Parametrization of Geopolymer Compressive Strength Obtained from Metakaolin Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterisation
3. Results and Discussion
3.1. XRD and FTIR Characterisation
3.2. Particle Size Distribution
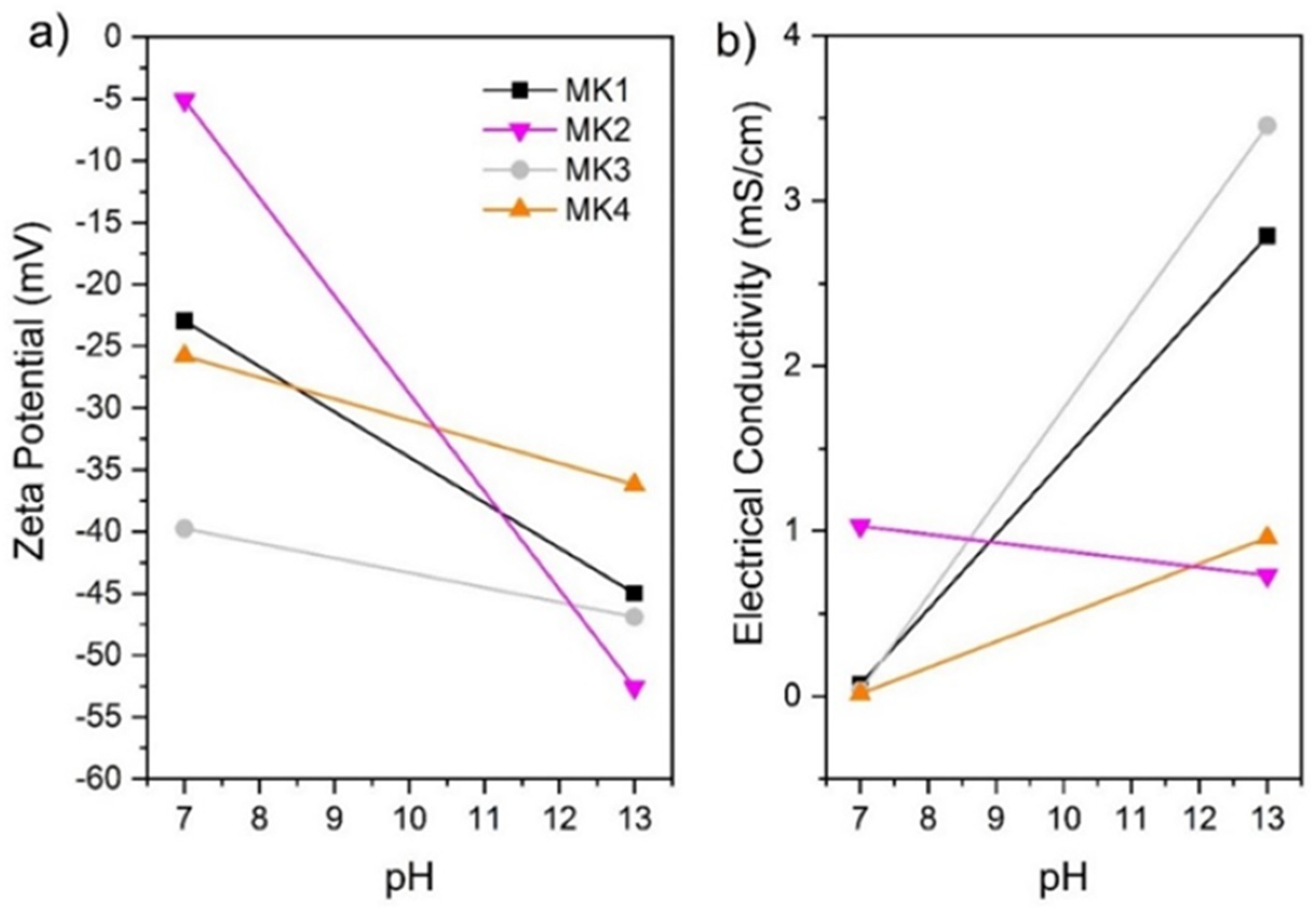
3.3. Zeta Potential (ZP) and Electrical Conductivity
3.4. Vitreous Phases Determination
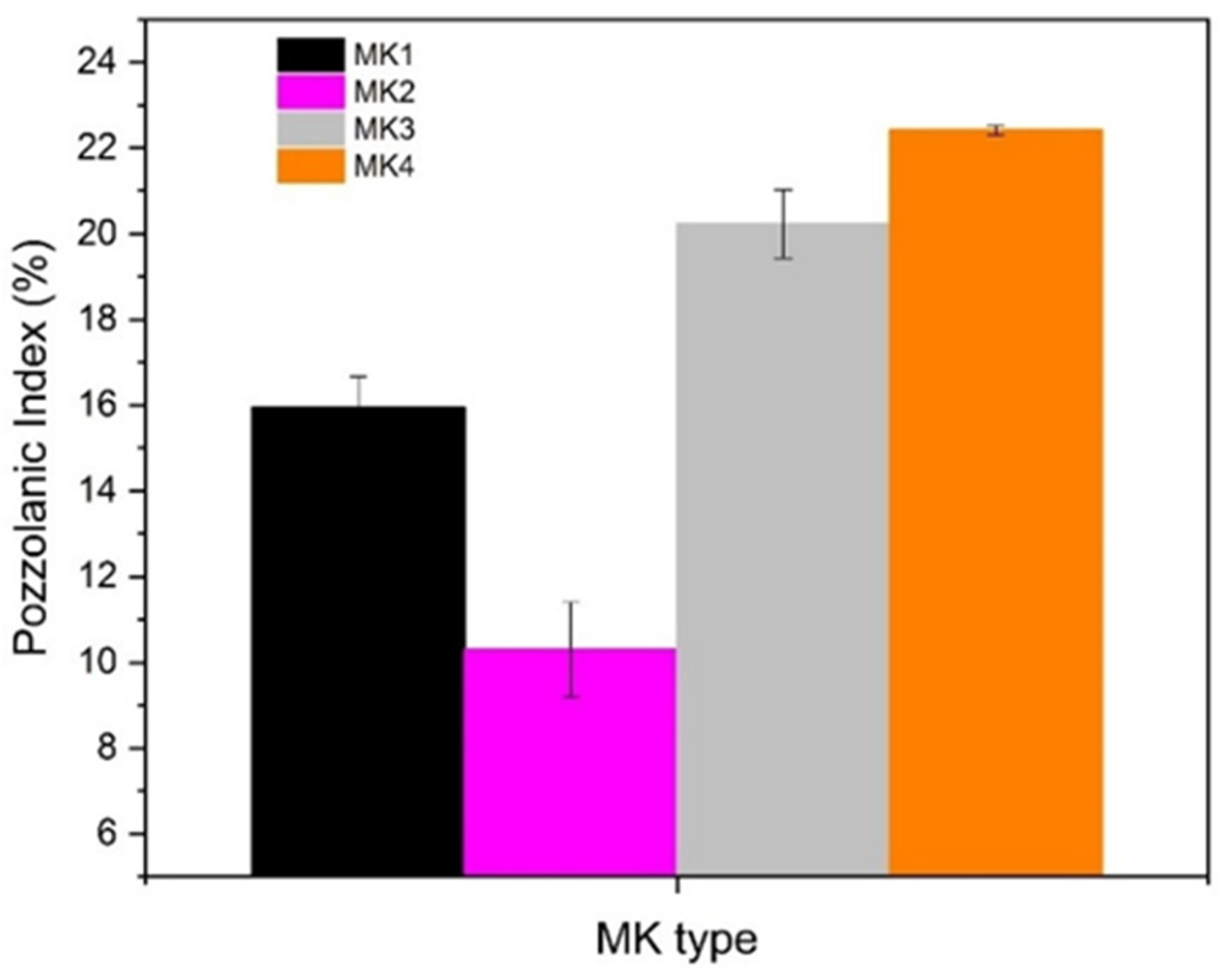
3.5. Pozzolanic Activity (PA)
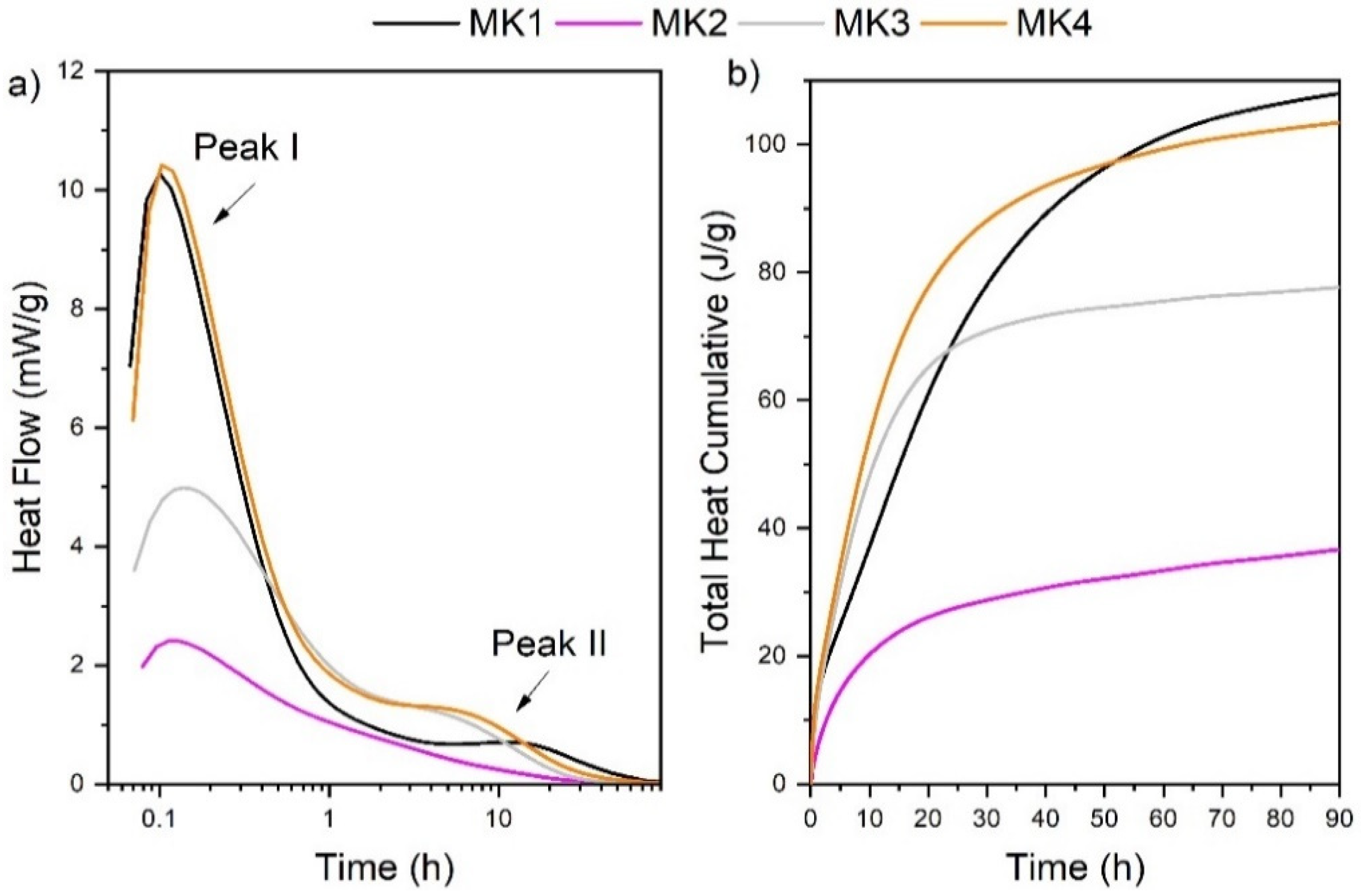
3.6. Isothermal Conduction Calorimetry (ICC)
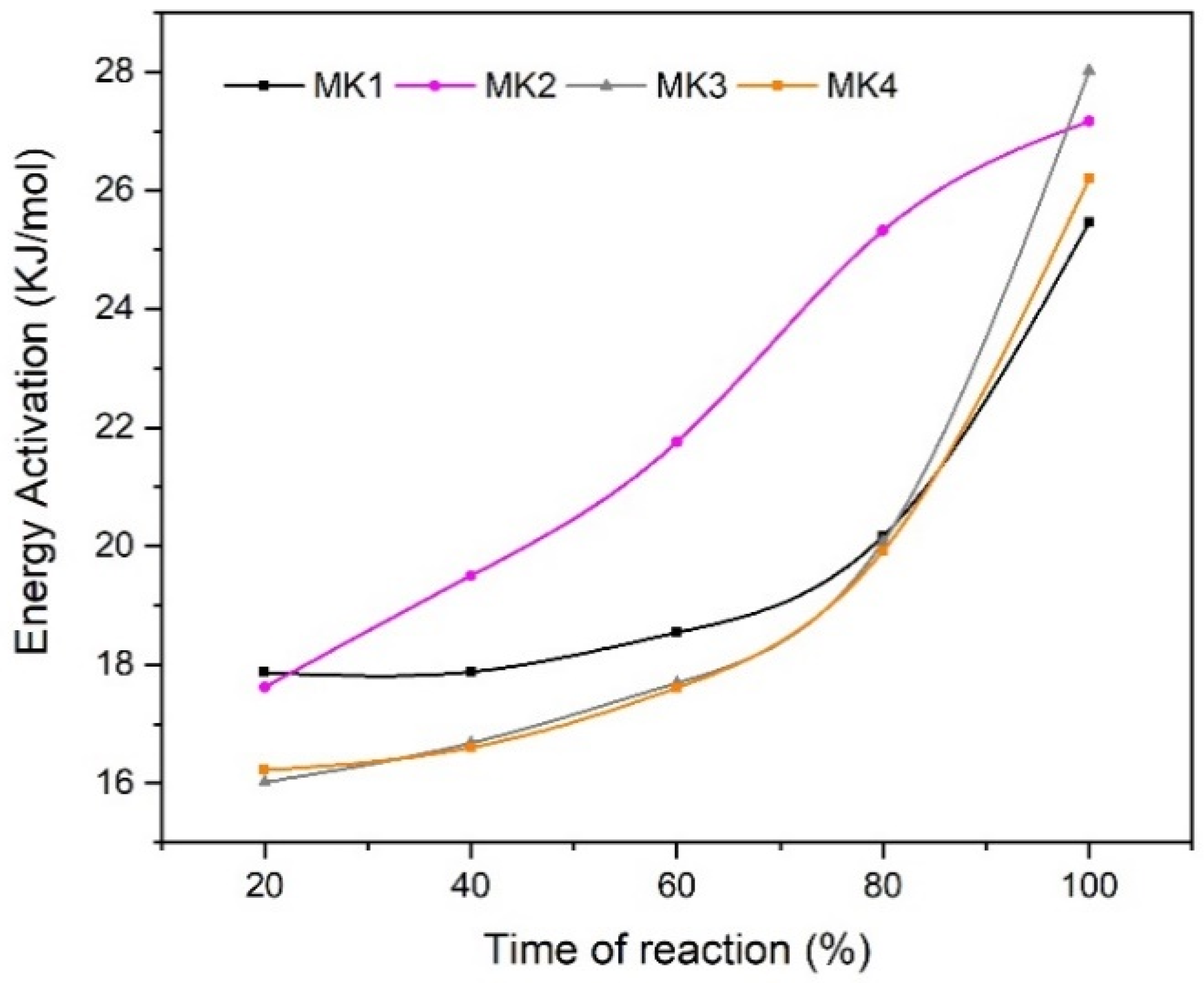
3.7. Apparent Activation Energy (EA)
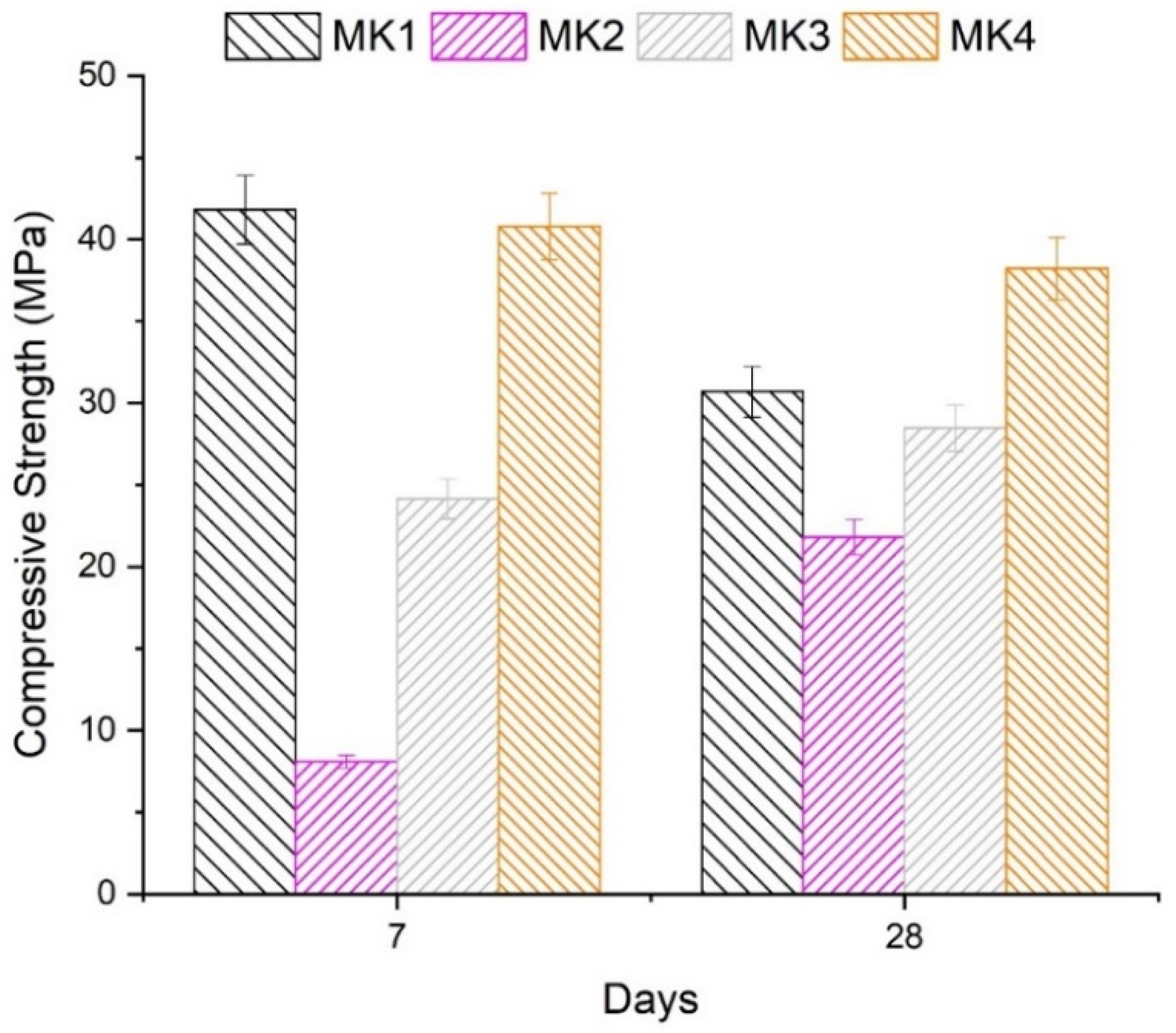
3.8. Compressive Strength
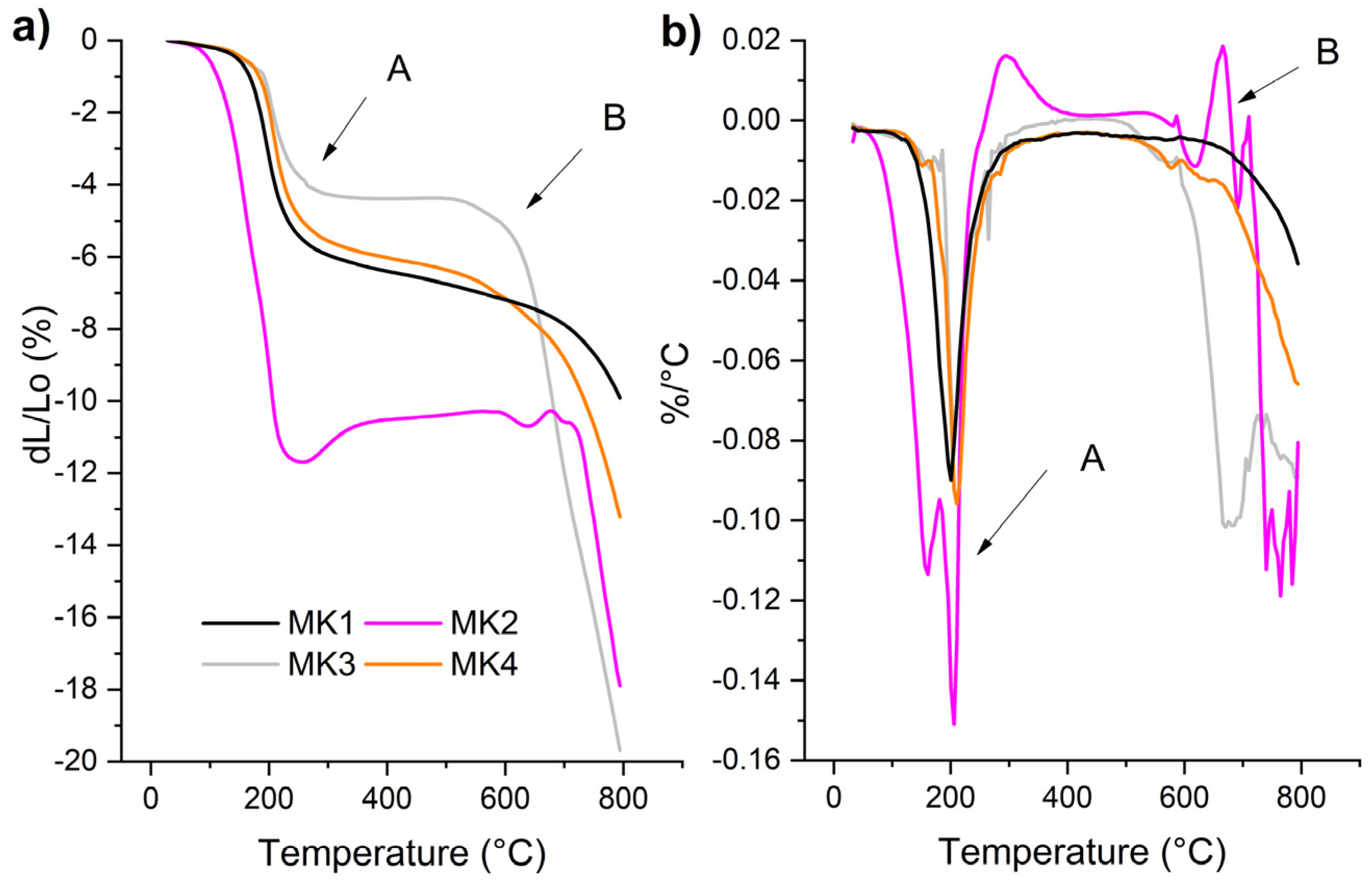
3.9. Dilatometry Analysis
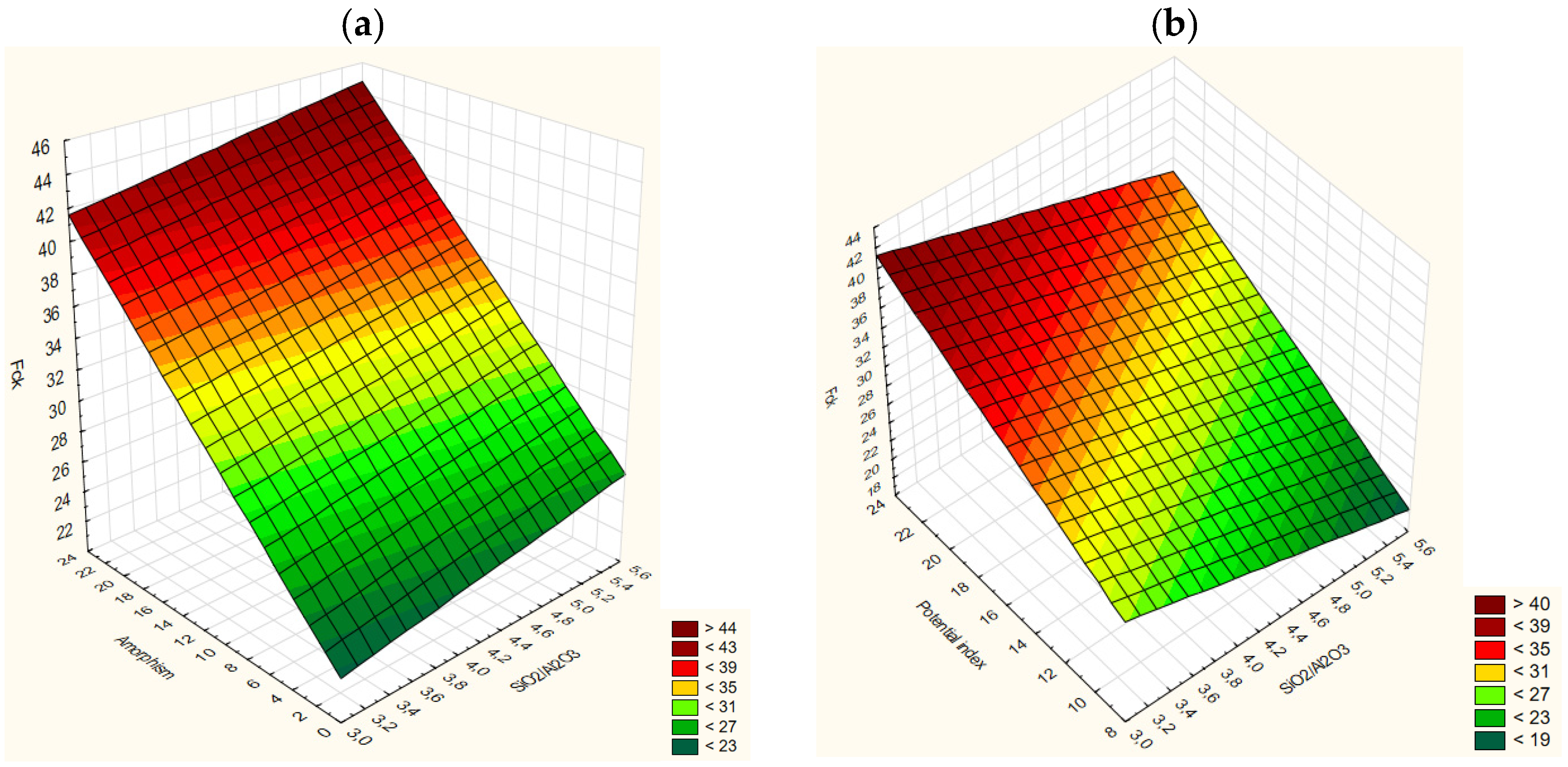
3.10. Mathematical Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 2. Reaction kinetic modeling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Yang, K.; White, C.E. Modeling the Formation of Alkali Aluminosilicate Gels at the Mesoscale Using Coarse-Grained Monte Carlo. Langmuir 2016, 32, 11580–11590. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Jalali, S. Ligantes Geopoliméricos. Uma Alternativa ao Cimento Portland? Ingenium 2010, 2, 66–68. [Google Scholar]
- Li, W.; Shumuye, E.D.; Shiying, T.; Wang, Z.; Zerfu, K. Eco-friendly fiber reinforced geopolymer concrete: A critical review on the microstructure and long-term durability properties. Case Stud. Constr. Mater. 2022, 16, e00894. [Google Scholar]
