Improved Quartz Flotation at Low Temperature by Amino Acid Lauryl Lysine as a Novel Green Collector
Abstract
:1. Introduction
2. Experimental
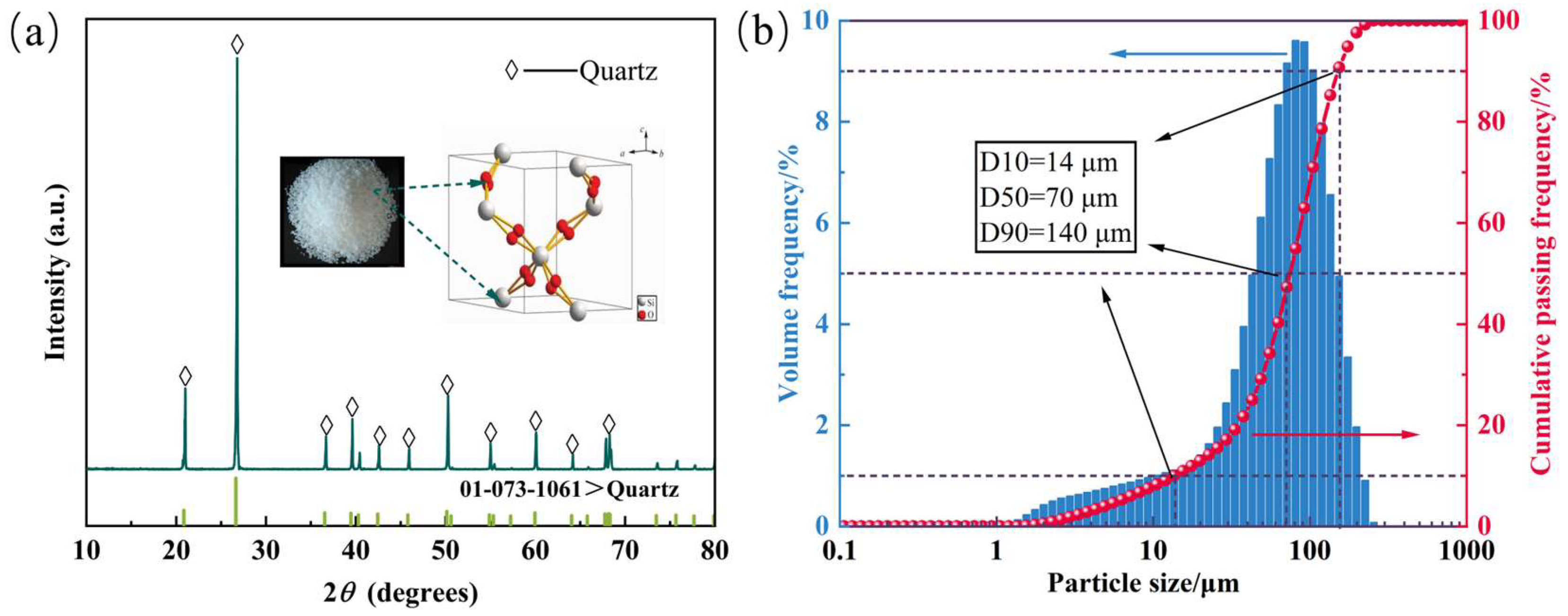
2.1. Materials
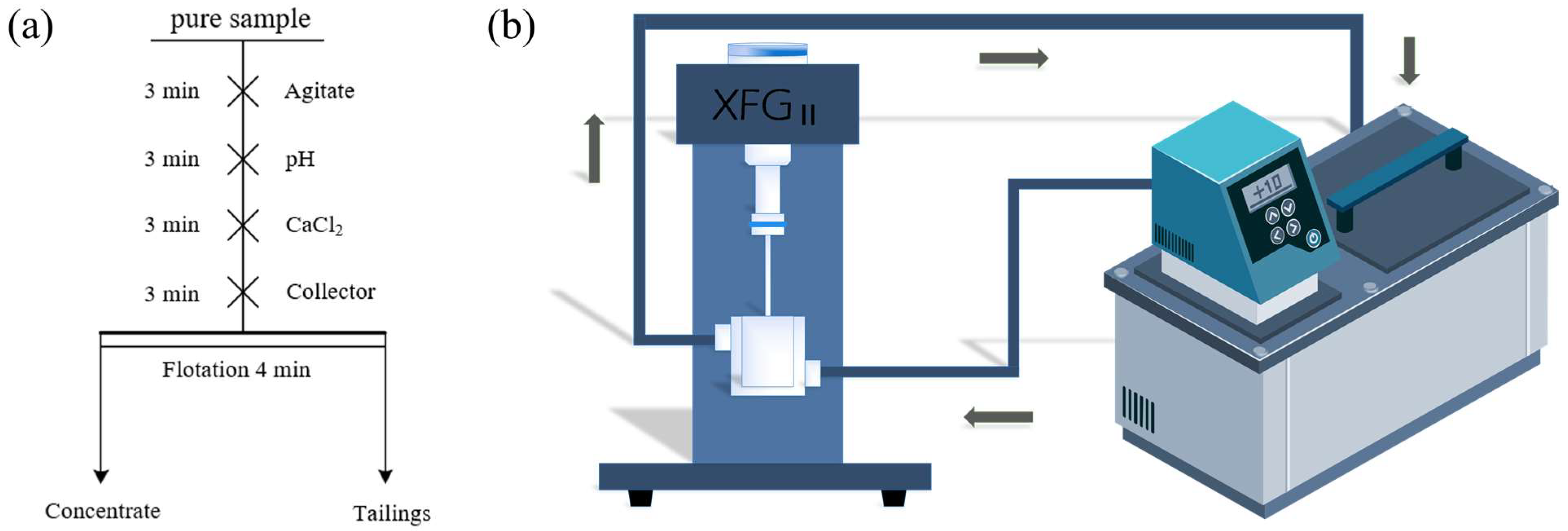
2.2. Flotation Tests
2.3. Adsorption Measurement
2.4. SEM-EDS Measurement
2.5. Zeta Potential Measurements
2.6. Fourier Transform Infrared Spectroscopy
2.7. X-ray Photoelectron Spectroscopy
3. Results and Discussion
3.1. Micro-Flotation Tests
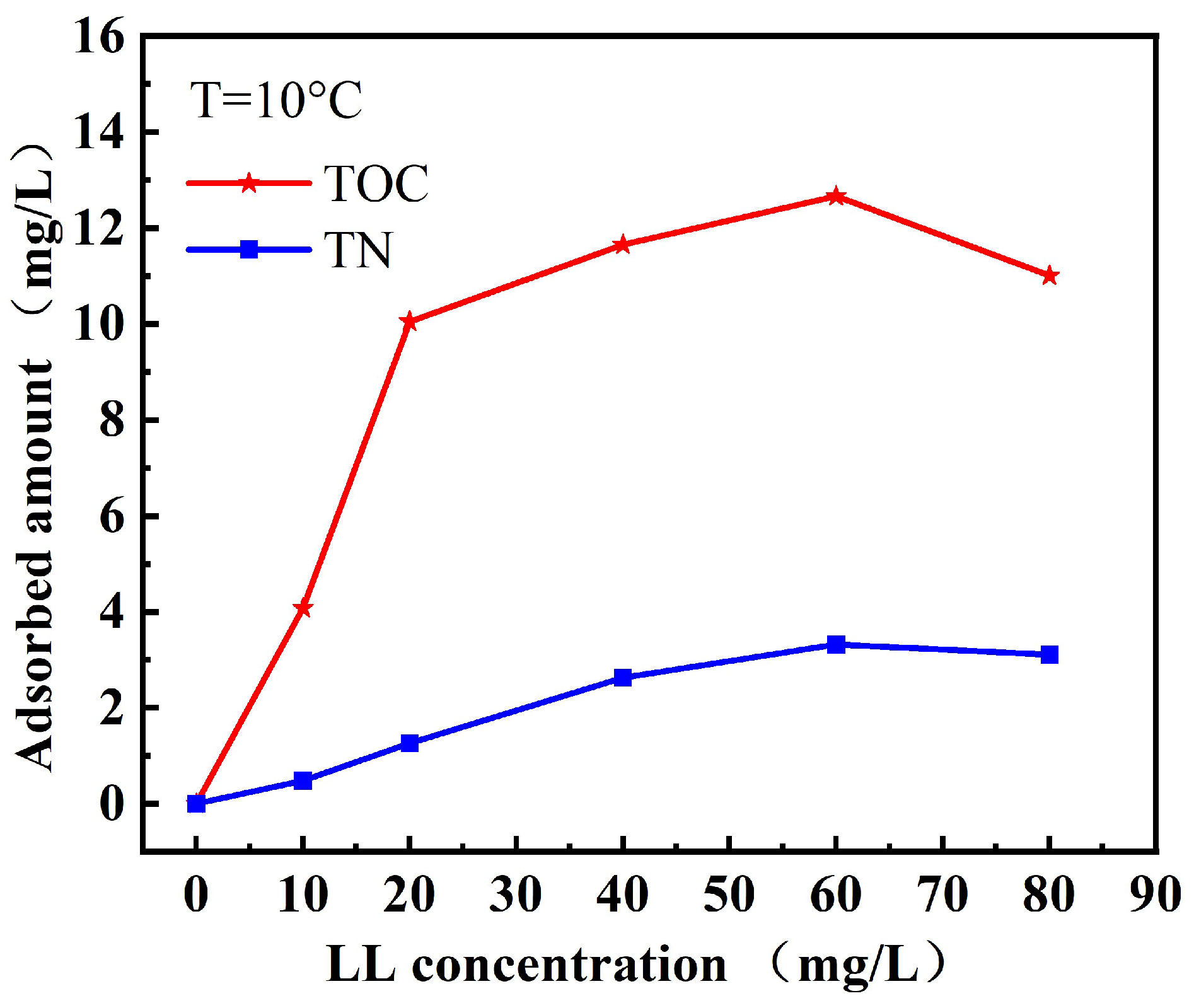
3.2. TOC Measurement Results
3.3. SEM-EDS Measurements
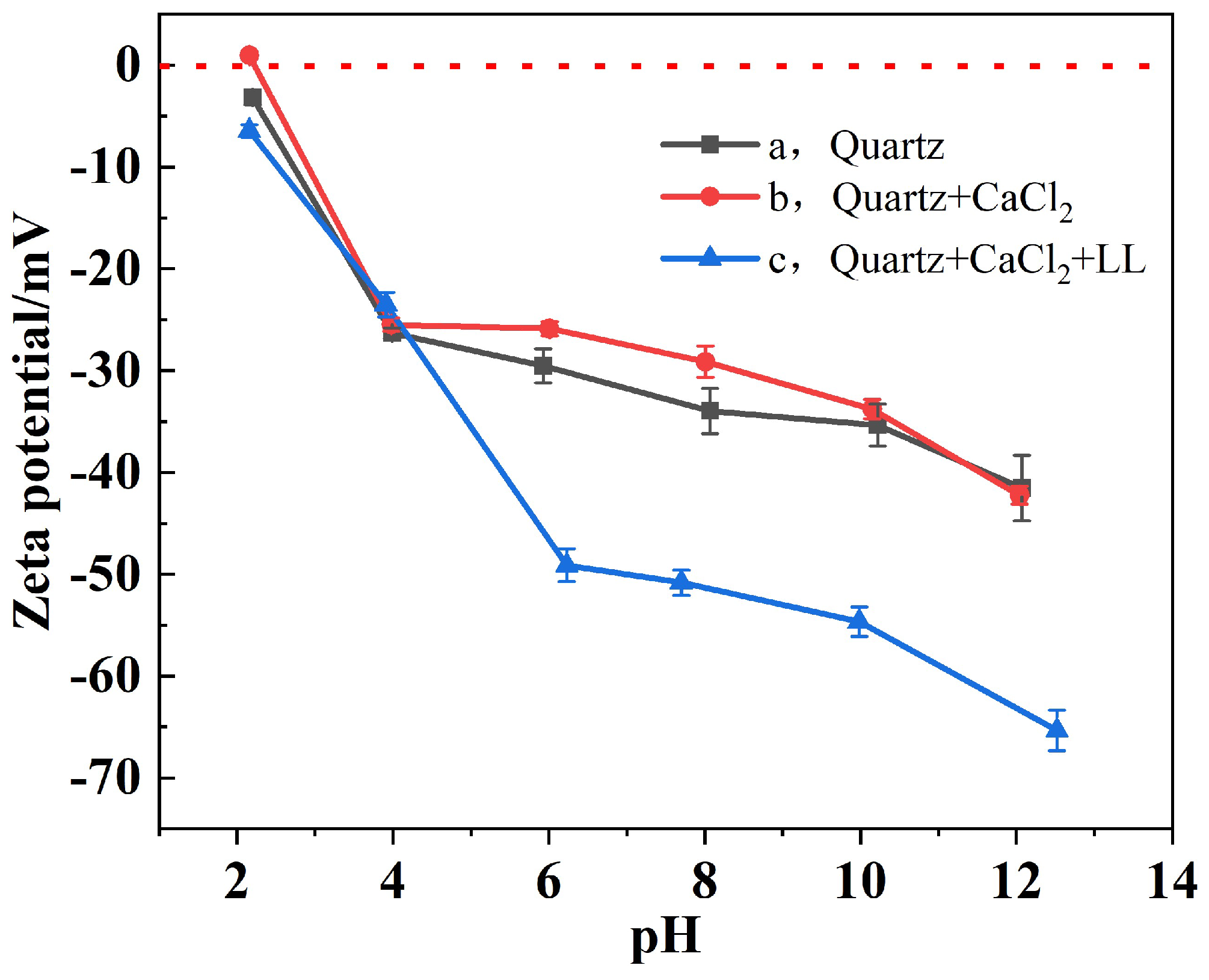
3.4. Zeta Potential Analyses
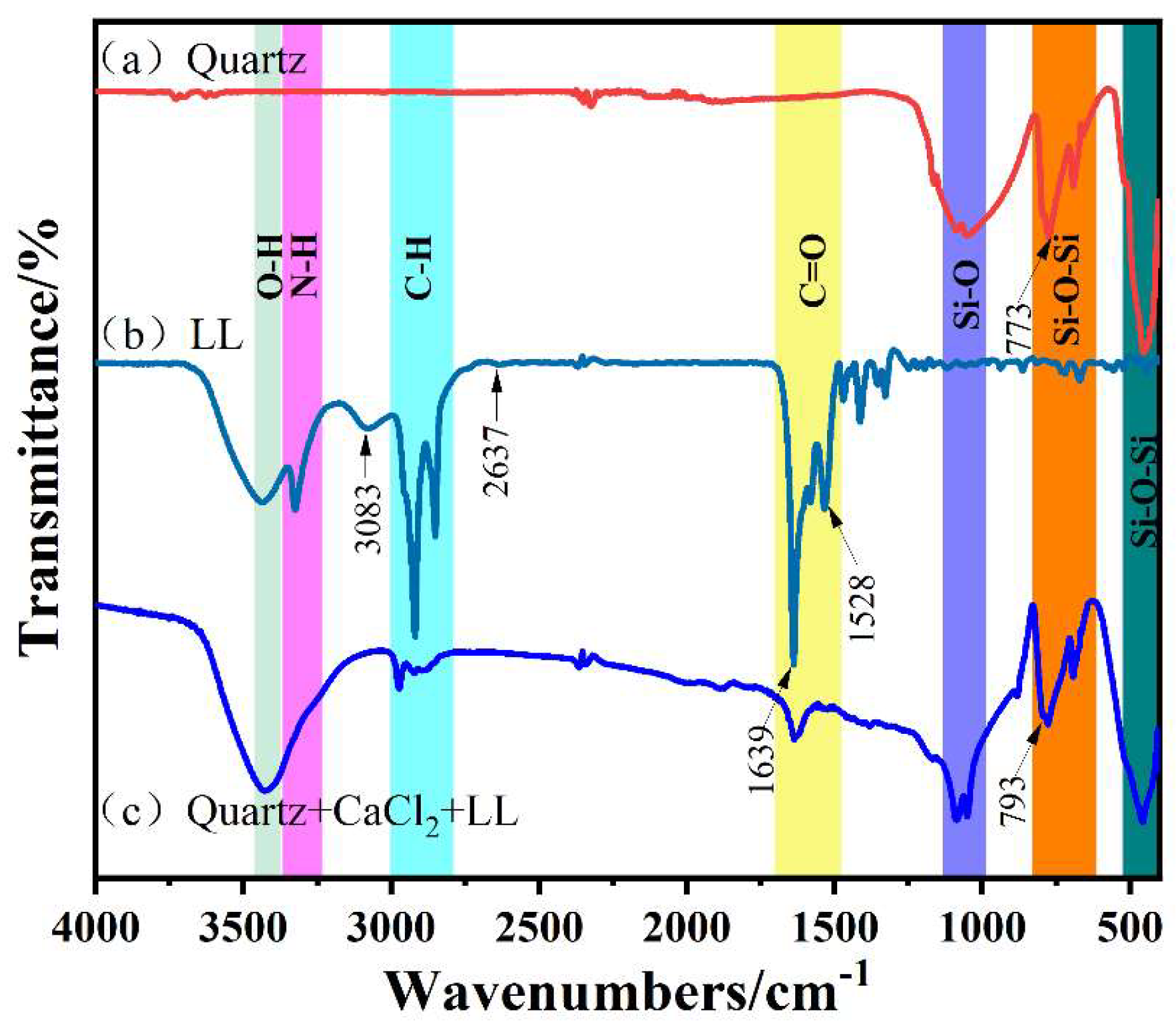
3.5. Fourier Transform Infrared Analyses
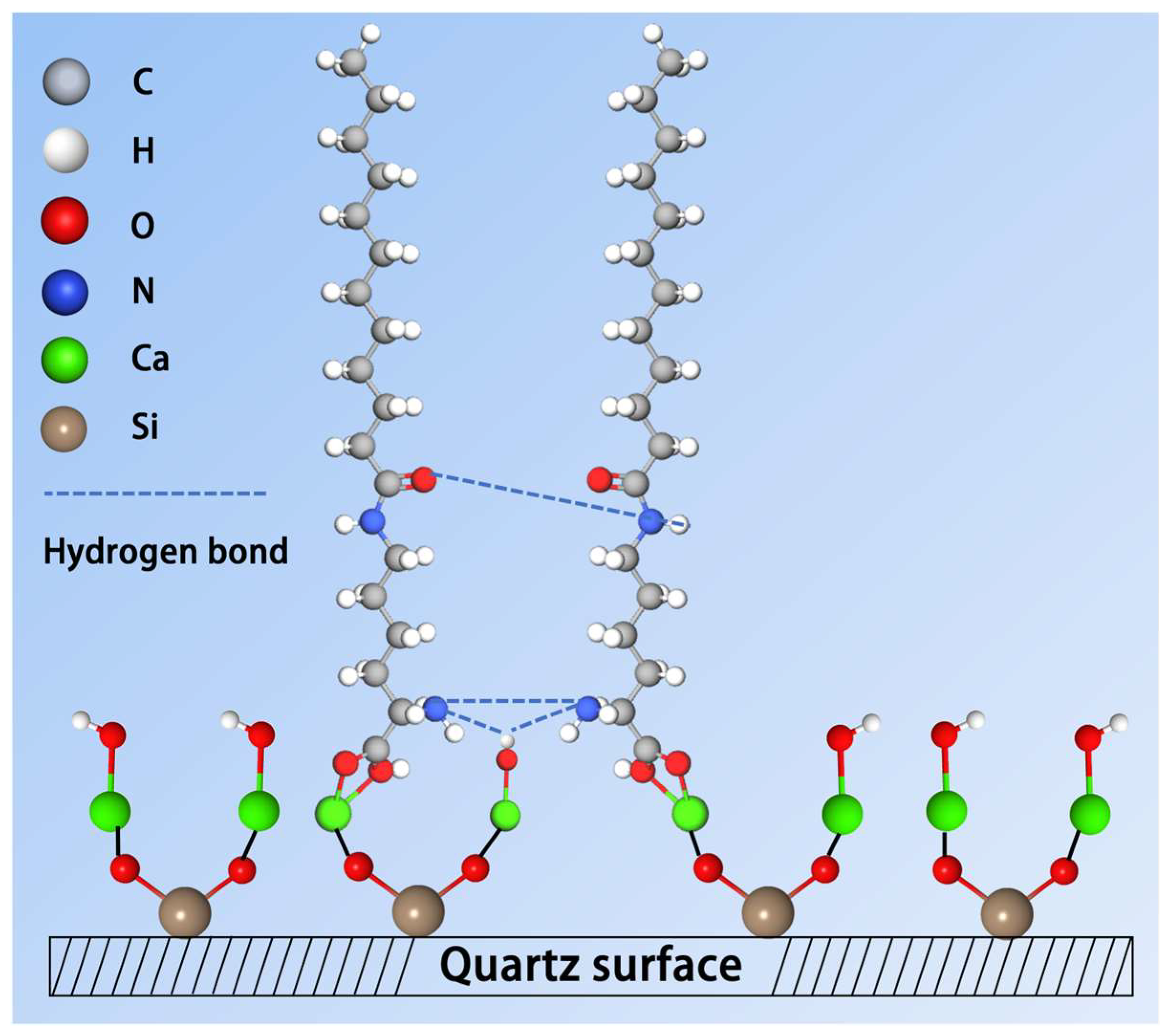
3.6. XPS Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Zhou, L.; Han, Y.; Zhu, Y.; Li, Y. Effect of carboxymethyl starch on fine-grained hematite recovery by high-intensity magnetic separation: Experimental investigation and theoretical analysis. Powder Technol. 2019, 343, 270–278. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Li, W.; Yan, X.; Zhang, H. Intensification of interfacial adsorption of dodecylamine onto quartz by ultrasonic method. Sep. Purif. Technol. 2019, 227, 115701. [Google Scholar] [CrossRef]
- Jia, K.; Ding, R.; Chen, Y.; Lu, T.; Li, G.; Cao, Y.; Wang, C. Green, multiple-ligand collector sodium myristoyl glutamate for flotation of smithsonite. Appl. Surf. Sci. 2024, 660, 159932. [Google Scholar] [CrossRef]
- Yang, B.; He, J. New insights into selective depression mechanism of Tamarindus indica kernel gum in flotation separation of fluorapatite and calcite. Sep. Purif. Technol. 2025, 354, 128787. [Google Scholar] [CrossRef]
