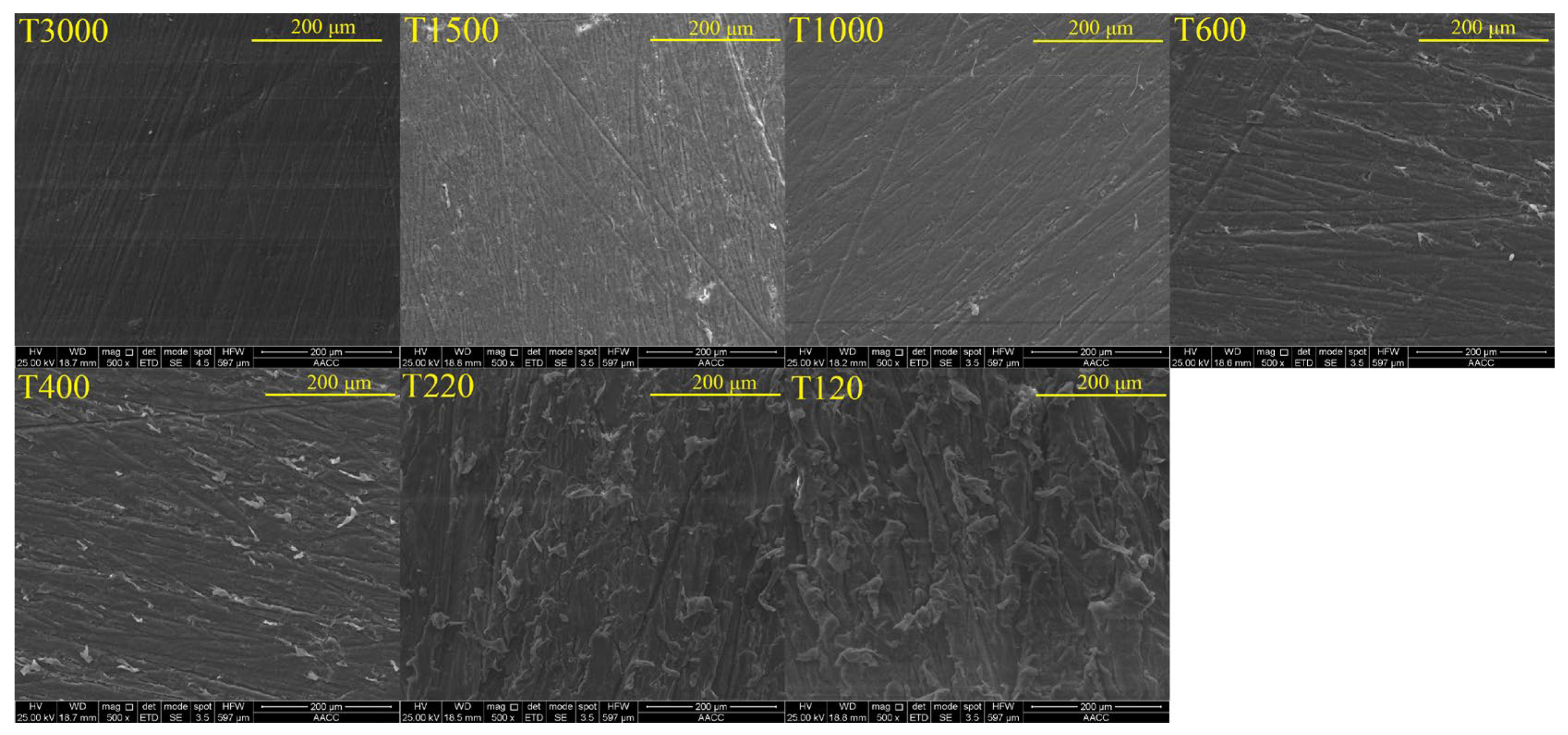
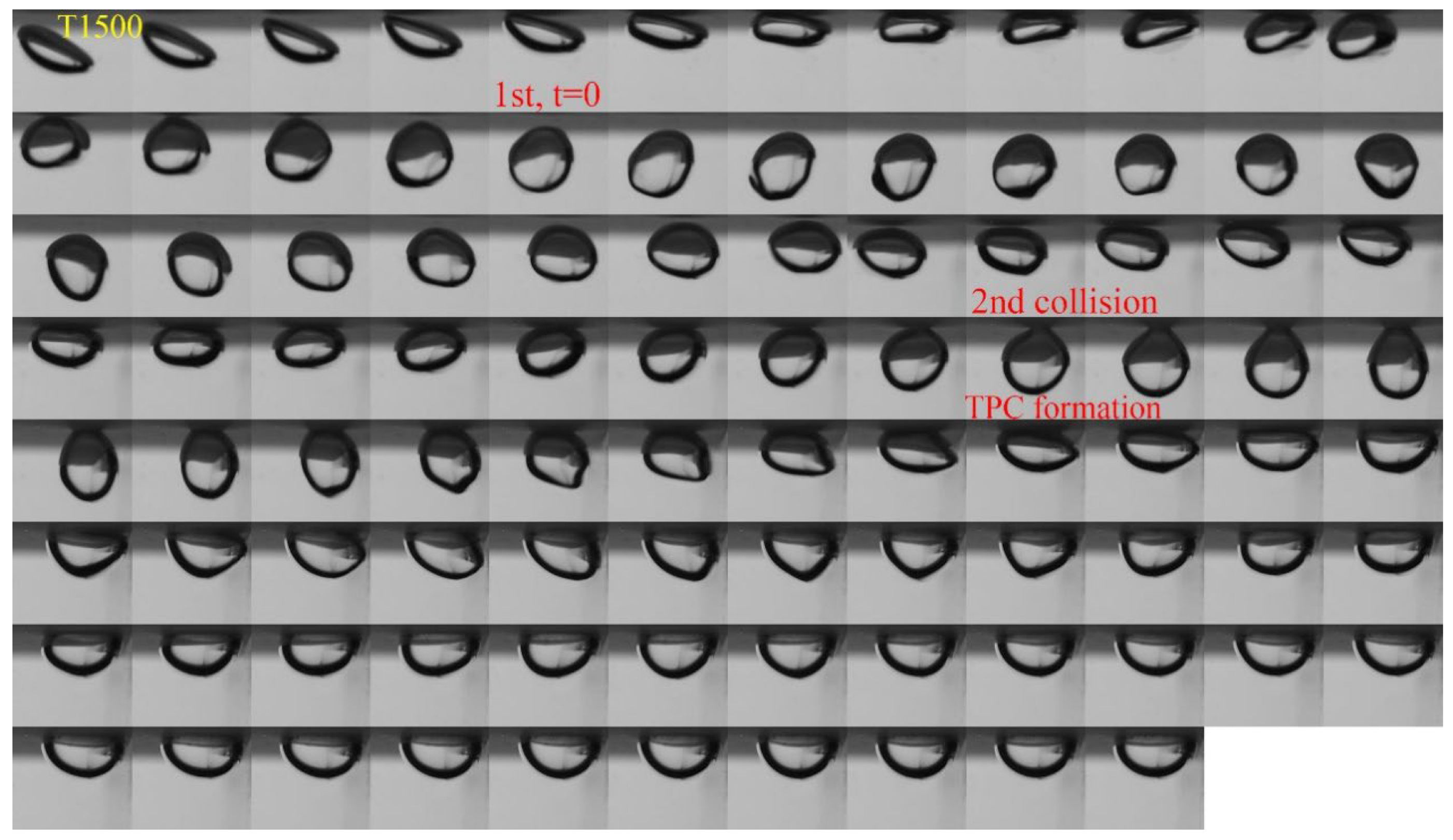
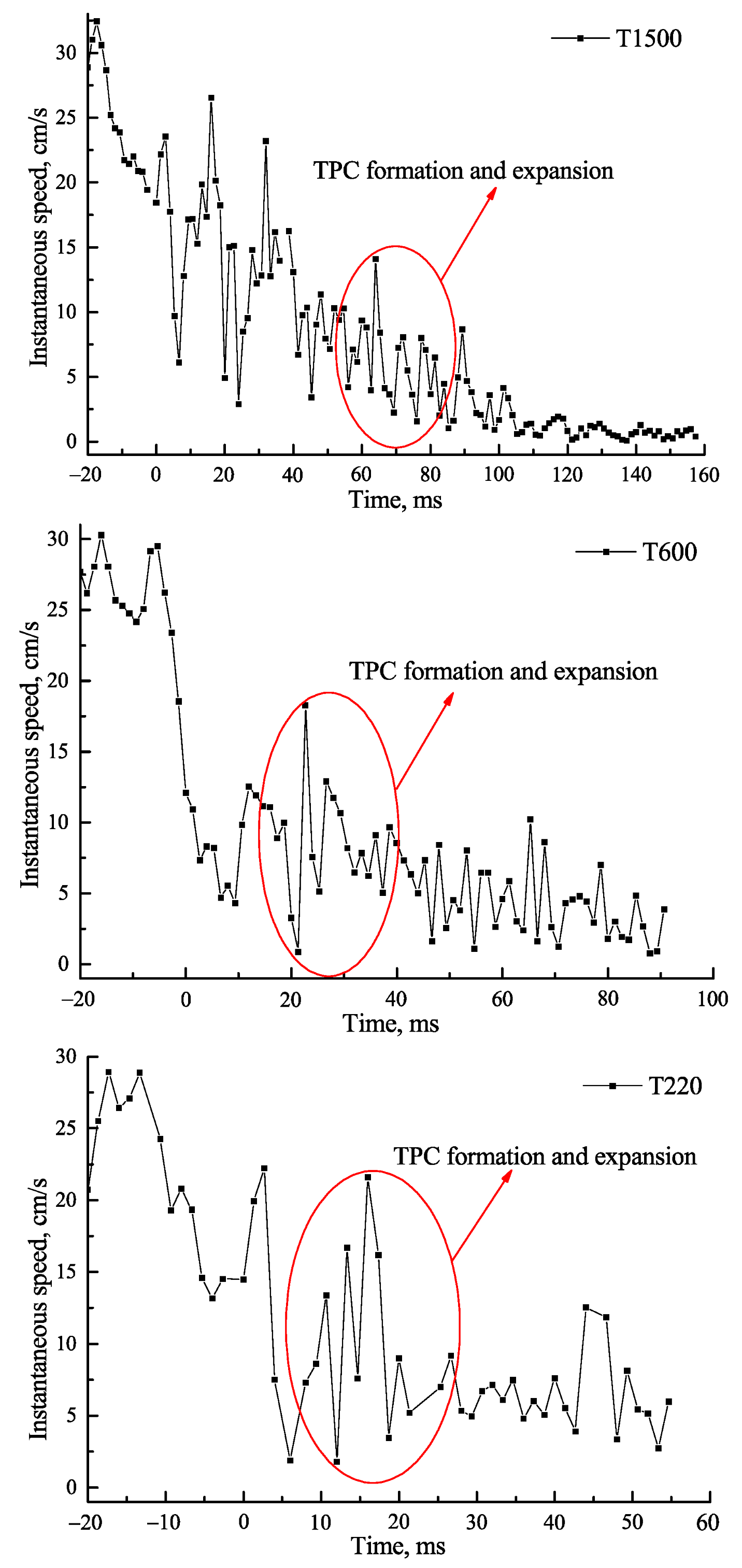
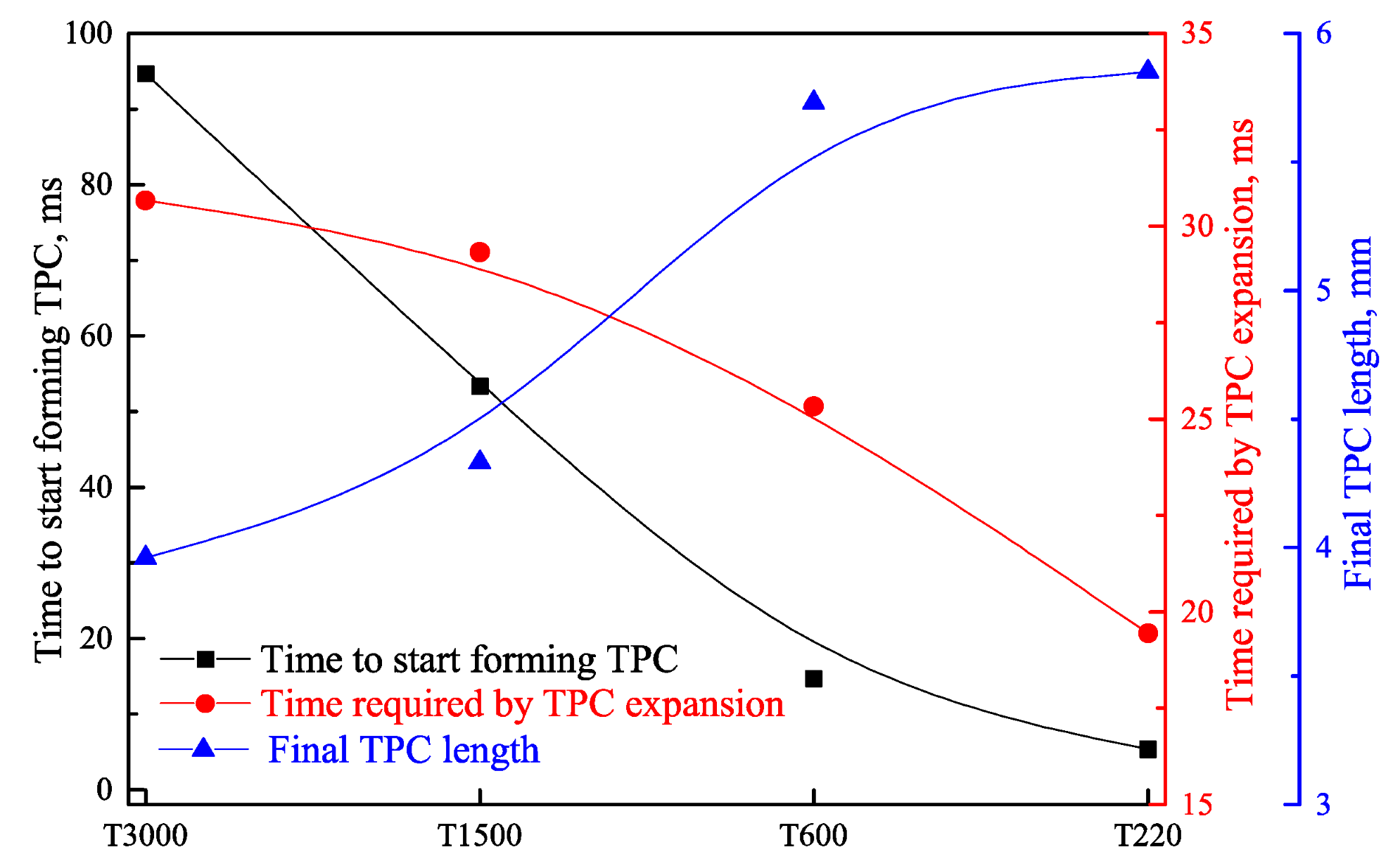
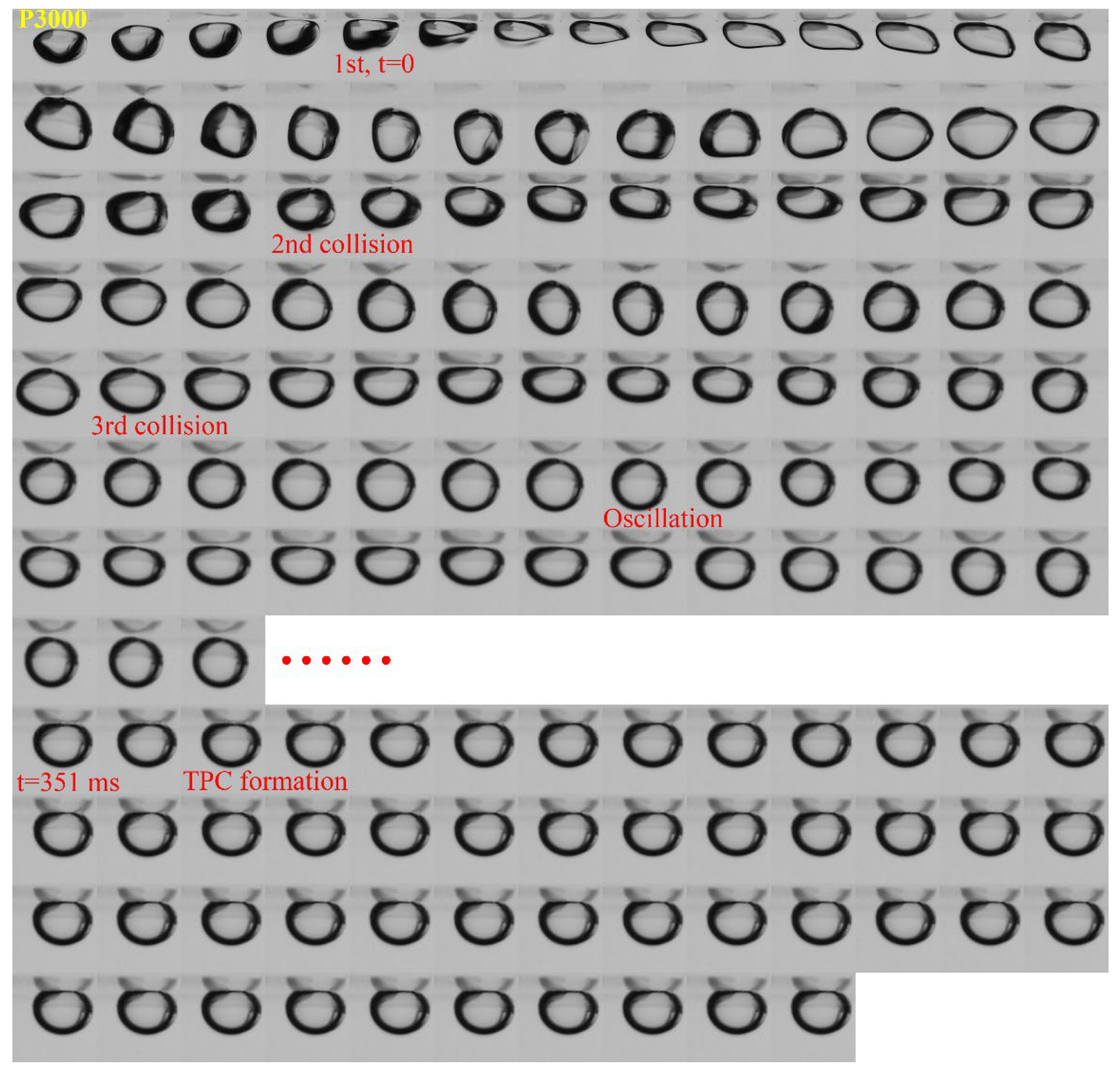
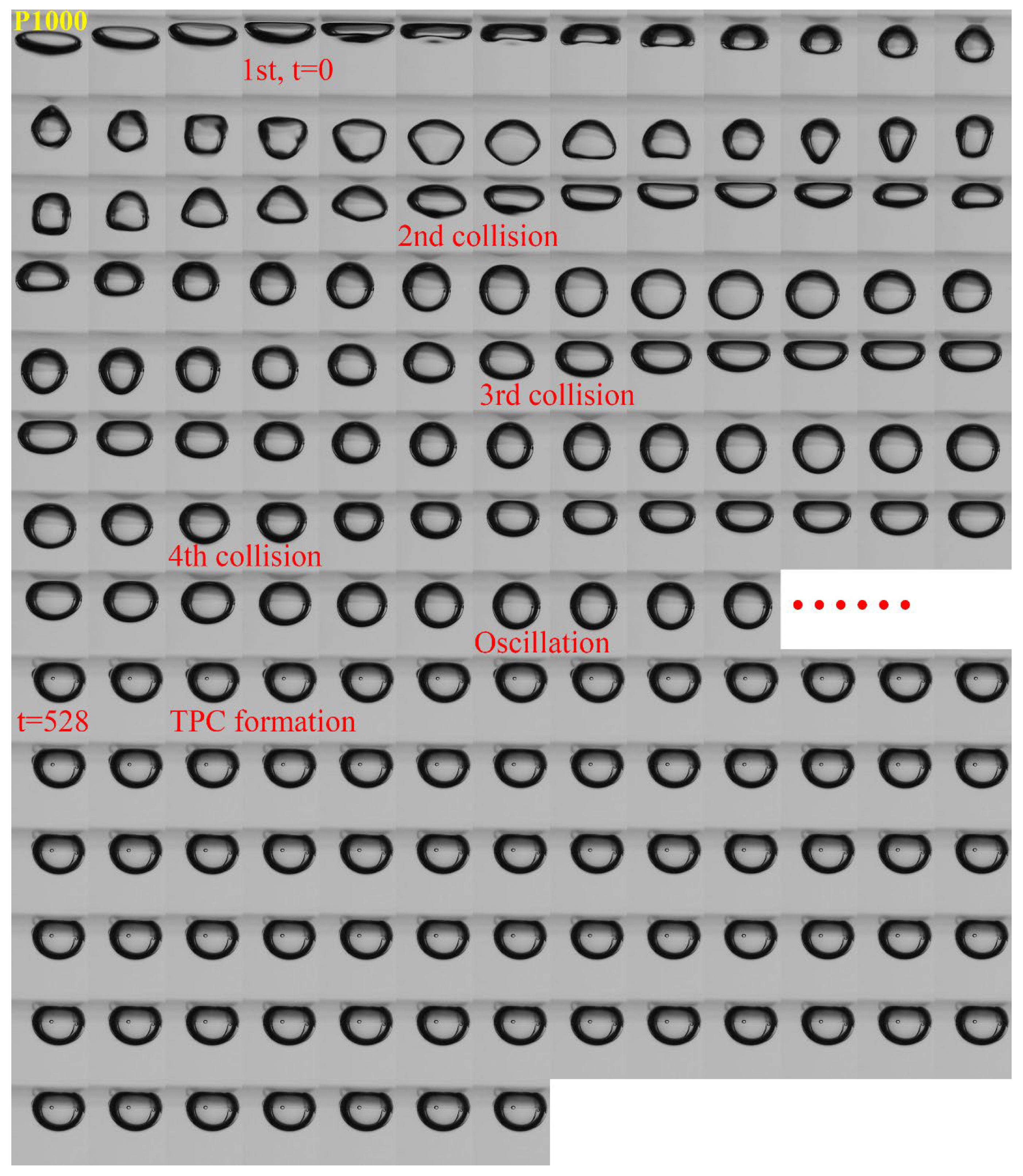
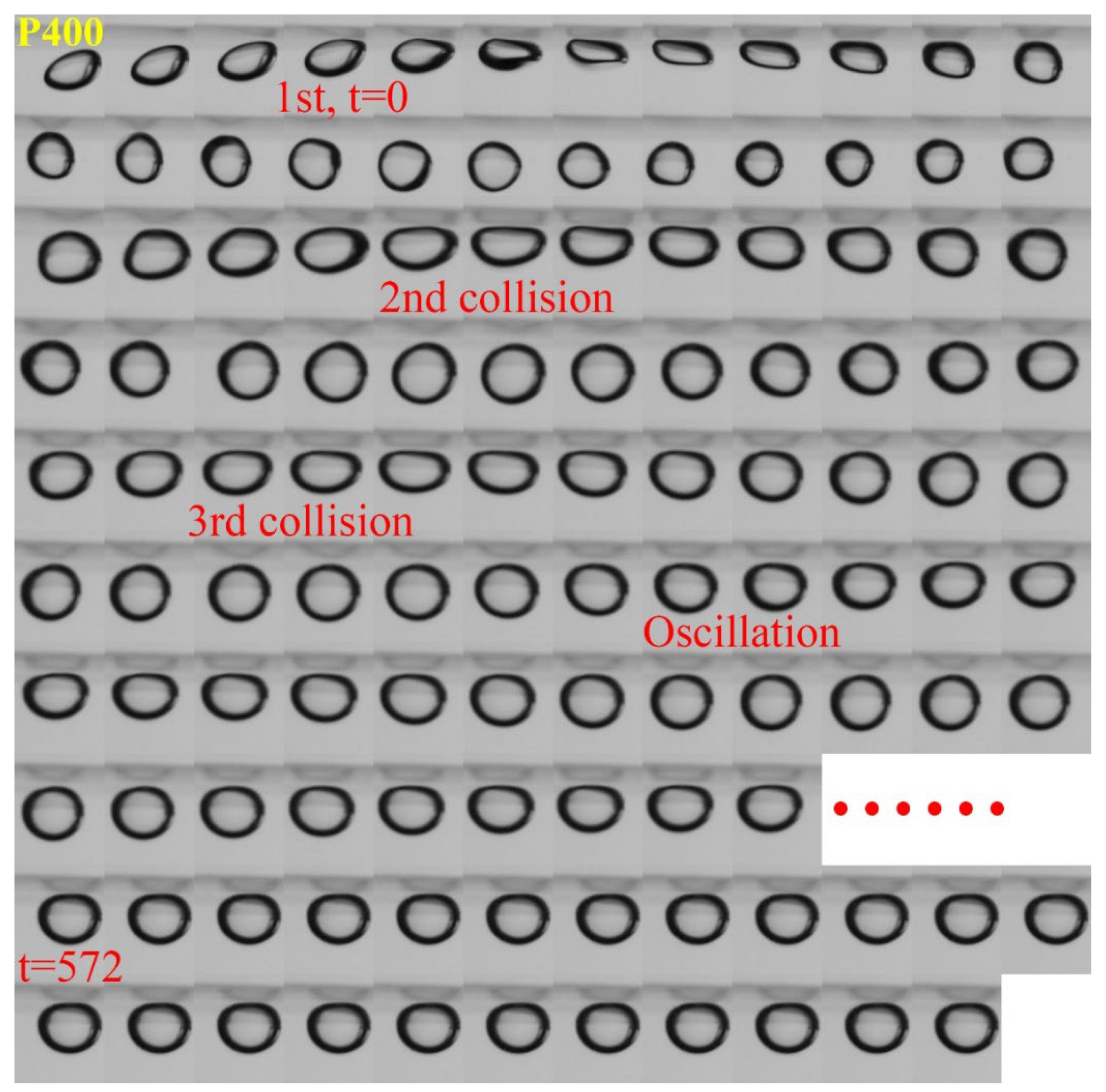
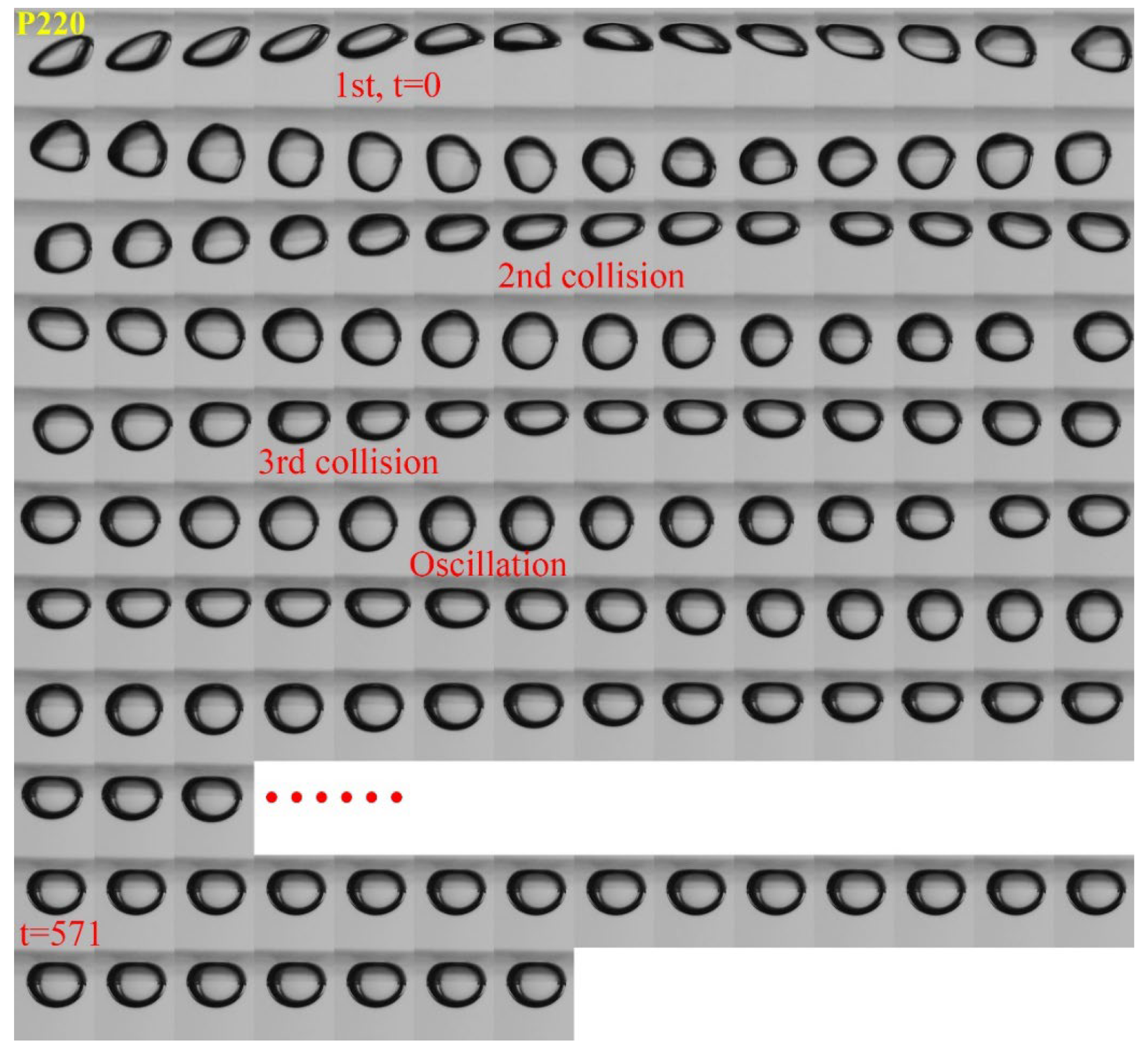
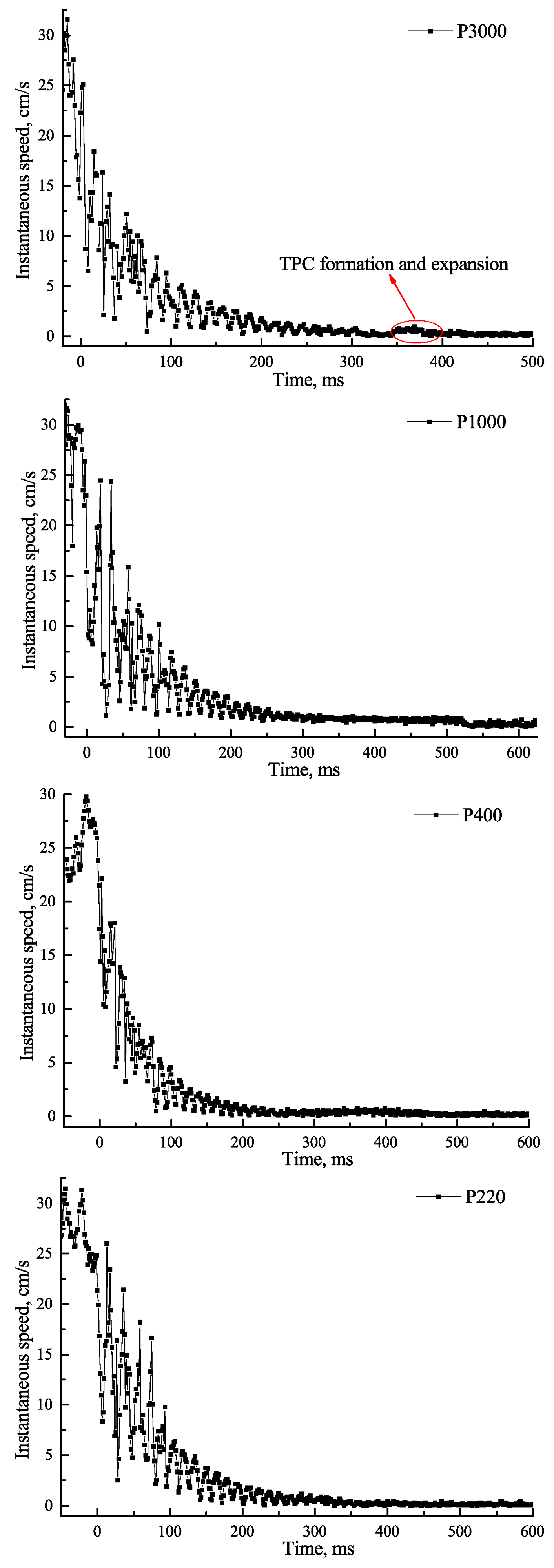
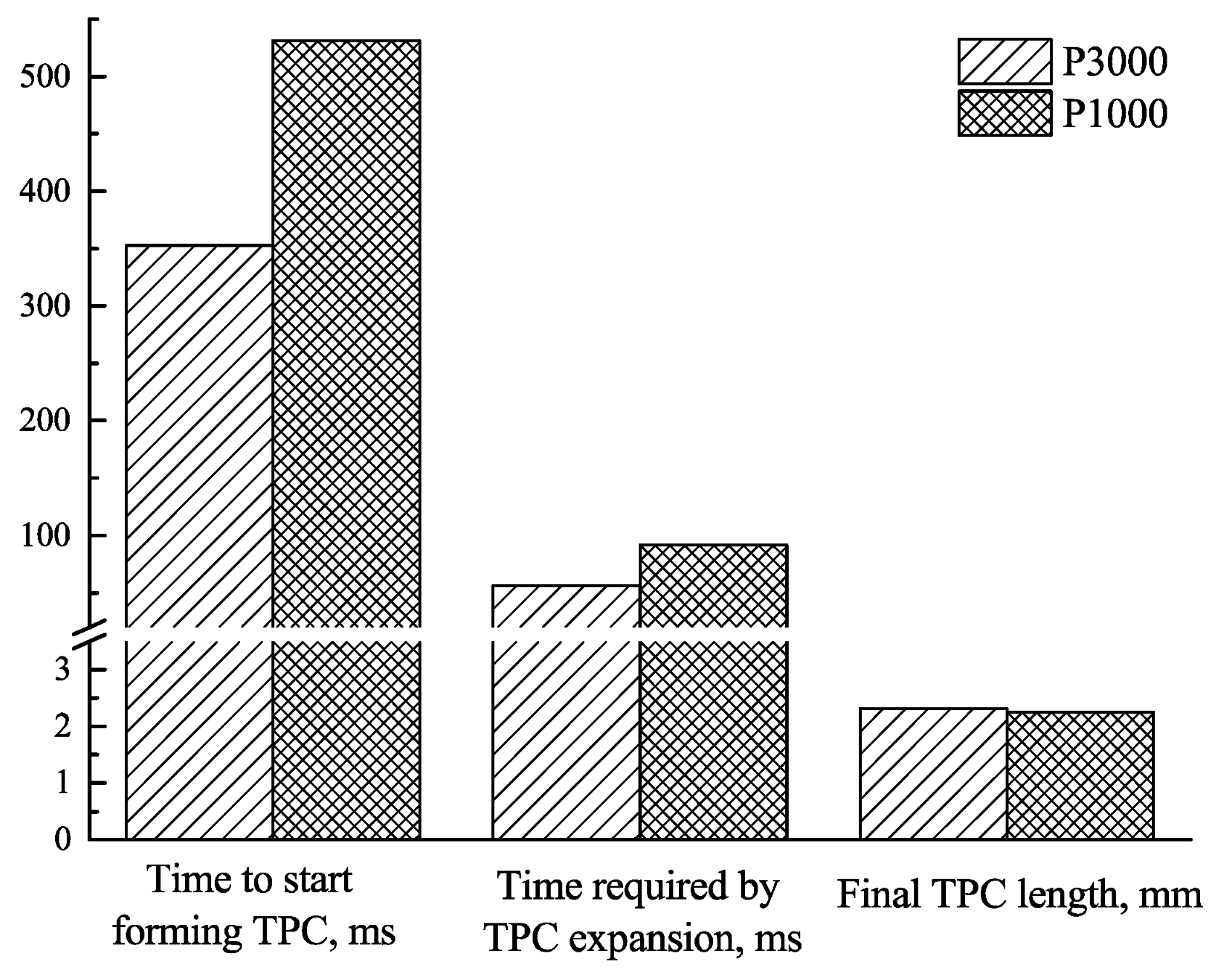
3.1. Effect of Roughness on Bubble Attachment to Hydrophobic Teflon Plates
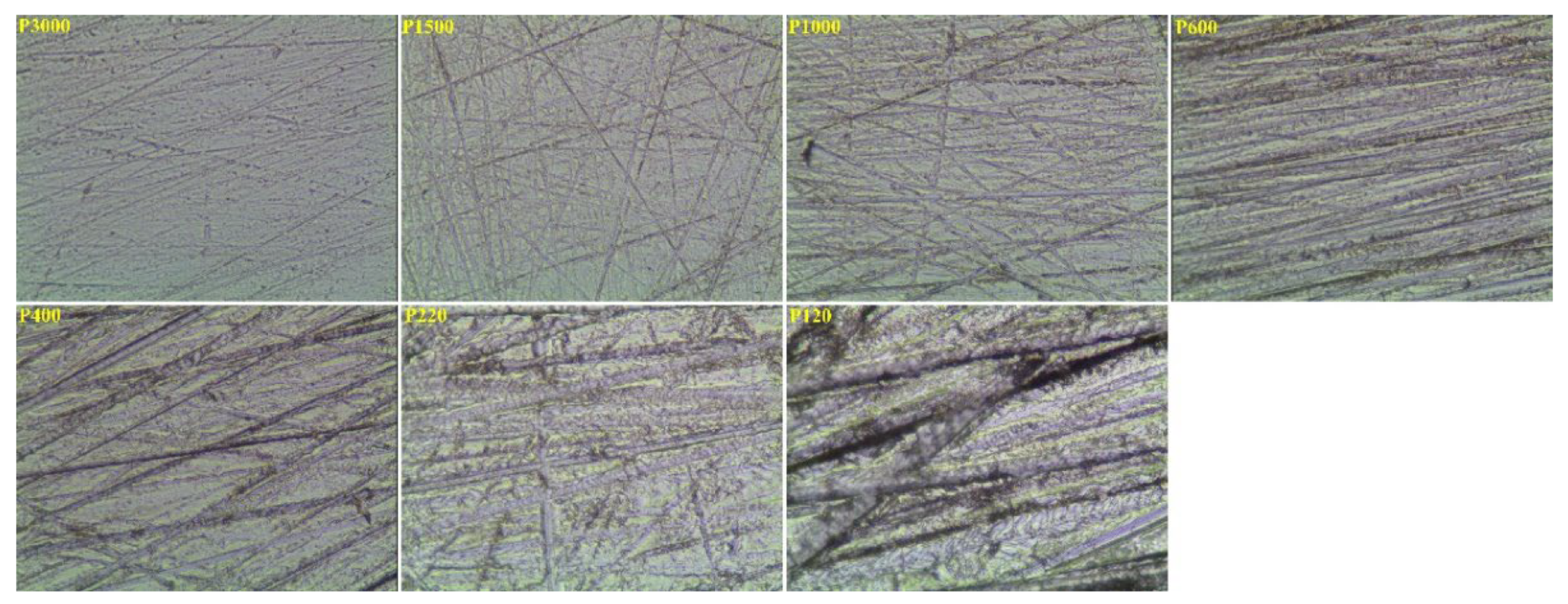
3.2. Effect of Roughness on Bubble Attachment to Hydrophilic Plexiglass Plates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, A.V. Flotation. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2007; pp. 1–27. [Google Scholar]
- Wei, Z.; Sun, W.; Han, H.; Gui, X.; Xing, Y. Flotation chemistry of scheelite and its practice: A comprehensive review. Miner. Eng. 2023, 204, 108404. [Google Scholar] [CrossRef]