- Hildebrando, E.A.; Angélica, R.S.; Neves, R.F.; Valenzuela-Diaz, F.R. Síntese de zeólita do tipo faujasita a partir de um rejeito de caulim. Ceramica 2012, 58, 453–458. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay reactivity: Production of alkali-activated cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Brown, T.L. Chemistry. The Central Science; Pearson: Glenview, IL, USA, 2012; Volume 12. [Google Scholar]
- Li, L.; Sun, W.; Feng, Z.; Li, Y.; Feng, T.; Liu, Z. Hydration kinetics and apparent activation energy of cement pastes containing high silica fume content at lower curing temperature. Constr. Build. Mater. 2024, 435, 136881. [Google Scholar] [CrossRef]
- Joseph, S.; Uppalapati, S.; Cizer, Ö. Instantaneous activation energy of alkali-activated materials. RILEM Tech. Lett. 2018, 3, 121–123. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Pelisser, F.; Bernardin, A.M.; Michel, M.D.; da Luz, C.A. Compressive strength, modulus of elasticity and hardness of geopolymeric cement synthetized from non-calcined natural kaolin. J. Clean. Prod. 2021, 280, 124293. [Google Scholar] [CrossRef]
- Du, X.; Pang, D.; Zhao, Y.; Hou, Z.; Wang, H.; Cheng, Y. Investigation into the adsorption of CO2, N2 and CH4 on kaolinite clay. Arab. J. Chem. 2022, 15, 103665. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, Y.; Xu, P.; Zhang, M.; Sun, L. Kaolinite-based form-stable phase change materials for thermal energy storage. J. Energy Storage 2024, 87, 111349. [Google Scholar] [CrossRef]
- Rosin, N.A.; Demattê, J.A.M.; Poppiel, R.R.; Silvero, N.E.Q.; Rodriguez-Albarracin, H.S.; Rosas, J.T.F.; Greschuk, L.T.; Bellinaso, H.; Minasny, B.; Gomez, C.; et al. Mapping Brazilian soil mineralogy using proximal and remote sensing data. Geoderma 2023, 432, 116413. [Google Scholar] [CrossRef]
