Peak Height Comparison K-Ar Method Applied to Zao Volcano Bomb Samples Younger than ca. 35 ka
Abstract
:1. Introduction
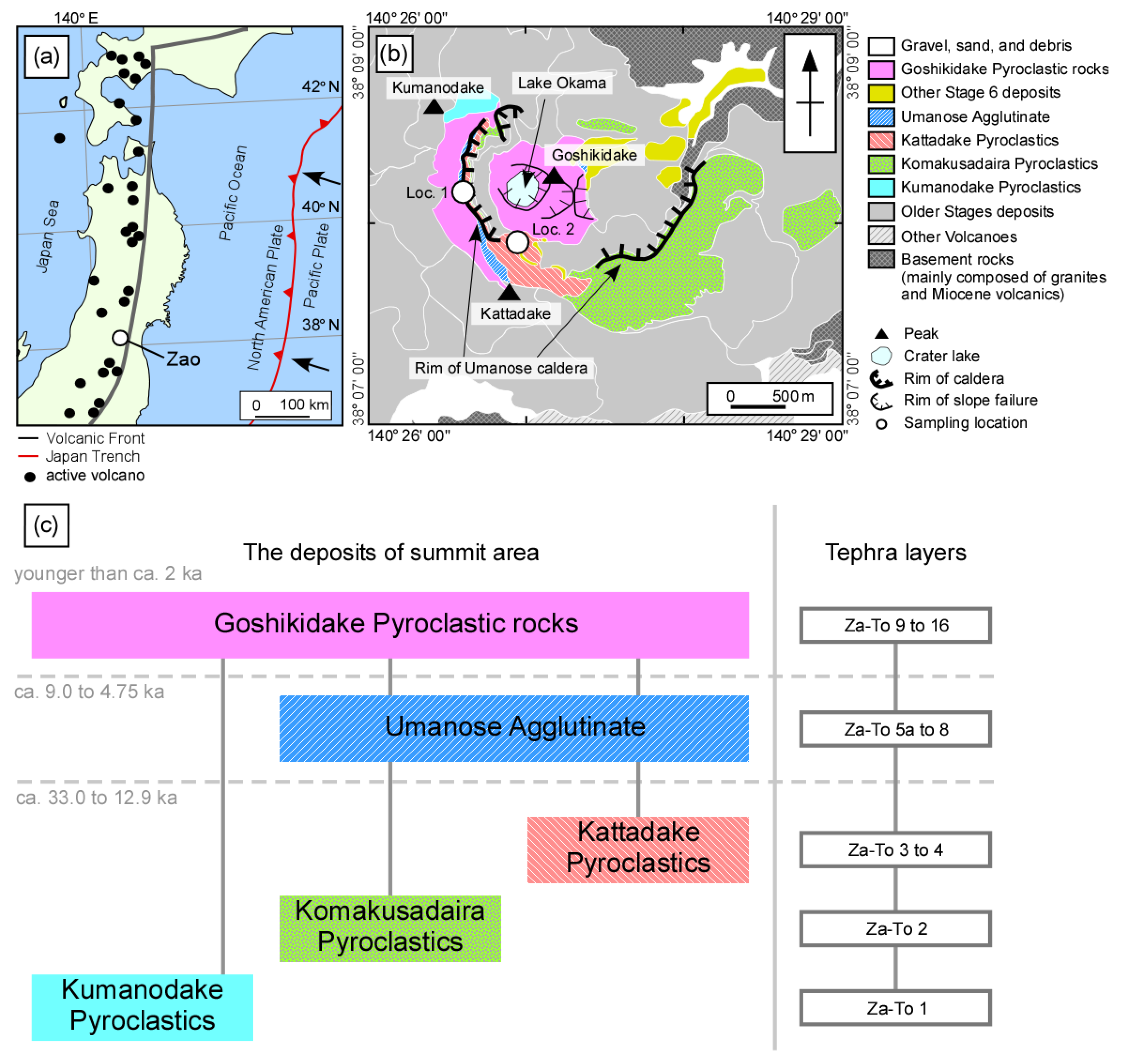
2. Geologic and Petrologic Outline of the Youngest Activity of Zao Volcano
3. Sample Descriptions
4. Materials and Methods
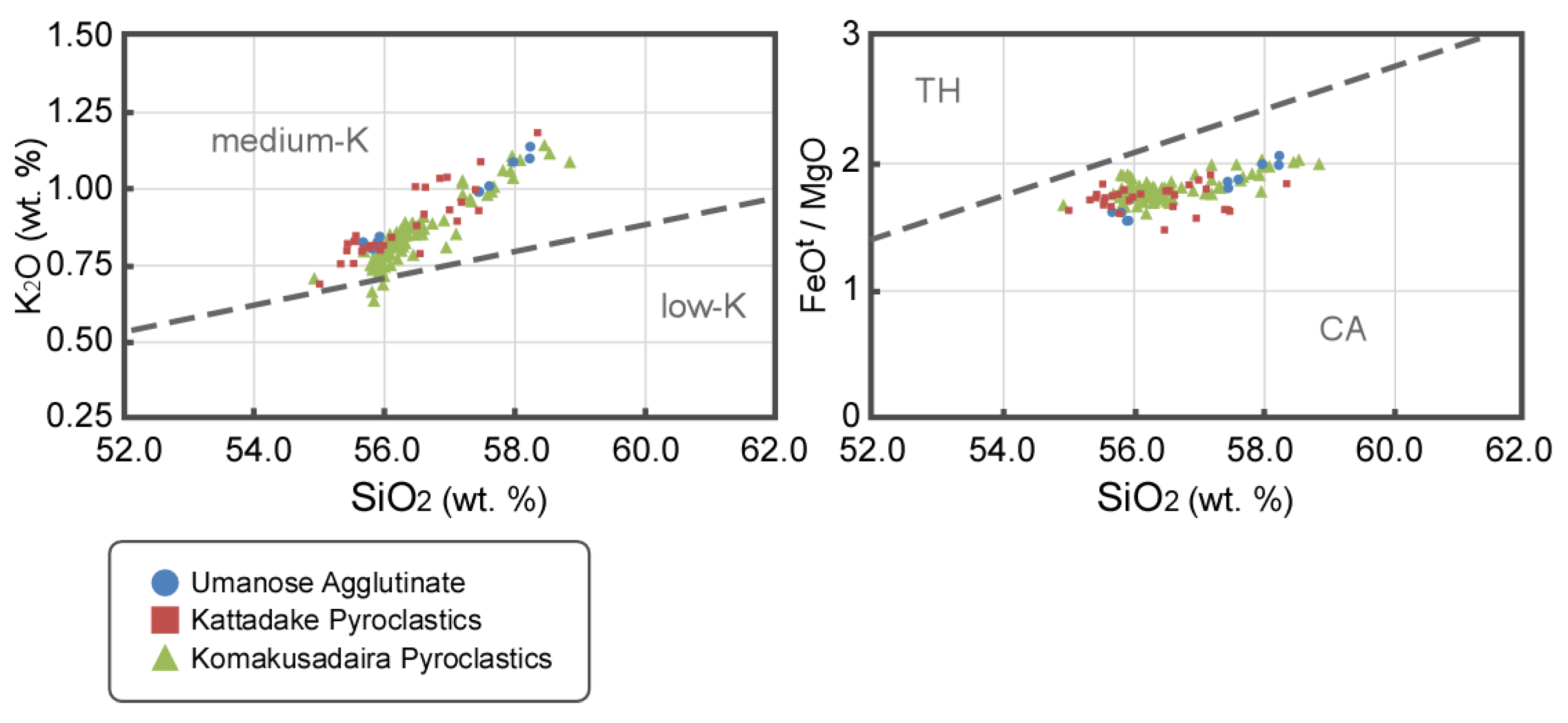
5. Results
6. Discussion
7. Conclusions
- The peak height comparison K-Ar method was applied on very young (younger than ca. 35 ka) bomb samples from Zao Volcano in order to verify the applicability of this method in such vesiculated cases.
- The errors in all the obtained ages exceed 100%, while none (zero) is involved within error range for all cases. However, the obtained data are able to constrain the upper age limits. A detailed comparison between the ages obtained in this study and those previously estimated based on 14C dating was not possible.
