The Ore-Forming Process of Washan Porphyrite Iron Deposits in the Ningwu District Associated with Iron Oxide Apatite (IOA) Deposits and Iron Oxide Copper Gold (IOCG) Deposits
Abstract
:1. Introduction
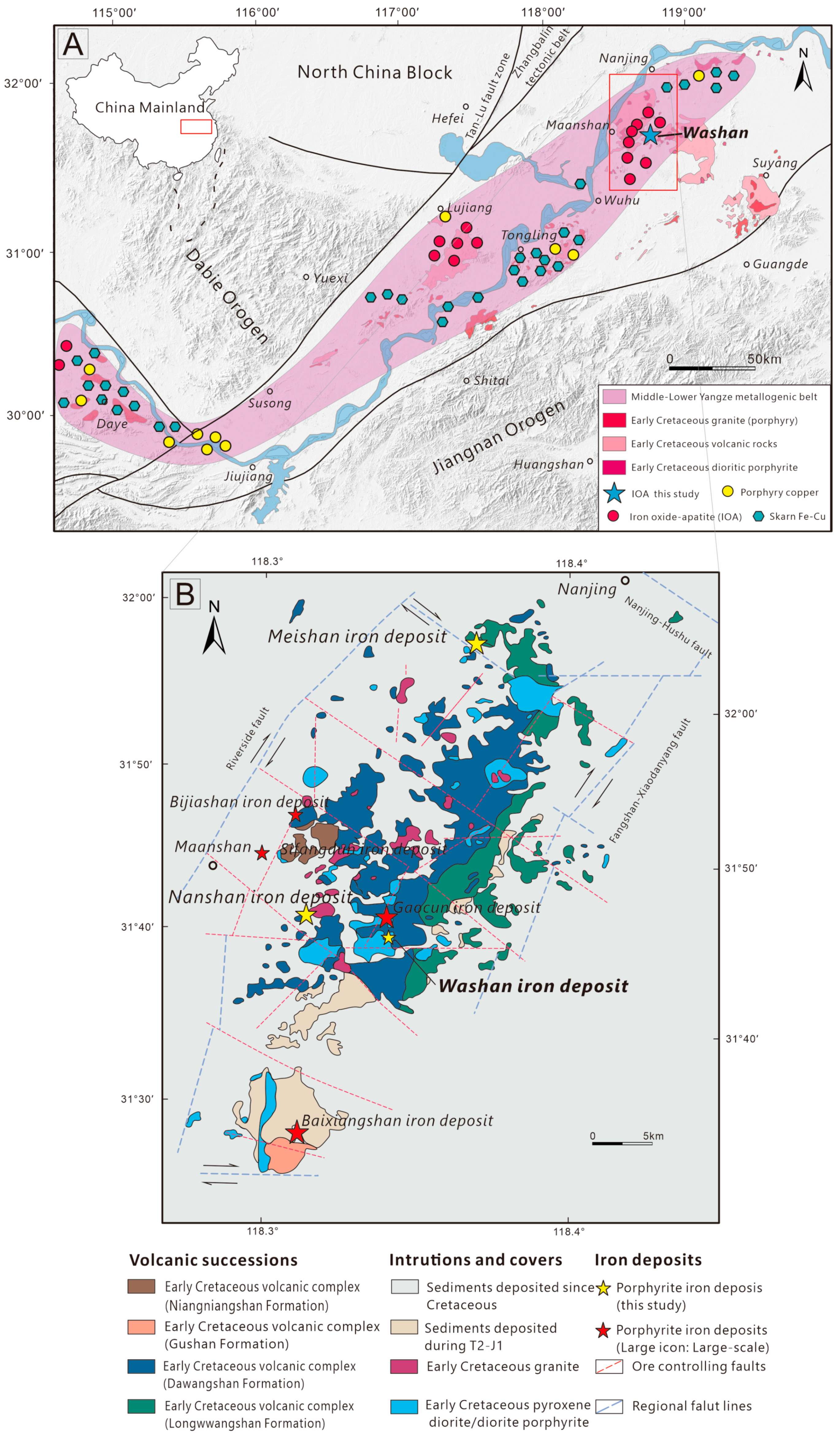
2. Geological Setting
2.1. Regional Geology
2.2. Iron Deposit Geology
3. Sample Selection and Analytical Methods
3.1. Sample Selection and Preparation for Analysis
3.2. EPMA Analysis with Associated SEM Photographing and WDS Mapping
3.3. Temperature Estimations
4. Results
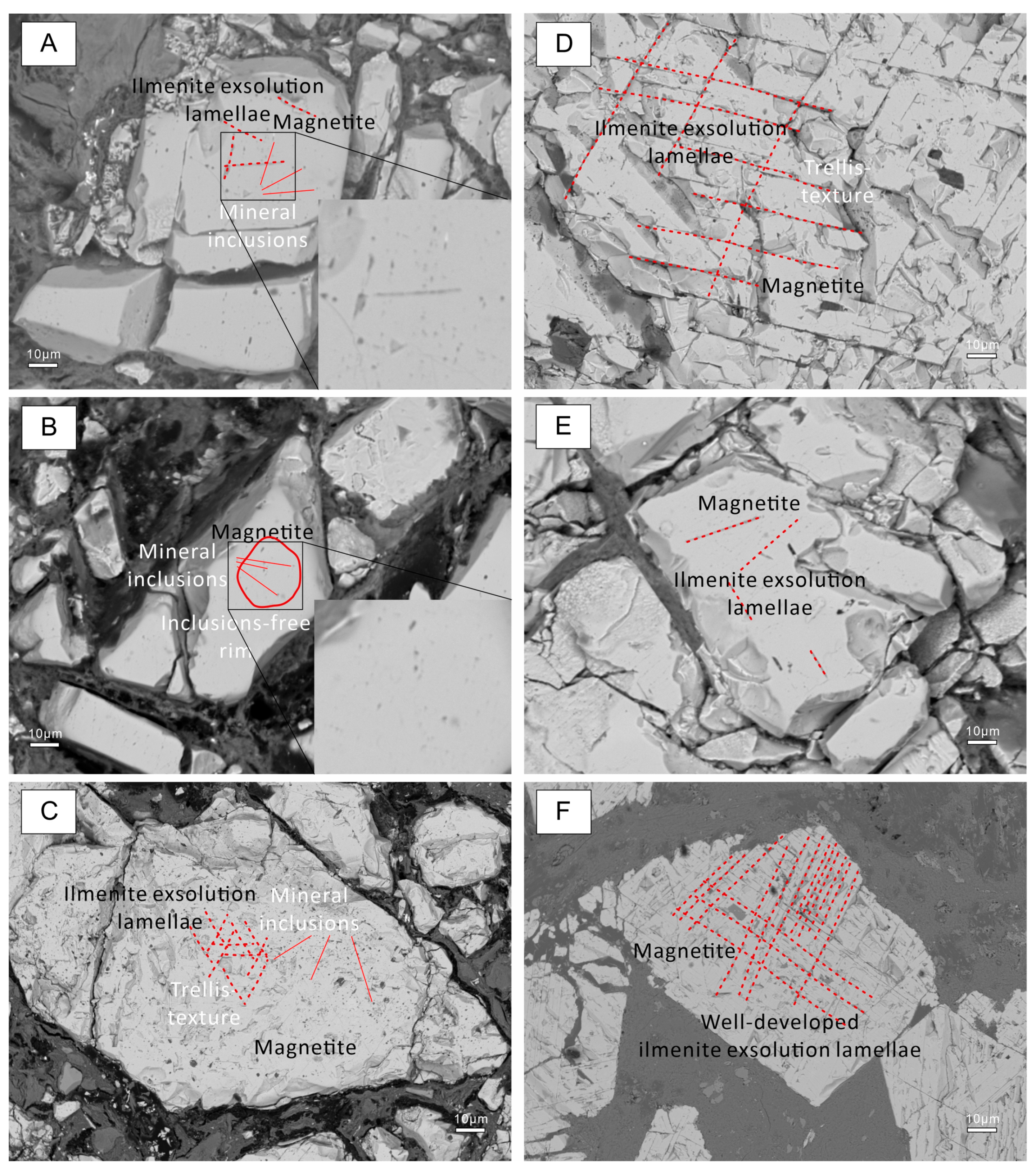
4.1. Magnetite Textures
4.2. Major Elements of Magnetite
4.3. Calculated Crystallization Temperatures of Magnetite
5. Discussion
5.1. A Transitional Mineralizing Process Based on Textural Features of Magnetites from Different Generations
5.2. Evolution of Magnetites from Different Generations
5.2.1. The Thermal Evolution of Magnetites
5.2.2. The Reconstruction of the Ore-Forming Process Based on the Thermal Features of Magnetites
6. Conclusions
- The ore magma-filled-type mineralization, relating to iron-rich high-salinity fluids, is a viable mechanism in the formation of high-Ti magnetites with porphyry genesis within the vein-style ore bodies filling in deep fractures in Washan.
- As temperatures drop, the mineralization evolution processes may split into two paths. The high-salinity fluids, originating from ore magma-filled-type ore bodies, generated medium-Ti magmatic–hydrothermal magnetites of IOA genesis. Decreased-salinity fluids can pass through cracks and react with rocks, causing the precipitation of low-Ti hydrothermal magnetites.