- Júnior, L.U.D.T.; Taborda-Barraza, M.; Cheriaf, M.; Gleize, P.J.P.; Rocha, J.C. Effect of bottom ash waste on the rheology and durability of alkali activation pastes. Case Stud. Constr. Mater. 2022, 16, e00790. [Google Scholar]
- Rocha, T.S.; Dias, D.P.; França, F.C.C.; Guerra, R.R.S.; Marques, L.R.C.O. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Neto, J.A.S.; Marçal, N.A.; Nóbrega, A.F.; Nóbrega, A.C.V.; Souza, J.J.N.; Malheiro, R. Use of metakaolin with a low surface area and rich in quartz and iron as a precursor in the production of structural alkali-activated concrete. Constr. Build. Mater. 2024, 430, 136418. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.S.; Faraj, R.H.; Qaidi, S.M.A.; Mohammed, A.A. Compressive strength of geopolymer concrete modified with nano-silica: Experimental and modeling investigations. Case Stud. Constr. Mater. 2022, 16, e01036. [Google Scholar] [CrossRef]
- Aouan, B.; Alehyen, S.; Fadil, M.; Alouani, M.E.L.; Khabbazi, A.; Atbir, A.; Taibi, M. Compressive strength optimization of metakaolin-based geopolymer by central composite design. Chem. Data Collect. 2021, 31, 100636. [Google Scholar] [CrossRef]
- Toufigh, V.; Jafari, A. Developing a comprehensive prediction model for compressive strength of fly ash-based geopolymer concrete (FAGC). Constr. Build. Mater. 2021, 277, 122241. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash. Part III: Effect of curing conditions on reaction and its graphical description. Fuel 2010, 89, 3185–3192. [Google Scholar] [CrossRef]
- Luxan, M.P.; Madruga, F.; Saavedra, J. Rapid Evaluation of Pozzolanic Activity of Natural Products. Cem. Concr. Res. 1989, 19, 63–68. [Google Scholar] [CrossRef]