- Deng, J.; Yang, S.; Liu, C.; Li, H. Effects of the calcite on quartz flotation using the reagent scheme of starch/dodecylamine. Colloids Surf. Physicochem. Eng. Asp. 2019, 583, 123983. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Dai, S.; Wang, B. Adsorption of bis(2-hydroxy-3-chloropropyl) dodecylamine on quartz surface and its implication on flotation. Results Phys. 2018, 9, 1096–1101. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, Y.; Li, Y.; Han, Y. Flotation and adsorption of quartz with the new collector butane-3-heptyloxy-1,2-diamine. Mineral. Petrol. 2019, 113, 207–216. [Google Scholar] [CrossRef]
- Gong, G.; Liu, J.; Han, Y.; Zhu, Y. An atomic scale investigation of the adsorption of sodium oleate on Ca2+ activated quartz surface. Physicochem. Probl. Miner. Process. 2019, 55, 426–436. [Google Scholar]
- Wang, L.; Wang, G.; Ge, P.; Sun, W.; Tang, H.; Hu, W. Activation mechanisms of quartz flotation with calcium ions and cationic/anionic mixed collectors under alkalescent conditions. Colloids Surf. Physicochem. Eng. Asp. 2022, 632, 127771. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Wang, D.; Yin, W.; Yao, J. Selective adsorption analysis of BAPTA depressants on the surface of carbonate minerals: Insights into flotation behavior and adsorption mechanism. Surf. Interfaces 2024, 45, 103872. [Google Scholar] [CrossRef]
- Wang, M.; Jin, S. Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation. Molecules 2023, 28, 5361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, Y.; Qiao, L.; Shen, P.; Mao, Y.; Dong, L.; Liu, D. Selective adsorption of soluble starch on the cassiterite surface for effective flotation separation of scheelite from cassiterite. Surf. Interfaces 2024, 48, 104238. [Google Scholar] [CrossRef]