- The 38Ar/36Ar ratio below 0.187 obtained from Kattadake Pyroclastic samples could be attributed to the magma’s interaction with the atmospheric argon transported to the reservoir via underground water. The 38Ar/36Ar ratio exceeding 0.187 obtained from the sample from Komakusadaira Pyroclastics is unusual for young volcanic rocks.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillou, H.; Turpin, L.; Garnier, F.; Charbit, S.; Thomas, D.M. Unspiked K–Ar dating of Pleistocene tholeiitic basalts from the deep core SOH-4, Kilauea, Hawaii. Chem. Geol. 1997, 140, 81–88. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kobayashi, T. K-Ar age determination of late Quaternary volcanic rocks using the ‘mass fractionation correction procedure’: Application to the Younger Ontake Volcano, central Japan. Chem. Geol. 1995, 125, 123–135. [Google Scholar] [CrossRef]
- Miyoshi, M.; Sumino, H.; Miyabuchi, Y.; Shinmura, T.; Mori, Y.; Hasenaka, T.; Furukawa, K.; Uno, K.; Nagao, K. K-Ar ages determined for post-caldera volcanic products from Aso volcano, central Kyushu, Japan. J. Volcanol. Geotherm. Res. 2012, 229–230, 64–73. [Google Scholar] [CrossRef]
- Shimura, T.; Miyoshi, M.; Sumino, H.; Ueda, Y.; Mori, Y.; Hasenaka, T.; Arakawa, Y.; Nagao, K. K-Ar ages of volcanic rocks from Nekodake Volcano in the eastern part of Aso Caldera, central Kyushu, Japan. Bull. Volcanol. Soc. Jpn. 2022, 67, 135–147, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Ban, M.; Oikawa, N.; Yamazaki, S. Geological map of Zao volcano. In Geological Survey of Japan; National Institute of Advanced Industrial Science and Technology (AIST): Tsukuba, Japan, 2015; (In Japanese with English Abstract). [Google Scholar]
- Itagaki, N.; Toyoshima, M.; Terado, T. The scoria bed of late Pleistocene in Sendai and its environs. Ann. Tohoku Geogr. Assoc. 1981, 33, 48–53, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Miura, K.; Ban, M.; Yagi, K. The tephra layers distributed around the eastern foot of the Zao volcano: Ages and volumes of the Za-To 1 to 4 tephras. Bull. Volcanol. Soc. Jpn. 2008, 53, 151–157. [Google Scholar] [CrossRef]
- Ban, M.; Oikawa, T.; Imura, T.; Tsunematsu, K.; Jin, H.; Takahashi, Y. An Example of the Magma Discharge Step-diagram: Zao Volcano. Tech. Note Natl. Res. Inst. Earth Sci. Disaster Resil. 2024, 500, 37–43, (In Japanese with English Abstract). [Google Scholar]
- Sakayori, A. Magmatic evolution of Zao volcano, Northeast Japan. Bull. Volcanol. Soc. Jpn. 1991, 36, 79–92, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Sakayori, A. Geology and petrology of Zao volcano, Northeast Japan. J. Mineral. Petrol. Econ. Geol. 1992, 87, 433–444, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Takebe, Y.; Ban, M. Evolution of magma feeding system in Kumanodake agglutinate activity, Zao Volcano, northeastern Japan. J. Volcanol. Geotherm. Res. 2015, 304, 62–74. [Google Scholar] [CrossRef]
- Takebe, M.; Ban, M.; Nishi, Y. Evolution of the calc-alkaline magma feeding system of the Komakusadaira pyroclastics in Zao volcano, NE Japan. Bull. Volcanol. 2021, 83, 12. [Google Scholar] [CrossRef]
- Takebe, M.; Ban, M.; Sato, M.; Nishi, Y. The Temporal Variation of Magma Plumbing System of the Kattadake Pyroclastics in the Zao Volcano, Northeastern Japan. Minerals 2021, 11, 430. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Ban, M.; Imura, T.; Takebe, M. Comparison of geologic and petrologic features between a pyroclastic unit in summit area and a tephra layer in eastern foot of early youngest stage of Zao volcano, NE Japan. In Proceedings of the Abstract Annual Meeting Bull. Volcanological Society Japan, Kagoshima, Japan, 18–20 October 2023. (In Japanese). [Google Scholar] [CrossRef]