- Kosmidi, D.; Panagiotopoulou, C.; Angelopoulos, P.; Taxiarchou, M. Thermal Activation of Kaolin: Effect of Kaolin Mineralogy on the Activation Process. Mater. Proc. 2021, 5, 18. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2020, 244, 118844. [Google Scholar] [CrossRef]
- Nadia, N.F.J.; Gharzouni, A.; Nait-Ali, B.; Ouamara, L.; Ndassa, I.M.; Bebga, G.; Elie, K.; Rossignol, S. Comparative study of laterite and metakaolin/hematite-based geopolymers: Effect of iron source and alkalization. Appl. Clay Sci. 2023, 233, 106824. [Google Scholar] [CrossRef]
- Juengsuwattananon, K.; Winnefeld, F.; Chindaprasirt, P.; Pimraksa, K. Correlation between initial SiO2/Al2O3, Na2O/Al2O3, Na2O/SiO2, and H2O/Na2O ratios on phase and microstructure of reaction products of metakaolin-rice husk ash geopolymer. Constr. Build. Mater. 2019, 226, 406–417. [Google Scholar] [CrossRef]
- Taborda-Barraza, M.; Padilha, F.; Silvestro, L.; Azevedo, A.R.G.; Gleize, P.J.P. Evaluation of CNTs and SiC Whiskers Effect on the Rheology and Mechanical Performance of Metakaolin-Based Geopolymers. Materials 2022, 15, 6099. [Google Scholar] [CrossRef]
- Sposito, R.; Maier, M.; Beuntner, N.; Thienel, K.C. Evaluation of zeta potential of calcined clays and time-dependent flowability of blended cements with customized polycarboxylate-based superplasticizers. Constr. Build. Mater. 2021, 308, 125061. [Google Scholar] [CrossRef]
- Peng, L.; Chen, B. Mechanical behavior, durability, thermal performances and microstructure of GGBFS—Modified MPC solidified dredged sludge. Constr. Build. Mater. 2021, 303, 124557. [Google Scholar] [CrossRef]