- The crystallization of magnetite from different generations connects the deep ore magma-filled-type mineralization and subaerial iron ores with IOA- and IOCG-like geneses in Washan.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ningwu Research Group. Ningwu Porphyrite Iron Deposit; Geoloigcal Publishing House: Beijing, China, 1978. [Google Scholar]
- Mao, J.W.; Yu, J.J.; Yuan, S.D.; Chen, Y.B.; Yang, Z.X. Iron Oxide-Copper-Gold Deposits: Characteristics, Present Research Situation and Ore Prospecting. Miner. Depos. 2008, 27, 267–278. [Google Scholar]
- Zhang, Y.; Guo, K.; Zeng, Y.; Chen, G.; Song, S. Comparation between Meishan Iron Deposit and IOCG Type Deposit in the Northern Part of Ningwu Basin. Miner. Depos. 2012, 31, 461–462. [Google Scholar]
- Zeng, L.; Zhao, X. Apatite Mineralogy and Chemistry of the Taocun Magnetite-Apatite Deposit in Ningwu Volcanic Basin: Implications for Ore Genesis of IOA Deposits. Acta Geol. Sin. Engl. Ed. 2014, 88, 1493. [Google Scholar] [CrossRef]
- Duan, C.; Li, Y.; Mao, J.; Wang, C.; Li, W. Study on the Ore-Forming Process of the Heshangqiao IOA Deposit in the Ningwu Ore District: Insight from Magnetite LA-ICP-MS in-Situ Analysis Data. Acta Petrol. Sin. 2017, 33, 3471–3483. [Google Scholar]
- Liu, Y.; Fan, Y.; Zhou, T.; White, N.C.; Hong, H.; Zhang, W.; Zhang, L. In-Situ LA-ICP-MS Trace Element Analysis of Magnetite from Mesozoic Iron Oxide Apatite (IOA) Deposits in the Luzong Volcanic Basin, Eastern China. J. Asian Earth Sci. 2018, 166, 233–246. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.; Zhou, T.; Fu, B.; Ireland, T.R.; Wang, J.; Zhang, L. Hydrothermal Fluid Characteristics and Implications of the Makou IOA Deposit in Luzong Basin, Eastern China. Ore Geol. Rev. 2020, 127, 103867. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, L.; Liao, W.; Li, W.; Hu, H.; Li, J. An Overview of Recent Advances in Porphyrite Iron (Iron Oxide-Apatite, IOA) Deposits in the Middle-Lower Yangtze River Valley Metallogenic Belt and Its Implication for Ore Genesis. Earth Sci. Front. 2020, 27, 197–217. [Google Scholar]
- Hu, H.; Li, J.-W.; Harlov, D.E.; Lentz, D.R.; McFarlane, C.R.; Yang, Y.-H. A Genetic Link between Iron Oxide-Apatite and Iron Skarn Mineralization in the Jinniu Volcanic Basin, Daye District, Eastern China: Evidence from Magnetite Geochemistry and Multi-Mineral U-Pb Geochronology. GSA Bull. 2020, 132, 899–917. [Google Scholar] [CrossRef]
- Henriquez, F.; Martin, F.R. Crystal-Growth Textures in Magnetite Flows and Feeder Dykes, El Laco, Chile. Can. Mineral. 1978, 16, 581–589. [Google Scholar]
- Frietsch, R. On the Magmatic Origin of Iron Ores of the Kiruna Type. Econ. Geol. 1978, 73, 478–485. [Google Scholar] [CrossRef]
- Nystroem, J.O.; Henriquez, F. Magmatic Features of Iron Ores of the Kiruna Type in Chile and Sweden; Ore Textures and Magnetite Geochemistry. Econ. Geol. 1994, 89, 820–839. [Google Scholar] [CrossRef]
- Jonsson, E.; Harlov, D.E.; Majka, J.; Högdahl, K.; Persson-Nilsson, K. Fluorapatite-Monazite-Allanite Relations in the Grängesberg Apatite-Iron Oxide Ore District, Bergslagen, Sweden. Am. Mineral. 2016, 101, 1769–1782. [Google Scholar] [CrossRef]