- Ren, L.; Qiu, H.; Zhang, Y.; Nguyen, A.V.; Zhang, M.; Wei, P.; Long, Q. Effects of alkyl ether amine and calcium ions on fine quartz flotation and its guidance for upgrading vanadium from stone coal. Powder Technol. 2018, 338, 180–189. [Google Scholar] [CrossRef]
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- Ogata, Y.; Sugimoto, T.; Inaishi, M. α-Chlorination of Long-chain Aliphatic Acids. Bull. Chem. Soc. Jpn. 1979, 52, 255–256. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Y.; Sun, C.; Li, Y.; Han, Y. Flotation and adsorption of a new collector α-Bromodecanoic acid on quartz surface. Miner. Eng. 2015, 77, 86–92. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, B.; Sun, C.; Li, Y.; Han, Y. Influence of bromine modification on collecting property of lauric acid. Miner. Eng. 2015, 79, 24–30. [Google Scholar] [CrossRef]
- Asgari, K.; Huang, Q.; Khoshdast, H.; Hassanzadeh, A. A Review on Bioflotation of Coal and Minerals: Classification, Mechanisms, Challenges, and Future Perspectives. Miner. Process. Extr. Metall. Rev. 2024, 45, 46–76. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological Avenues in Mineral Processing: Fundamentals, Applications and Advances in Bioleaching and Bio-beneficiation. Miner. Process. Extr. Metall. Rev. 2023, 44, 22–51. [Google Scholar] [CrossRef]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants—Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.; Qiu, X. Stimulatory effects of amino acids on γ-polyglutamic acid production by Bacillus subtilis. Sci. Rep. 2018, 8, 17934. [Google Scholar] [CrossRef] [PubMed]
- Khoso, S.A.; Hu, Y.; Lyu, F.; Liu, R.; Sun, W. Selective separation of chalcopyrite from pyrite with a novel non-hazardous biodegradable depressant. J. Clean. Prod. 2019, 232, 888–897. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Fei, L.; Cao, Z.; Zhong, H.; Ma, X. The selective flotation separation of rhodochrosite against quartz and calcite with dicarboxylic amino acid-based surfactants as a novel collector. Miner. Eng. 2022, 182, 107559. [Google Scholar] [CrossRef]