- Zentar, R.; Wang, H.; Wang, D. Comparative study of stabilization/solidification of dredged sediments with ordinary Portland cement and calcium sulfo-aluminate cement in the framework of valorization in road construction material. Constr. Build. Mater. 2012, 279, 122447. [Google Scholar] [CrossRef]
- Korolev, V.A.; Nesterov, D.S. Regulation of clay particles charge for design of protective electrokinetic barriers. J. Hazard. Mater. 2018, 358, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhu, Z.; Liu, R.; Chen, M.; Shao, C.; Zhang, C.; Li, X. A multi-perspective study on the influence of physical and chemical properties of 5 types of fly ash on the performance of high-volume blended fly ash cementitious slurry. Constr. Build. Mater. 2024, 411, 134301. [Google Scholar] [CrossRef]
- Siline, M.; Mehsas, B. Effect of increasing the Blaine fineness of Metakaolin on its chemical reactivity. J. Build. Eng. 2022, 56, 104778. [Google Scholar] [CrossRef]
- Tole, I.; Delogu, F.; Qoku, E.; Habermehl-Cwirzen, K.; Cwirzen, A. Enhancement of the pozzolanic activity of natural clays by mechanochemical activation. Constr. Build. Mater. 2022, 352, 128739. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta. 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Guggenheim, S.; Chang, Y.H.; Van Groos, A.F.K. Muscovite dehydroxylation: High-temperature studies. Am. Mineral. 1987, 72, 537–550. [Google Scholar]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash, and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zhu, H.; Wu, Q. Reaction kinetics and mechanical properties of a mineral-micropowder/metakaolin-based geopolymer. Ceram. Int. 2022, 48, 14173–14181. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Chen, X.; Niu, Z.; Wang, J.; Zhu, G.R.; Zhou, M. Effect of sodium polyacrylate on mechanical properties and microstructure of metakaolin-based geopolymer with different SiO2/Al2O3 ratio. Ceram. Int. 2018, 44, 18173–18180. [Google Scholar] [CrossRef]
- Kaze, C.R.; Jiofack, S.B.K.; Cengiz, Ö.; Alomayri, T.S.; Adesina, A.; Rahier, H. Reactivity and mechanical performance of geopolymer binders from metakaolin/meta-halloysite blends. Constr. Build. Mater. 2022, 336, 15–19. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Z.; Wang, D.; Lv, Y.; Gao, C.; Xue, G. Effects of alkaline activators on pore structure and mechanical properties of ultrafine metakaolin geopolymers cured at room temperature. Constr. Build. Mater. 2022, 361, 129678. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M.; Sulekar, S.; Davis, C.; Nino, J.C. Thermal properties of novel binary geopolymers based on metakaolin and alternative silica sources. Appl. Clay Sci. 2015, 118, 276–282. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniemi, J.; Ismailov, A.; Levanen, E.; Tanskanen, P.; Kinnunen, P.; Roning, J.; Illikainen, M. Thermal stability of one-part metakaolin geopolymer composites containing a high volume of spodumene tailings and glass wool. Cem. Concr. Compos. 2020, 114, 103792. [Google Scholar] [CrossRef]
- Provis, J.L.; Harrex, R.M.; Bernal, S.A.; Duxson, P.; Van Deventer, J.S.J. Dilatometry of geopolymers as a means of selecting desirable fly ash sources. J. Non. Cryst. Solids. 2012, 358, 1930–1937. [Google Scholar] [CrossRef]