- Mungall, J.; Long, K.; Brenan, J.; Smythe, D.; Naslund, H. Immiscible Shoshonitic and Fe-P-Oxide Melts Preserved in Unconsolidated Tephra at El Laco Volcano, Chile. Geology 2018, 46, 255–258. [Google Scholar] [CrossRef]
- Parente, C.V.; Veríssimo, C.U.V.; Botelho, N.F.; Xavier, R.P.; Menez, J.; de Oliveira Lino, R.; da Silva, C.D.A.; dos Santos, T.J.S. Geology, Petrography and Mineral Chemistry of Iron Oxide-Apatite Occurrences (IOA Type), Western Sector of the Neoproterozoic Santa Quiteria Magmatic Arc, Ceará Northeast, Brazil. Ore Geol. Rev. 2019, 112, 103024. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, Q.-C.; Du, Y.-S. Deep Ore Magma Hydrothermal System of Zhonggu Ore Field in Southern Part of Ningwu Basin. Earth Sci. Front. 2010, 17, 186. [Google Scholar]
- Li, Y.H.; Duan, C.; Han, D.; Chen, X.W.; Liu, F. Effect of Sulfate Evaporate Salt Layer for Formation of Porphyrite Iron Ores in the Middle-Lower Yangtze River Area. Acta Petrol. Sin. 2014, 30, 1355–1368. [Google Scholar]
- Wang, Y.; Zhu, X.K.; Mao, J.W.; Cheng, Y.B. Preliminary Fe Isotopic Study of Gushan Ore Magma Deposit in Anhui Province. Miner. Depos. 2014, 33, 689–696. [Google Scholar]
- Li, Y.; Duan, C.; Dan, H.; Liu, F.; Wan, D.; Wang, C. Oxygen Isotopic Discriminant Marker of Magmatic Iron Deposits: Ningwu Porphyrite Iron Ore as an Example. Acta Petrol. Sin. 2017, 33, 3411–3421. [Google Scholar]
- Hou, T.; Zhang, Z.; Kusky, T. Gushan Magnetite–Apatite Deposit in the Ningwu Basin, Lower Yangtze River Valley, SE China: Hydrothermal or Kiruna-Type? Ore Geol. Rev. 2011, 43, 333–346. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, Z.; Du, Y.; Li, S. Geology of the Gushan Iron Oxide Deposit Associated with Dioritic Porphyries, Eastern Yangtze Craton, SE China. Int. Geol. Rev. 1981, 51, 520–541. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, J.; Ai, Y. Meishan Iron Deposit—An Ore Magma-Hydrothermal Deposit. Bull. Inst. Miner. Depos. Chin. Acad. Geol. Sci. 1981, 2, 26–48. [Google Scholar]
- Zhu, Z. Study on Migration of the Iron-Rich Magma of the Meishan Iron Deposit. Chin. J. Geol. 1987, 22, 276–281. [Google Scholar]
- Yuan, J.; Zhang, F.; Yin, C.; Shao, H. Systematical Study on Ore-Magma Genesis of Meishan Iron Ore Deposits. Geoscience 1997, 11, 170–176. [Google Scholar]
- Yu, J.; Chen, Y.; Mao, J.; Pirajno, F.; Duan, C. Review of Geology, Alteration and Origin of Iron Oxide–Apatite Deposits in the Cretaceous Ningwu Basin, Lower Yangtze River Valley, Eastern China: Implications for Ore Genesis and Geodynamic Setting. Ore Geol. Rev. 2011, 43, 170–181. [Google Scholar] [CrossRef]
- Yu, J.J. Sr Isotope of Apatites from the Washan and Taishan Iron Deposits in the Nanjing-Wuhu Area and Its Implications. Geol. Rev. 2003, 43, 272–277. [Google Scholar]
- Ma, F.; Jiang, S.Y.; Jiang, Y.H.; Ni, P.; Ling, H.F. Fluid Inclusions and H-O Isotopic Compositions in the Washan and Dongshan Iron Deposits, Ningwu Basin, China. Acta Petrol. Sin. 2006, 22, 2581–2589. [Google Scholar]