- Karlkvist, T.; Patra, A.; Rao, K.H.; Bordes, R.; Holmberg, K. Flotation selectivity of novel alkyl dicarboxylate reagents for apatite–calcite separation. J. Colloid Interface Sci. 2015, 445, 40–47. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Pooley, S.G.; Wang, J.; Liu, Y.; Xu, F.; Wang, Q.; Zeng, L.; Wu, Y. New role of the conventional foamer sodium N-lauroylsarcosinate as a selective collector for the separation of calcium minerals. J. Mol. Liq. 2020, 318, 114031. [Google Scholar] [CrossRef]
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Wang, Y.; Xu, M.; Wang, B. A novel reusable nanocomposite adsorbent, xanthated Fe3O4-chitosan grafted onto graphene oxide, for removing Cu(II) from aqueous solutions. Appl. Surf. Sci. 2016, 367, 327–334. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; He, Y.; Huang, J.; Xu, H. Preparation of Pickering emulsion stabilized by lauroyl lysine. Tenside Surfactants Deterg. 2023, 60, 21–35. [Google Scholar] [CrossRef]
- Gesslein, B.W.; Oshimura, E.; Popova, K. Water Dispersible Lauroyl Lysine in Cleansing Applications. SOFW J. Int. J. Angew. Wiss. 2014, 140, 32, 34–36. [Google Scholar]
- Cao, S.; Yin, W.; Yang, B.; Zhu, Z.; Sun, H.; Sheng, Q.; Chen, K. Insights into the influence of temperature on the adsorption behavior of sodium oleate and its response to flotation of quartz. Int. J. Min. Sci. Technol. 2022, 32, 399–409. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Xia, W.; Peng, Y.; Xie, G. Adsorption of sodium oleate on coal surface with different oxidation degrees and its effect on flotation. Colloids Surf. Physicochem. Eng. Asp. 2021, 611, 125801. [Google Scholar] [CrossRef]