- Selmani, S.; Essaidi, N.; Gouny, F.; Bouaziz, S.; Joussein, E.; Driss, A.; Sdiri, A.; Rossignol, S. Physical-chemical characterization of Tunisian clays for the synthesis of geopolymers materials. J. Afr. Earth Sci. 2015, 103, 113–120. [Google Scholar] [CrossRef]
- Muracchioli, M.; Menardi, G.; D’Agostini, M.; Franchin, G.; Colombo, P. Modeling the compressive strength of metakaolin-based geopolymers based on the statistical analysis of experimental data. Appl. Clay Sci. 2023, 242, 107020. [Google Scholar] [CrossRef]
- Ghanbari, M.; Hadian, A.M.; Nourbakhsh, A.A.; MacKenzie, K.J.D. Modeling and optimization of compressive strength and bulk density of metakaolin-based geopolymer using central composite design: A numerical and experimental study. Ceram. Int. 2017, 43, 324–335. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | CaO | Others | Blaine (m2/kg) | |
|---|---|---|---|---|---|---|---|---|
| MK1 | 53.73 | 42.20 | 0.57 | 0.11 | 2.15 | 0.11 | 1.13 | 2039.94 |
| MK2 | 63.93 | 30.94 | 1.53 | 1.56 | 0.54 | 0.14 | 1.36 | 701.68 |
| MK3 | 65.32 | 28.95 | 2.15 | 2.10 | 0.31 | 0.09 | 1.08 | 801.39 |
| MK4 | 51.28 | 38.27 | 7.18 | 1.63 | 1.04 | 0.10 | 0.50 | 1118.20 |
| Molar Ratio | MK1 | MK2 | MK3 | MK4 |
|---|---|---|---|---|
| Na2O/SiO2 | 0.18 | 0.16 | 0.16 | 0.18 |
| SiO2/Al2O3 | 3.28 | 5.04 | 5.46 | 3.51 |
| H2O/Na2O | 18.03 | 18.03 | 18.03 | 18.03 |
| Na2O/Al2O3 | 0.58 | 0.79 | 0.85 | 0.64 |
| Average EA (kJ/mol) | MK1 | MK2 | MK3 | MK4 |
| 19.98 | 22.27 | 19.70 | 19.32 |
| Sample | SiO2/Al2O3 | Specific Area (m2/kg) | %P. Index | Apparent Activation Energy (kJ/mol) | Amorphism (Points) | Fck (MPa) | Aver. Fck 28 Days |