- Wang, G.; Bai, X.; Wu, C.; Li, W.; Liu, K.; Kiani, A. Recent advances in the beneficiation of ultrafine coal particles. Fuel Process. Technol. 2018, 178, 104–125. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Schulze, H.J. Colloidal Science of Flotation; CRC Press: Boca Raton, FL, USA, 2004; Volume 118, pp. 1–850. [Google Scholar]
- Albijanic, B.; Ozdemir, O.; Nguyen, A.V.; Bradshaw, D. A review of induction and attachment times of wetting thin films between air bubbles and particles and its relevance in the separation of particles by flotation. Adv. Colloid Interface Sci. 2010, 159, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Gui, X.; Cao, Y.; Liu, J. Bubble–particle attachment science: Advances and dilemma in bubble–particle attachment on a macroscopic scale. J. China Coal Soc. 2019, 44, 582–587. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Ralston, J.; Schulze, H.J. On modelling of bubble–particle attachment probability in flotation. Int. J. Miner. Process. 1998, 53, 225–249. [Google Scholar] [CrossRef]
- Gu, G.; Xu, Z.; Nandakumar, K.; Masliyah, J. Effects of physical environment on induction time of air–bitumen attachment. Int. J. Miner. Process. 2003, 69, 235–250. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Koh, P.T.L.; Nguyen, A.V. Particle–bubble interaction and attachment in flotation. Chem. Eng. Sci. 2011, 66, 5910–5921. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Bruckard, W.J.; Koh, P.T.L.; Schwarz, M.P.; Follink, B. Particle shape effects in flotation. Part 1: Microscale experimental observations. Miner. Eng. 2014, 58, 80–89. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Caliskan, H.; Guven, O.; Karakas, F.; Cinar, M.; Celik, M.S. Effect of roughness and shape factor on flotation characteristics of glass beads. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 88–99. [Google Scholar] [CrossRef]
- Malysa, K.; Krasowska, M.; Krzan, M. Influence of surface active substances on bubble motion and collision with various interfaces. Adv. Colloid Interface Sci. 2005, 114, 205–225. [Google Scholar] [CrossRef]
- Krasowska, M.; Malysa, K. Wetting films in attachment of the colliding bubble. Adv. Colloid Interface Sci. 2007, 134, 138–150. [Google Scholar] [CrossRef]