- Kuang, J.; Zou, Z.; Huang, Z.; Liu, P.; Yuan, W.; Zhu, L. Surface dissolution of scheelite under different regulators and its effect on flotation behavior. Miner. Eng. 2021, 164, 106811. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, X.; Yue, T.; Sun, W. A novel GCMS method for the quantitative analysis of sodium oleate in froth flotation. Miner. Eng. 2022, 176, 107317. [Google Scholar] [CrossRef]
- Schaefer, C.E.G.R.; Gilkes, R.J.; Fernandes, R.B.A. EDS/SEM study on microaggregates of Brazilian Latosols, in relation to P adsorption and clay fraction attributes. Geoderma 2004, 123, 69–81. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B.; Marois, J.; Ourriban, M. Study of pyrochlore matrix composition effects on froth flotation by SEM–EDX. Miner. Eng. 2012, 30, 62–66. [Google Scholar] [CrossRef]
- Xu, L.; Liu, D.; Sun, R.; Wang, Y.; Liu, Z.; Shao, P.; Wang, C.; Wen, S. Flotation separation of smithsonite and calcite in sodium oleate system using soluble starch as depressant. Miner. Eng. 2024, 205, 108490. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, L.; He, J.; Yin, W.; Yao, J. A feasible strategy for depressant-free flotation separation of siderite from magnesiohornblende using a highly selective collector. J. Mol. Liq. 2024, 394, 123689. [Google Scholar] [CrossRef]
- Zhang, D.; Kang, J.; Zhu, W. Selective flotation separation of fluorite and calcite by utilising a novel anionic/nonionic collector. Colloids Surf. Physicochem. Eng. Asp. 2022, 642, 128687. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, B.; Tian, C.; Sheng, Q.; Li, Z.; Zhang, N.; Wang, J.; Yang, X. Polyaspartic acid, an eco-friendly regulator for the improvement of coking coal flotation: Applications and mechanism. Int. J. Coal Prep. Util. 2024, 1–20. [Google Scholar] [CrossRef]