- Ban, M.; Takebe, Y.; Adachi, T.; Matsui, R.; Nishi, Y. Eruption Histories of Zao and Azuma Volcanoes and Their Magma Feeding Systems for Recent Activities. Bull. Earthq. Res. Inst. Univ. Tokyo 2016, 91, 25–39. [Google Scholar] [CrossRef]
- Nishi, Y.; Ban, M.; Takebe, M.; Álvarez-Valero, A.M.; Oikawa, T.; Yamasaki, S. Structure of the shallow magma chamber of the active volcano Mt. Zao, NE Japan: Implications for its eruptive time scales. J. Volcanol. Geotherm. Res. 2019, 371, 137–161. [Google Scholar] [CrossRef]
- Sato, M.; Ban, M.; Yuguchi, T.; Adachi, T. Pre-eruptive magmatic processes of historical activities at Zao volcano, northeastern Japan: Insights from compositional zoning in orthopyroxene phenocrysts. J. Volcanol. Geotherm. Res. 2022, 432, 107686. [Google Scholar] [CrossRef]
- Tsuchiyama, A. Dissolution kinetics of plagioclase in the melt of the system diopside-albite-anorthite, and origin of dusty plagioclase in andesites. Contrib. Mineral. Petrol. 1985, 89, 137–161. [Google Scholar] [CrossRef]
- Ban, M.; Sagawa, H.; Miura, K.; Hirotani, S. Evidence for short-lived stratified magma chamber: Petrology of Z-To tephra layer (~5.8ka) at Zao volcano, NE Japan. In Dynamics of Crustal Magma Transfer, Storage, and Differentiation Integrating Geochemical and Geophysical Constraints; Special Publication; Zellmer, G., Annen, C., Eds.; The Geological Society of London: London, UK, 2005; Volume 304, pp. 149–168. [Google Scholar] [CrossRef]
- Gill, J.B. Orogenic Andesites and Plate Tectonics; Springer: Berlin/Heidelberg, Germany, 1981; pp. 1–392. [Google Scholar] [CrossRef]
- Miyashiro, A. Volcanic rock series in island arcs and active continental margins. Am. J. Sci. 1974, 274, 321–355. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Sato, K.; Tamura, H.; Nishio, Y.; Kumagai, H.; Hanyu, T. Application of K-Ar Dating Performed by New Technique of Ultra Low Potassium Analysis with New Zeeman Atomic Absorption Photometry in Clean Room Laboratory. Frontier Research on Earth Evolution 2, IFREE Report for 2003–2004. 2005, Volume 2, pp. 1–6. Available online: https://www.researchgate.net/profile/Hidenori-Kumagai/publication/267689576_Application_of_K-Ar_dating_performed_by_new_technique_of_ultra_low_potassium_analysis_with_new_Zeeman_atomic_absorption_photometry_in_clean_room_laboratory/links/5467f7a40cf20dedafcf52d4/Application-of-K-Ar-dating-performed-by-new-technique-of-ultra-low-potassium-analysis-with-new-Zeeman-atomic-absorption-photometry-in-clean-room-laboratory.pdf (accessed on 25 September 2024).
- Sato, K.; Tamura, H.; Kumagai, H.; Hanyu, T. Application of K–Ar Dating System to Be Performed by New Noble Gas Mass Spectrometry and Its Calibration from Standard Air Analysis. Frontier Research on Earth Evolution, IFREE Report 2003–2004. 2005, Volume 2, pp. 1–6. Available online: https://d1wqtxts1xzle7.cloudfront.net/70816935/Application_of_K-Ar_dating_system_to_be_20210930-2558-108m2ff.pdf?1633050261=&response-content-disposition=inline%3B+filename%3DApplication_of_K_Ar_dating_system_to_be.pdf&Expires=1727575826&Signature=Zen~lR2WECdK1-DTAXaLnFJmgLOKjb9BOQh~BeTxeOC~3DaPitlb3QGwlx0xPaWbNYIVgycOHY2ykw823AQxHGWzye0mDqEGOMCJxgqbqs23b4WLw9wIMB92CJ2kplMX7b4n-5b15oKiFLl0VsdOZfwHH4rNROu827VeR6SNNzOEwe5jkCj-EUYhfcXs8dfmMQAHmf7oBWcXfBQF0l5Y71ozD1yo0~JS1NP7G4QdqQUGKWxdJ4d-NOHlE9t2yZpX~eENdHhHVZmKAaDQ1JPSB6twsuxnZFh1XTrYTzvvayikOnxPR4YR0R-QWpnV9duOBB3kHSQQIY58wX9uLAlQ0w__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 25 September 2024).