- Krasowska, M.; Malysa, K. Kinetics of bubble collision and attachment to hydrophobic solids: I. Effect of surface roughness. Int. J. Miner. Process. 2007, 81, 205–216. [Google Scholar] [CrossRef]
- Krasowska, M.; Zawala, J.; Malysa, K. Air at hydrophobic surfaces and kinetics of three phase contact formation. Adv. Colloid Interface Sci. 2009, 147, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Zawala, J.; Kosior, D. Dynamics of dewetting and bubble attachment to rough hydrophobic surfaces—Measurements and modelling. Miner. Eng. 2016, 85, 112–122. [Google Scholar] [CrossRef]
- Zawala, J.; Dabros, T. Analysis of energy balance during collision of an air bubble with a solid wall. Phys. Fluids 2013, 25, 123101. [Google Scholar] [CrossRef]
- Zawala, J.; Krasowska, M.; Dabros, T.; Malysa, K. Influence of bubble kinetic energy on its bouncing during collisions with various interfaces. Can. J. Chem. Eng. 2007, 85, 669–678. [Google Scholar] [CrossRef]
- Niecikowska, A.; Zawala, J.; Miller, R.; Malysa, K. Dynamic adsorption layer formation and time of bubble attachment to a mica surface in solutions of cationic surfactants (CnTABr). Colloids Surf. A Physicochem. Eng. Asp. 2010, 365, 14–20. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Zawala, J.; Drzymala, J.; Malysa, K. Influence of hexylamine on kinetics of flotation and bubble attachment to the quartz surface. Sep. Sci. 2016, 51, 2681–2690. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Q.; Guo, H.; Li, M.; Xing, Y.; Gui, X.; Cao, Y. Bubble adhesion dynamics on solid surfaces: Interfacial behavior, force, and energy perspectives using a self–developed dynamic force testing system. Sep. Purif. Technol. 2024, 355, 129651. [Google Scholar] [CrossRef]
- Li, M.; Xing, Y.; Zhu, C.; Liu, Q.; Yang, Z.; Zhang, R.; Zhang, Y.; Xia, Y.; Gui, X. Effect of roughness on wettability and floatability: Based on wetting film drainage between bubbles and solid surfaces. Int. J. Min. Sci. Technol. 2022, 32, 1389–1396. [Google Scholar] [CrossRef]
- Snoswell, D.R.E.; Duan, J.; Fornasiero, D.; Ralston, J. Colloid Stability and the Influence of Dissolved Gas. J. Phys. Chem. B 2003, 107, 2986–2994. [Google Scholar] [CrossRef]
- Parker, J.L.; Yaminsky, V.V.; Claesson, P.M. Surface forces between glass surfaces in cetyltrimethylammonium bromide solutions. J. Phys. Chem. 1993, 97, 7706–7710. [Google Scholar] [CrossRef]
- Pashley, R.M.; McGuiggan, P.M.; Ninham, B.W.; Evans, D.F. Attractive Forces Between Uncharged Hydrophobic Surfaces: Direct Measurements in Aqueous Solution. Science 1985, 229, 1088–1089. [Google Scholar] [CrossRef]
- Bérard, D.R.; Attard, P.; Patey, G.N. Cavitation of a Lennard–Jones fluid between hard walls, and the possible relevance to the attraction measured between hydrophobic surfaces. J. Chem. Phys. 1993, 98, 7236–7244. [Google Scholar] [CrossRef]
- Krasowska, M.; Krastev, R.; Rogalski, M.; Malysa, K. Air–facilitated three–phase contact formation at hydrophobic solid surfaces under dynamic conditions. Langmuir 2007, 23, 549–557. [Google Scholar] [CrossRef]
- Attard, P. Nanobubbles and the hydrophobic attraction. Adv. Colloid Interface Sci. 2003, 104, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Schönherr, H.; Xing, Y.; Yang, H.; Gui, X.; Cao, Y. Influence of the concentration of dissolved air in unsaturated water on the interaction between hydrophobic surfaces. J. Mol. Liq. 2024, 412, 125475. [Google Scholar] [CrossRef]