|---|---|---|---|---|---|---|---|
| MK1 | 3.28 | 2104.11 | 15.57 | 19.98 | 8.948 | 31.68 | 30.69 |
| MK1 | 3.28 | 2013.42 | 14.10 | 19.98 | 9.143 | 32.05 | |
| MK1 | 3.28 | 2002.30 | 16.14 | 19.98 | 8.545 | 28.35 | |
| MK2 | 5.04 | 692.50 | 10.33 | 22.27 | 0.915 | 25.15 | 23.75 |
| MK2 | 5.04 | 711.39 | 10.82 | 22.27 | 1.068 | 24.05 | |
| MK2 | 5.04 | 701.14 | 10.51 | 22.27 | 0.895 | 22.05 | |
| MK3 | 5.46 | 812.90 | 20.23 | 19.70 | 1.339 | 28.25 | 28.53 |
| MK3 | 5.46 | 810.27 | 20.15 | 19.70 | 1.320 | 29.35 | |
| MK3 | 5.46 | 781.01 | 20.35 | 19.70 | 1.348 | 27.99 | |
| MK4 | 3.51 | 1120.21 | 22.42 | 19.32 | 21.658 | 36.75 | 38.20 |
| MK4 | 3.51 | 1118.41 | 22.46 | 19.32 | 21.167 | 40.11 | |
| MK4 | 3.51 | 1116.11 | 22.30 | 19.32 | 21.932 | 37.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taborda-Barraza, M.; Tambara, L.U.D., Jr.; Vieira, C.M.; de Azevedo, A.R.G.; Gleize, P.J.P. Parametrization of Geopolymer Compressive Strength Obtained from Metakaolin Properties. Minerals 2024, 14, 974. https://doi.org/10.3390/min14100974
Taborda-Barraza M, Tambara LUD Jr., Vieira CM, de Azevedo ARG, Gleize PJP. Parametrization of Geopolymer Compressive Strength Obtained from Metakaolin Properties. Minerals. 2024; 14(10):974. https://doi.org/10.3390/min14100974
Chicago/Turabian StyleTaborda-Barraza, Madeleing, Luis U. D. Tambara, Jr., Carlos M. Vieira, Afonso R. Garcez de Azevedo, and Philippe J. P. Gleize. 2024. "Parametrization of Geopolymer Compressive Strength Obtained from Metakaolin Properties" Minerals 14, no. 10: 974. https://doi.org/10.3390/min14100974