- Cherif, O.M.; Bouhenguel, M.; Amirech, A.; Gherraf, N.; Bouchemma, A. Adsorption of tetradecylamine on quartz and its implication on flotation. Chem. Pap. 2023, 77, 5183–5197. [Google Scholar] [CrossRef]
- Yin, Z.; Hu, Y.; Sun, W.; Zhang, C.; He, J.; Xu, Z.; Zou, J.; Guan, C.; Zhang, C.; Guan, Q.; et al. Adsorption Mechanism of 4-Amino-5-mercapto-1,2,4-triazole as Flotation Reagent on Chalcopyrite. Langmuir 2018, 34, 4071–4083. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xue, J.; Li, D.; Sun, Q.; Yao, J.; Huang, S. Flotation of heavily oxidized pyrite in the presence of fine digenite particles. Miner. Eng. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, M.; Xia, L.; Fu, W.; Zhou, W.; Yang, S. The utilization of citric acid as a depressant for the flotation separation of barite from fluorite. Miner. Eng. 2020, 156, 106491. [Google Scholar] [CrossRef]
- Forbes, E.; Bradshaw, D.J.; Franks, G.V. Temperature sensitive polymers as efficient and selective flotation collectors. Miner. Eng. 2011, 24, 772–777. [Google Scholar] [CrossRef]
- Luo, B.; Zhu, Y.; Sun, C.; Li, Y.; Han, Y. The flotation behavior and adsorption mechanisms of 2-((2-(decyloxy)ethyl)amino)lauric acid on quartz surface. Miner. Eng. 2018, 117, 121–126. [Google Scholar] [CrossRef]
- Guo, W.; Han, Y.; Zhu, Y.; Li, Y.; Tang, Z. Effect of amide group on the flotation performance of lauric acid. Appl. Surf. Sci. 2020, 505, 144627. [Google Scholar] [CrossRef]
- Kitadai, N.; Yokoyama, T.; Nakashima, S. In situ ATR-IR investigation of L-lysine adsorption on montmorillonite. J. Colloid Interface Sci. 2009, 338, 395–401. [Google Scholar] [CrossRef]