- Ishida, N.; Inoue, T.; Miyahara, M.; Higashitani, K. Nano bubbles on a hydrophobic surface in water observed by tapping–mode atomic force microscopy. Langmuir 2000, 16, 6377–6380. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, H.; Fan, G.; Gui, X.; Xing, Y.; Cao, Y. The role of interfacial nanobubbles in the flotation performance of microfine particles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 699, 134633. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, H.; Cheng, Y.; Xing, Y.; Cao, Y.; Gui, X. Solid–liquid interfacial nanobubble nucleation dynamics influenced by surface hydrophobicity and gas oversaturation. J. Mol. Liq. 2024, 411, 125758. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the nanobubble bridging capillary force. Adv. Colloid Interface Sci. 2010, 154, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, O.I.; Belyaev, A.V. Wetting, roughness and flow boundary conditions. J. Phys. Condens. Matter 2011, 23, 184104. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H. Recent experimental advances for understanding bubble–particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Bhushan, B. Boundary slip and nanobubble study in micro/nanofluidics using atomic force microscopy. Soft Matter 2010, 6, 29–66. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, J.; Lei, G.; Ma, W.; Zhang, N.; Yu, Y.; Zhu, Z.; Li, Z. Study on the Dynamic Process of the Attachment of a Single Bubble to Rough Surfaces with Different Hydrophobicity. Minerals 2024, 14, 963. https://doi.org/10.3390/min14100963
Chen S, Wang J, Lei G, Ma W, Zhang N, Yu Y, Zhu Z, Li Z. Study on the Dynamic Process of the Attachment of a Single Bubble to Rough Surfaces with Different Hydrophobicity. Minerals. 2024; 14(10):963. https://doi.org/10.3390/min14100963
Chicago/Turabian StyleChen, Songjiang, Jiarui Wang, Gang Lei, Wanqi Ma, Ningning Zhang, Yuexian Yu, Zhanglei Zhu, and Zhen Li. 2024. "Study on the Dynamic Process of the Attachment of a Single Bubble to Rough Surfaces with Different Hydrophobicity" Minerals 14, no. 10: 963. https://doi.org/10.3390/min14100963