- Duan, C. Metallogeny Study of Washan Porphyry Iron Deposit in Ningwu Ore District. Doctoral Dissertation, Hefei University of Technology, Hefei, China, 2012. [Google Scholar]
- Yu, J.; Lu, B.; Wang, T.; Che, L. Cretaceous Cu–Au, Pyrite, and Fe-Oxide–Apatite Deposits in the Ningwu Basin, Lower Yangtze Area, Eastern China. J. Asian Earth Sci. 2015, 103, 150–168. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Z.; Hou, T.; Cheng, Z.; Campos, E.; Wang, Z.; Fei, X. New Insights for the Formation of Kiruna-Type Iron Deposits by Immiscible Hydrous Fe-P Melt and High-Temperature Hydrothermal Processes: Evidence from El Laco Deposit. Econ. Geol. 2019, 114, 35–46. [Google Scholar] [CrossRef]
- Mokhtari, M.A.A.; Zadeh, G.H.; Emami, M.H. Genesis of Iron-Apatite Ores in Posht-e-Badam Block (Central Iran) Using REE Geochemistry. J. Earth Syst. Sci. 2013, 122, 795–807. [Google Scholar] [CrossRef]
- Tornos, F.; Velasco, F.; Hanchar, J.M. Iron-Rich Melts, Magmatic Magnetite, and Superheated Hydrothermal Systems: The El Laco Deposit, Chile. Geology 2016, 44, 427–430. [Google Scholar] [CrossRef]
- Günther, T.; Klemd, R.; Zhang, X.; Horn, I.; Weyer, S. In-Situ Trace Element and Fe-Isotope Studies on Magnetite of the Volcanic-Hosted Zhibo and Chagangnuoer Iron Ore Deposits in the Western Tianshan, NW China. Chem. Geol. 2017, 453, 111–127. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Burrows, D.R. New Field Evidence Bearing on the Origin of the El Laco Magnetite Deposit, Northern Chile. Econ. Geol. 2002, 97, 1101–1109. [Google Scholar]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G. Did the Massive Magnetite “Lava Flows” of El Laco (Chile) Form by Magmatic or Hydrothermal Processes? New Constraints from Magnetite Composition by LA-ICP-MS. Miner. Depos. 2015, 50, 607–617. [Google Scholar] [CrossRef]
- Chen, H.; Clark, A.H.; Kyser, T.K. The Marcona Magnetite Deposit, Ica, South-Central Peru: A Product of Hydrous, Iron Oxide-Rich Melts? Econ. Geol. 2010, 105, 1441–1456. [Google Scholar] [CrossRef]
- Pietruszka, D.K.; Hanchar, J.M.; Tornos, F.; Whitehouse, M.J.; Velasco, F. Tracking Isotopic Sources of Immiscible Melts at the Enigmatic Magnetite-(Apatite) Deposit at El Laco, Chile, Using Pb Isotopes. Bulletin 2024, 136, 513–530. [Google Scholar] [CrossRef]
- Knipping, J.L.; Bilenker, L.D.; Simon, A.C.; Reich, M.; Barra, F.; Deditius, A.P.; Lundstrom, C.; Bindeman, I.; Munizaga, R. Giant Kiruna-Type Deposits Form by Efficient Flotation of Magmatic Magnetite Suspensions. Geology 2015, 43, 591–594. [Google Scholar] [CrossRef]
- Knipping, J.L.; Webster, J.D.; Simon, A.C.; Holtz, F. Accumulation of Magnetite by Flotation on Bubbles during Decompression of Silicate Magma. Sci. Rep. 2019, 9, 3852. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mustafa, M.A.; Simon, A.C.; del Real, I.; Thompson, J.F.; Bilenker, L.D.; Barra, F.; Bindeman, I.; Cadwell, D. A Continuum from Iron Oxide Copper-Gold to Iron Oxide-Apatite Deposits: Evidence from Fe and O Stable Isotopes and Trace Element Chemistry of Magnetite. Econ. Geol. 2020, 115, 1443–1459. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Zeng, L.-P.; Liao, W.; Fan, Y.-Z.; Hofstra, A.H.; Emsbo, P.; Hu, H.; Wen, G.; Li, J.-W. Iron Oxide–Apatite Deposits Form from Hydrosaline Liquids Exsolved from Subvolcanic Intrusions. Miner. Depos. 2024, 59, 655–669. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, Z.; Encarnacion, J.; Huang, H.; Wang, M. Geochronology/Geochemistry of the Washan Dioritic Porphyry Associated with Kiruna-Type Iron Ores, Middle-Lower Yangtze River Valley, Eastern China: Implications for Petrogenesis/Mineralization. Int. Geol. Rev. 2012, 54, 1332–1352. [Google Scholar] [CrossRef]
- Duan, C.; Li, Y.; Mao, J.; Hou, K.; Wang, C.; Yang, B.; Wang, Q.; Li, W. Ore Formation at the Washan Iron Oxide–Apatite Deposit in the Ningwu Ore District, Eastern China: Insights from in Situ LA-ICP-MS Magnetite Trace Element Geochemistry. Ore Geol. Rev. 2019, 112, 103064. [Google Scholar] [CrossRef]
- Canil, D.; Lacourse, T. Geothermometry Using Minor and Trace Elements in Igneous and Hydrothermal Magnetite. Chem. Geol. 2020, 541, 119576. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Liu, C.; Huang, D.; Zhou, W.; Teng, X.; Liang, E.; Dai, T. Genesis of Early Cretaceous Porphyrite-Type Iron Deposits and Related Sub-Volcanic Rocks in the Ningwu Volcanic Basin, Middle-Lower Yangtze Metallogenic Belt, Southeast China. Int. Geol. Rev. 2018, 60, 1507–1528. [Google Scholar] [CrossRef]
- Bai, Z.; Zhong, H.; Li, C.; Zhu, W.; He, D.; Qi, L. Contrasting Parental Magma Compositions for the Hongge and Panzhihua Magmatic Fe-Ti-V Oxide Deposits, Emeishan Large Igneous Province, SW China. Econ. Geol. 2014, 109, 1763–1785. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, H.; Yang, S.; Yu, X. Mineral Characteristics and Metallogenesis of the Wajilitag Layered Mafic–Ultramafic Intrusion and Associated Fe–Ti–V Oxide Deposit in the Tarim Large Igneous Province, Northwest China. J. Asian Earth Sci. 2012, 49, 161–174. [Google Scholar] [CrossRef]
- Buddington, A.F.; Lindsley, D. Iron-Titanium Oxide Minerals and Synthetic Equivalents. J. Petrol. 1964, 5, 310–357. [Google Scholar] [CrossRef]
- Von Gruenewaldt, G.; Klemm, D.; Henckel, J.; Dehm, R. Exsolution Features in Titanomagnetites from Massive Magnetite Layers and Their Host Rocks of the Upper Zone, Eastern Bushveld Complex. Econ. Geol. 1985, 80, 1049–1061. [Google Scholar] [CrossRef]
- Huang, X.-W.; Beaudoin, G. Nanoinclusions in Zoned Magnetite from the Sossego IOCG Deposit, Carajás, Brazil: Implication for Mineral Zoning and Magnetite Origin Discrimination. Ore Geol. Rev. 2021, 139, 104453. [Google Scholar] [CrossRef]
- Groves, D.I.; Bierlein, F.P.; Meinert, L.D.; Hitzman, M.W. Iron Oxide Copper-Gold (IOCG) Deposits through Earth History: Implications for Origin, Lithospheric Setting, and Distinction from Other Epigenetic Iron Oxide Deposits. Econ. Geol. 2010, 105, 641–654. [Google Scholar] [CrossRef]
- Nadoll, P.; Mauk, J.L.; Leveille, R.A.; Koenig, A.E. Geochemistry of Magnetite from Porphyry Cu and Skarn Deposits in the Southwestern United States. Miner. Depos. 2015, 50, 493–515. [Google Scholar] [CrossRef]