- Sahoo, H.; Sinha, N.; Rath, S.S.; Das, B. Ionic liquids as novel quartz collectors: Insights from experiments and theory. Chem. Eng. J. 2015, 273, 46–54. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, Y.; Han, Y.; Li, Y.; Yuan, S. Flotation performance and adsorption mechanism of a new collector 2-(carbamoylamino) lauric acid on quartz surface. Miner. Eng. 2020, 153, 106343. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wei, D.; Li, M.; Zhao, Q.; Xu, S. Synthesis of N,N-Bis(2-hydroxypropyl)laurylamine and its flotation on quartz. Chem. Eng. J. 2017, 309, 63–69. [Google Scholar] [CrossRef]
- Nan, H.; Huang, R.; Zhang, X.; Wang, C. How does ball-milling elevate biochar as a value-added peroxydisulfate activator for antibiotics removal? Ind. Crops Prod. 2024, 214, 118569. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Show, P.L.; Mahlknecht, J.; Wang, C. Degradation of tetracycline by nitrogen-doped biochar as a peroxydisulfate activator: Nitrogen doping pattern and non-radical mechanism. Sustain. Horiz. 2024, 10, 100091. [Google Scholar] [CrossRef]
- Buckley, A.N.; Parker, G.K. Adsorption of n-octanohydroxamate collector on iron oxides. Int. J. Miner. Process. 2013, 121, 70–89. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. Mechanism of starch adsorption on Fe–Mg–Al-bearing amphiboles. Int. J. Miner. Process. 2013, 123, 120–128. [Google Scholar] [CrossRef]
- Ray, S.K.; Maiti, C.K.; Lahiri, S.K.; Chakrabarti, N.B. Properties of silicon dioxide films deposited at low temperatures by microwave plasma enhanced decomposition of tetraethylorthosilicate. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1992, 10, 1139–1150. [Google Scholar] [CrossRef]