- Takaoka, N.; Konno, K.; Oba, Y.; Konda, T. K-Ar dating lavas from Zao Volcano, North-eastern Japan. J. Geol. Soc. Jpn. 1989, 95, 157–170, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Sudo, M.; Tagami, T.; Sato, K.; Hasebe, N.; Nishimura, S. Calibration of a new Ar analytical system for the K-Ar dating method and analytical results of K-Ar age known samples. Mem. Fac. Sci. Kyoto Univ. Ser. Geol. Mineral. 1996, 58, 21–40. Available online: http://hdl.handle.net/2433/186680 (accessed on 25 September 2024).
- Sudo, M.; Uto, K.; Anno, K.; Ishizuka, O.; Uchimi, S. SORI93 biotite: A new mineral standard for K-Ar dating. Geochem. J. 1998, 32, 49–58. [Google Scholar] [CrossRef]
- Imai, N.; Terashima, S.; Itoh, S.; Ando, A. 1994 compilation of analytical data for minor and trace elements in seventeen gsj geochemical reference samples, “igneous rock series”. Geostand. Newsl. 1995, 19, 135–213. [Google Scholar] [CrossRef]
- Steiger, R.H.; Jäger, E. Subcommission on geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth Planet. Sci. Lett. 1977, 36, 359–362. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sawada, R.; Ozawa, A.; Tagami, T.; Watanabe, Y.; Takahashi, E. Unspiked K–Ar dating of Koolau lavas, Hawaii: Evaluation of the influence of weathering/alteration on age determinations. Chem. Geol. 2011, 287, 41–53. [Google Scholar] [CrossRef]
- Ozawa, A.; Tagami, T.; Garcia, M.O. Unspiked K–Ar dating of the Honolulu rejuvenated and Ko‘olau shield volcanism on O‘ahu, Hawai‘i. Earth Planet. Sci. Lett. 2005, 232, 1–11. [Google Scholar] [CrossRef]
- Nier, A.O. Redetermination of the relative abundances of the isotopes of carbon, nitrogen, oxygen, argon, and potassium. Phys. Rev. 1950, 77, 789. [Google Scholar] [CrossRef]
- Matsumoto, A.; Uno, K.; Shibata, K. Argon isotopic ratios in historic lavas-Importance of correction for the initial argon in K-Ar dating of young volcanic rocks. J. Mass. Spectrom. Soc. Jpn. 1989, 37, 353–363, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Kaneoka, I. Noble gas signatures of magmatic sources and processes. Geochem. J. 1993, 27, 201–211, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Ozawa, A.; Tagami, T.; Kamata, H. Argon isotopic composition of some Hawaiian historical lavas. Chem. Geol. 2006, 226, 66–72. [Google Scholar] [CrossRef]
- Ryu, S.; Kitagawa, H.; Nakamura, E.; Itaya, T.; Watanabe, K. K–Ar analyses of the post-caldera lavas of Bratan volcano in Bali Island, Indonesia—Ar isotope mass fractionation to light isotope enrichment. J. Volcanol. Geotherm. Res. 2013, 264, 107–116. [Google Scholar] [CrossRef]
- Itaya, T.; Nagao, K. K-Ar age determination of volcanic rocks younger than 1 Ma. Mem. Geol. Soc. Jpn. 1988, 29, 143–161, (In Japanese with English Abstract). Available online: http://dl.ndl.go.jp/info:ndljp/pid/10809696 (accessed on 25 September 2024).