- Palma, G.; Barra, F.; Reich, M.; Simon, A.C.; Romero, R. A Review of Magnetite Geochemistry of Chilean Iron Oxide-Apatite (IOA) Deposits and Its Implications for Ore-Forming Processes. Ore Geol. Rev. 2020, 126, 103748. [Google Scholar] [CrossRef]
- Di, S. Geological and Geochemical Characteristics and Genesis of Baixiangshan Iron Deposit in Ningwu Basin. Master Dissertation, Hefei University of Technology, Hefei, China, 2020. [Google Scholar]
- Carmichael, I.S. The Iron-Titanium Oxides of Salic Volcanic Rocks and Their Associated Ferromagnesian Silicates. Contrib. Mineral. Petrol. 1966, 14, 36–64. [Google Scholar] [CrossRef]
- Pang, K.; Zhou, M.; Lindsley, D.; Zhao, D.; Malpas, J. Origin of Fe–Ti Oxide Ores in Mafic Intrusions: Evidence from the Panzhihua Intrusion, SW China. J. Petrol. 2008, 49, 295–313. [Google Scholar] [CrossRef]
- Nold, J.L.; Davidson, P.; Dudley, M.A. The Pilot Knob Magnetite Deposit in the Proterozoic St. Francois Mountains Terrane, Southeast Missouri, USA: A Magmatic and Hydrothermal Replacement Iron Deposit. Ore Geol. Rev. 2013, 53, 446–469. [Google Scholar] [CrossRef]
- Huang, X.; Beaudoin, G. Textures and Chemical Compositions of Magnetite from Iron Oxide Copper-Gold (IOCG) and Kiruna-Type Iron Oxide-Apatite (IOA) Deposits and Their Implications for Ore Genesis and Magnetite Classification Schemes. Econ. Geol. 2019, 114, 953–979. [Google Scholar] [CrossRef]
- James, B.R. Chromium. In Encyclopedia of Water Science; Stewart, B.A., Howell, T.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 77–82. [Google Scholar]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G. Variation in Trace Element Content of Magnetite Crystallized from a Fractionating Sulfide Liquid, Sudbury, Canada: Implications for Provenance Discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Palma, G.; Reich, M.; Barra, F.; Ovalle, J.T.; Del Real, I.; Simon, A.C. Thermal Evolution of Andean Iron Oxide–Apatite (IOA) Deposits as Revealed by Magnetite Thermometry. Sci. Rep. 2021, 11, 18424. [Google Scholar] [CrossRef] [PubMed]
- Childress, T.M.; Simon, A.C.; Reich, M.; Barra, F.; Arce, M.; Lundstrom, C.C.; Bindeman, I.N. Formation of the Mantoverde Iron Oxide-Copper-Gold (IOCG) Deposit, Chile: Insights from Fe and O Stable Isotopes and Comparisons with Iron Oxide-Apatite (IOA) Deposits. Miner. Depos. 2020, 55, 1489–1504. [Google Scholar] [CrossRef]
- Mukherjee, R.; Venkatesh, A.S.; Fareeduddin. Geochemical Characterization of Mineralized Albitite from Paleoproterozoic Bhukia IOCG-IOA Deposit of Aravalli-Delhi Fold Belt, Rajasthan, Western India: Genetic Linkage to the Gold (\pmCu\pmU) Mineralization. Geol. J. 2020, 55, 4203–4225. [Google Scholar] [CrossRef]
- Sepidbar, F.; Ghorbani, G.; Simon, A.C.; Ma, J.; Palin, R.M.; Homam, S.M. Formation of the Chah-Gaz Iron Oxide-Apatite Ore (IOA) Deposit, Bafq District, Iran: Constraints from Halogens, Trace Element Concentrations, and Sr-Nd Isotopes of Fluorapatite. Ore Geol. Rev. 2022, 140, 104599. [Google Scholar] [CrossRef]