- Gardner, S.D.; Singamsetty, C.S.K.; Booth, G.L.; He, G.-R.; Pittman, C.U. Surface characterization of carbon fibers using angle-resolved XPS and ISS. Carbon 1995, 33, 587–595. [Google Scholar] [CrossRef]
- Fujita, K.; Oya, A.; Benoit, R.; Beguin, F. Structure and mechanical properties of methyltrimethoxysilane-treated taeniolite films. J. Mater. Sci. 1996, 31, 4609–4615. [Google Scholar] [CrossRef]





| SiO2 | Al2O3 | Na2O | K2O | MgO | Fe2O3 | CaO | Loss |
|---|---|---|---|---|---|---|---|
| 99.82 | 0.062 | 0.020 | 0.021 | 0.0062 | 0.014 | 0.0127 | 0.0441 |
| Element | Atomic Conc.% | ||||
|---|---|---|---|---|---|
| Si | O | C | N | Ca | |
| Quartz | 57.064 | 42.936 | - | - | - |
| Quartz + CaCl2 + LL | 14.256 | 46.582 | 30.757 | 8.283 | 0.122 |
| Sample | Element at.% (BE, eV) | |||
|---|---|---|---|---|
| C | O | Si | N | |
| Quartz | 16.84 (284.8) | 41.32 (531.71) | 22.72 (102.32) | - |
| - | 7.99 (532.46) | 9.72 (103.35) | - | |
| Quartz + CaCl2 + LL | 28.13 (284.8) | 6.23 (530.65) | 9.09 (102.36) | 3.66 (399.30) |
| 5.73 (286.23) | 24.36 (531.81) | 2.99 (102.67) | - | |
| 5.83 (288.49) | 8.50 (532.50) | 6.69 (103.28) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Cao, S.; Yin, W.; Fu, Y.; Li, C.; Cao, Y. Improved Quartz Flotation at Low Temperature by Amino Acid Lauryl Lysine as a Novel Green Collector. Minerals 2024, 14, 972. https://doi.org/10.3390/min14100972
Wu F, Cao S, Yin W, Fu Y, Li C, Cao Y. Improved Quartz Flotation at Low Temperature by Amino Acid Lauryl Lysine as a Novel Green Collector. Minerals. 2024; 14(10):972. https://doi.org/10.3390/min14100972
Chicago/Turabian StyleWu, Fei, Shaohang Cao, Wanzhong Yin, Yafeng Fu, Chao Li, and Yijun Cao. 2024. "Improved Quartz Flotation at Low Temperature by Amino Acid Lauryl Lysine as a Novel Green Collector" Minerals 14, no. 10: 972. https://doi.org/10.3390/min14100972