- Itaya, T.; Okada, T.; Onoe, T.; Isshiki, N. K-Ar ages of the Middle Pleistocene Takahara Volcano, central Japan, and argon release processes in cooling lava. J. Mass Spectrom. Soc. Jpn. 1989, 37, 365–374. [Google Scholar] [CrossRef]
- Kaneoka, I. The effect of water on noble gas signatures of volcanic materials. In Noble Gas Geochemistry and Cosmochemistry; Matsuda, J., Ed.; TERRAPUB: Tokyo, Japan, 1994; pp. 205–215. [Google Scholar]
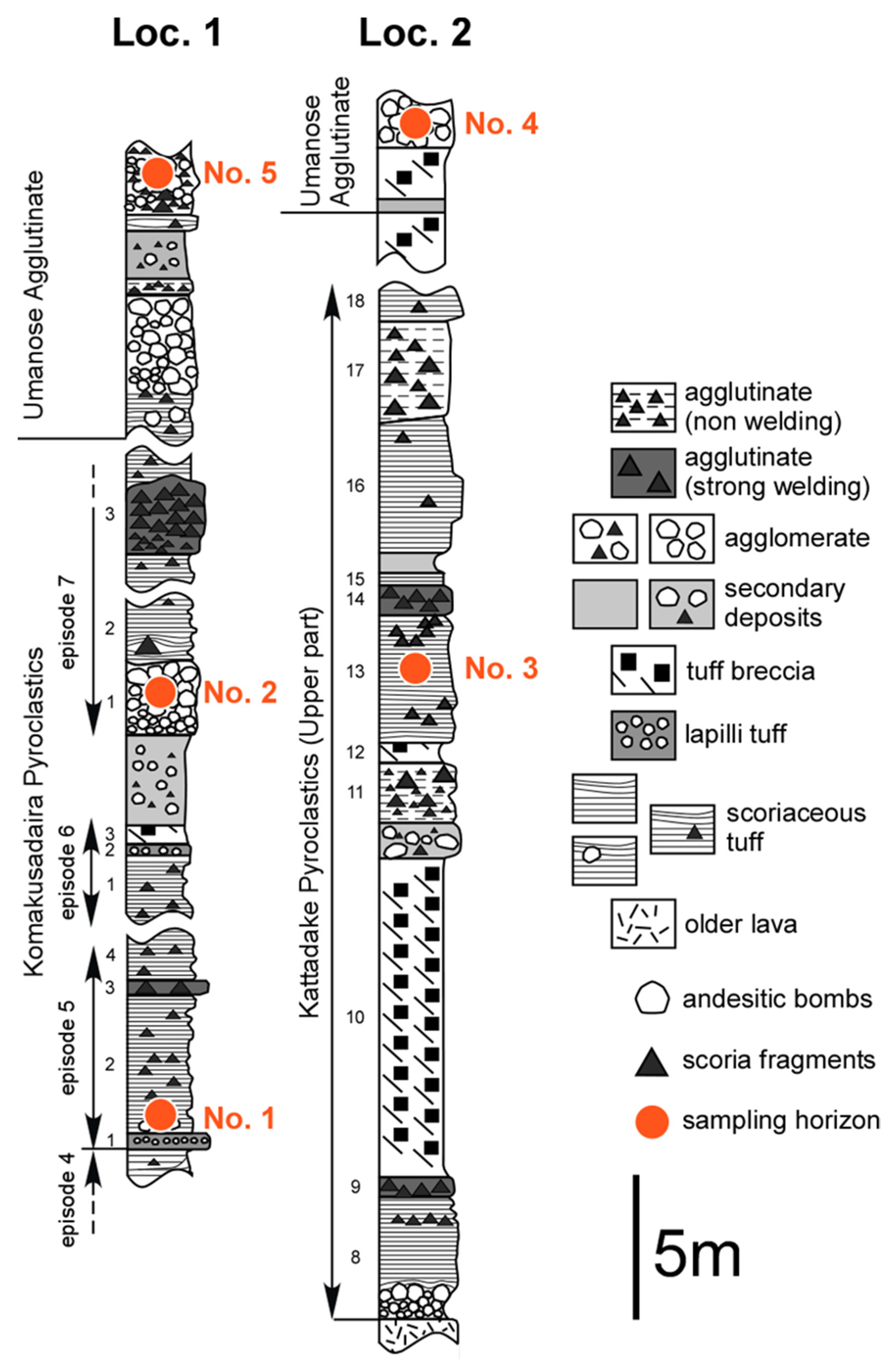




| Sample Name | Measurement No. | Formation | Layer or Bed No. | Location | Sample | Bubble (vol.%) | Rock Type | |
|---|---|---|---|---|---|---|---|---|
| Longitude (N) | Latitude (E) | |||||||
| 5 | 5-1 | Umanose Agglutinate | - | 38°08′24.6″ | 140°26′38.2″ | moderately vesiculated bomb | 17.6 | cpx-opx andesite |
| 4 | 4-1 and 4-2 | Umanose Agglutinate | - | 38°07′55.9″ | 140°26′49.9″ | weakly vesiculated bomb | <0.1 | cpx-opx andesite |
| 3 | 3-1 | Kattadake Pyroclastics | bed 13 | 38°07′55.8″ | 140°27′02.0″ | highly vesiculated bomb | 38.4 | cpx-opx basaltic andesite |
| 2 | 2-1 and 2-2 | Komakusadaira Pyroclastics | layer 1 of episode 7 | 38°08′12.9″ | 140°26′37.2″ | weakly vesiculated bomb | 9.8 | ol-cpx-opx basaltic andesite |
| 1 | 1-1 | Komakusadaira Pyroclastics | layer 2 of episode 5 | 38°08′14.7″ | 140°26′37.7″ | weakly vesiculated bomb | 0.2 | ol-cpx-opx andesite |
| Unit Name | No. | wt. (g) | K2O (wt.%) | 40Ar/36Ar (Total) | 38Ar/36Ar | 40Ar rad. (×10−9 cc/g) | Air con. (%) | 36Ar (×10−9 cc/g) | Applied 40Ar/36Ar Initial | Corrected Age (ka) |
|---|---|---|---|---|---|---|---|---|---|---|
| Umanose | 5-1 | 0.2898 | 1.28 ± 0.03 | 296.0 ± 1.2 | 0.1869 ± 0.0007 | 0.72 ± 3.28 | 99.8 | 1.22 ± 5.56 | 295.4 ± 2.4 | 17.3 ± 79.3 |
| 4-1 | 0.2781 | 1.39 ± 0.03 | 295.7 ± 1.2 | 0.1869 ± 0.0008 | 0.24 ± 2.01 | 99.9 | 0.72 ± 6.10 | 295.4 ± 2.5 | 5.3 ± 44.9 | |
| 4-2 | 0.2968 | 1.39 ± 0.03 | 295.8 ± 0.9 | 0.1867 ± 0.0006 | 0.25 ± 0.64 | 99.7 | 0.31 ± 0.79 | 295.0 ± 1.9 | 5.7 ± 14.3 | |
| Kattadake | 3-1 | 0.1649 | 1.33 ± 0.03 | 290.3 ± 0.9 | 0.1850 ± 0.0006 | 0.54 ± 2.01 | 99.8 | 0.92 ± 3.39 | 289.7 ± 2.0 | 12.6 ± 46.7 |
| Komakusadaira | 2-1 | 0.1777 | 1.50 ± 0.03 | 301.6 ± 1.9 | 0.1880 ± 0.0013 | 1.81 ± 3.15 | 99.1 | 0.68 ± 1.18 | 298.9 ± 4.2 | 37.4 ± 64.8 |
| 2-2 | 0.1968 | 1.50 ± 0.03 | 298.5 ± 2.1 | 0.1870 ± 0.0014 | 1.60 ± 2.81 | 99.1 | 0.57 ± 0.99 | 295.7 ± 4.5 | 33.0 ± 58.0 | |
| 1-1 | 0.1651 | 1.48 ± 0.03 | 300.6 ± 1.0 | 0.1878 ± 0.0007 | 1.43 ± 1.54 | 99.2 | 0.62 ± 0.66 | 298.3 ± 2.3 | 30.0 ± 32.2 | |
| Standard sample | YZ1.7 | 0.0736 | 1.69 ± 0.03 | 312.0 ± 1.7 | 0.1850 ± 0.0011 | 12.3 ±1.19 | 94.7 | 0.75 ± 0.07 | 295.5 * | 225.4 ± 22.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takebe, M.; Sato, K.; Kumagai, H.; Iwata, N.; Ban, M. Peak Height Comparison K-Ar Method Applied to Zao Volcano Bomb Samples Younger than ca. 35 ka. Minerals 2024, 14, 979. https://doi.org/10.3390/min14100979
Takebe M, Sato K, Kumagai H, Iwata N, Ban M. Peak Height Comparison K-Ar Method Applied to Zao Volcano Bomb Samples Younger than ca. 35 ka. Minerals. 2024; 14(10):979. https://doi.org/10.3390/min14100979
Chicago/Turabian StyleTakebe, Mirai, Keiko Sato, Hidenori Kumagai, Naoyoshi Iwata, and Masao Ban. 2024. "Peak Height Comparison K-Ar Method Applied to Zao Volcano Bomb Samples Younger than ca. 35 ka" Minerals 14, no. 10: 979. https://doi.org/10.3390/min14100979