- Ovalle, J.T.; La Cruz, N.L.; Reich, M.; Barra, F.; Simon, A.C.; Konecke, B.A.; Rodriguez-Mustafa, M.A.; Deditius, A.P.; Childress, T.M.; Morata, D. Formation of Massive Iron Deposits Linked to Explosive Volcanic Eruptions. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, M.; Zhang, X.; Xia, Y.; Algeo, T.J.; Li, H. An Evolving Magmatic-Hydrothermal System in the Formation of the Mesozoic Meishan Magnetite-Apatite Deposit in the Ningwu Volcanic Basin, Eastern China. J. Asian Earth Sci. 2018, 158, 1–17. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Z.; Bai, Z. On the Conditions of Formation of Gushan Iron Ore Deposit. Geochemistry 1984, 3, 45–55. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, G. A Thermodynamic Study on Physicochemical Conditions of Formation of Meishan Iron Deposit. Geochemistry 1985, 4, 141–149. [Google Scholar] [CrossRef]
- Duan, C.; Li, Y.H.; Yuan, S.D.; Hu, M.Y.; Liu, J.L. Geochemical Characteristics of Magnetite from Washan Iron Deposit in Ningwu Ore District and Its Constraints on Ore-Forming. Acta Petrol. Sin. 2012, 28, 243–257. [Google Scholar]
- Suleimenov, O.; Seward, T. Spectrophotometric Measurements of Metal Complex Formation at High Temperatures: The Stability of Mn (II) Chloride Species. Chem. Geol. 2000, 167, 177–192. [Google Scholar] [CrossRef]
- Iveson, A.A.; Webster, J.D.; Rowe, M.C.; Neill, O.K. Fluid-Melt Trace-Element Partitioning Behaviour between Evolved Melts and Aqueous Fluids: Experimental Constraints on the Magmatic-Hydrothermal Transport of Metals. Chem. Geol. 2019, 516, 18–41. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant Diagrams for Iron Oxide Trace Element Fingerprinting of Mineral Deposit Types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace Elements in Magnetite as Petrogenetic Indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Pettke, T.; Kessel, R. Chromium Mobility in Hydrous Fluids at Upper Mantle Conditions. Lithos 2011, 125, 122–130. [Google Scholar] [CrossRef]
- Watenphul, A.; Schmidt, C.; Scholten, L. First Insights into Cr3+ Solubility in Aqueous Fluids at Elevated P and T by μ-XRF. In Proceedings of the 1st European Mineralogical Conference-EMC, Frankfurt, Germany, 2–6 September 2012. [Google Scholar]
- Zeng, L.-P.; Zhao, X.-F.; Spandler, C.; Mavrogenes, J.A.; Mernagh, T.P.; Liao, W.; Fan, Y.-Z.; Hu, Y.; Fu, B.; Li, J.-W. The Role of Iron-Rich Hydrosaline Liquids in the Formation of Kiruna-Type Iron Oxide–Apatite Deposits. Sci. Adv. 2024, 10, eadk2174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xie, G.; Mao, J.; Li, W.; Li, Y.; Wang, J.; Zhang, P. Mineralogical and Sulfur Isotopic Evidence for the Incursion of Evaporites in the Jinshandian Skarn Fe Deposit, Edong District, Eastern China. J. Asian Earth Sci. 2015, 113, 1253–1267. [Google Scholar] [CrossRef]
- Hou, T.; Charlier, B.; Holtz, F.; Veksler, I.; Zhang, Z.; Thomas, R.; Namur, O. Immiscible Hydrous Fe–Ca–P Melt and the Origin of Iron Oxide-Apatite Ore Deposits. Nat. Commun. 2018, 9, 1415. [Google Scholar] [CrossRef] [PubMed]


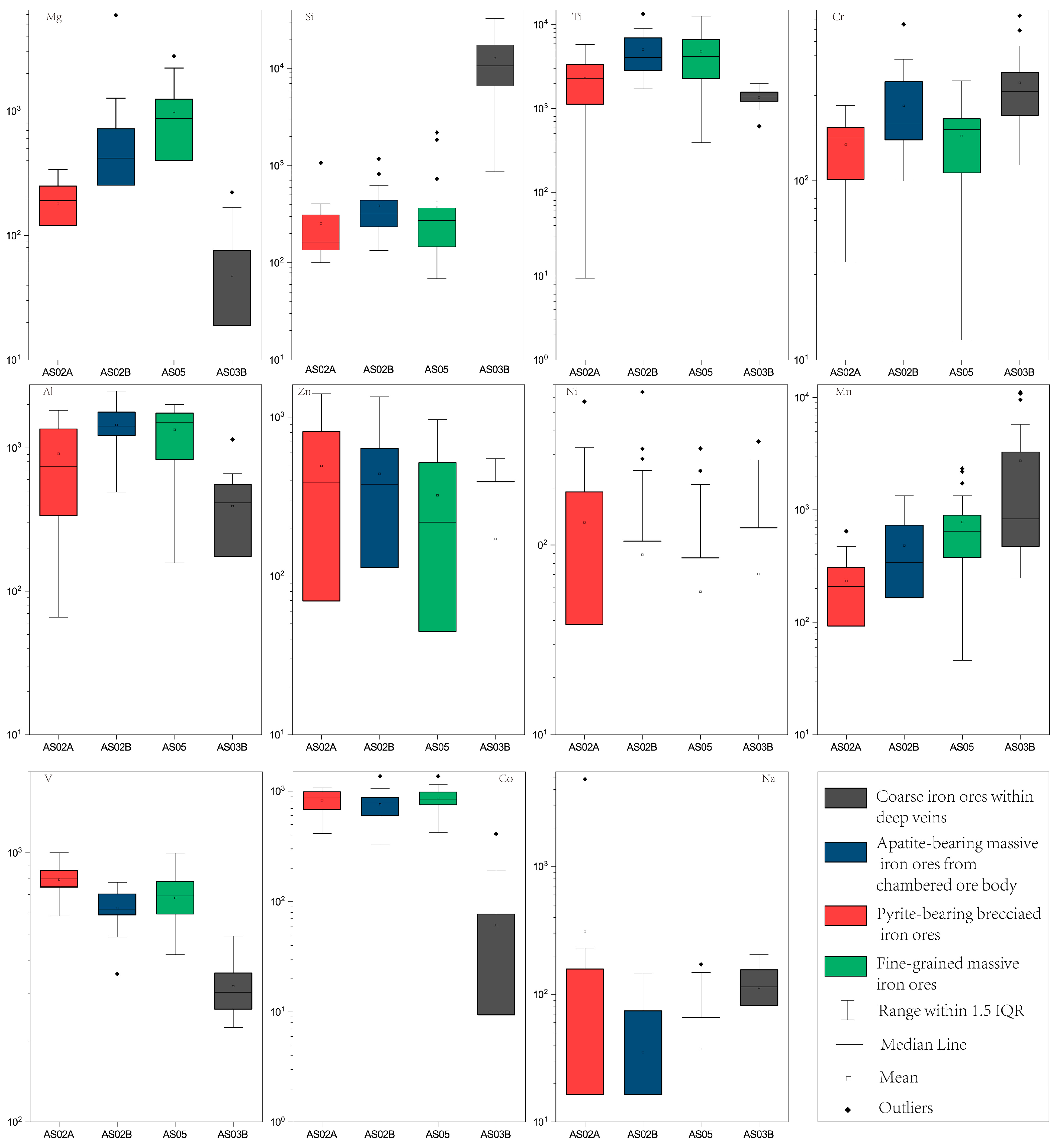
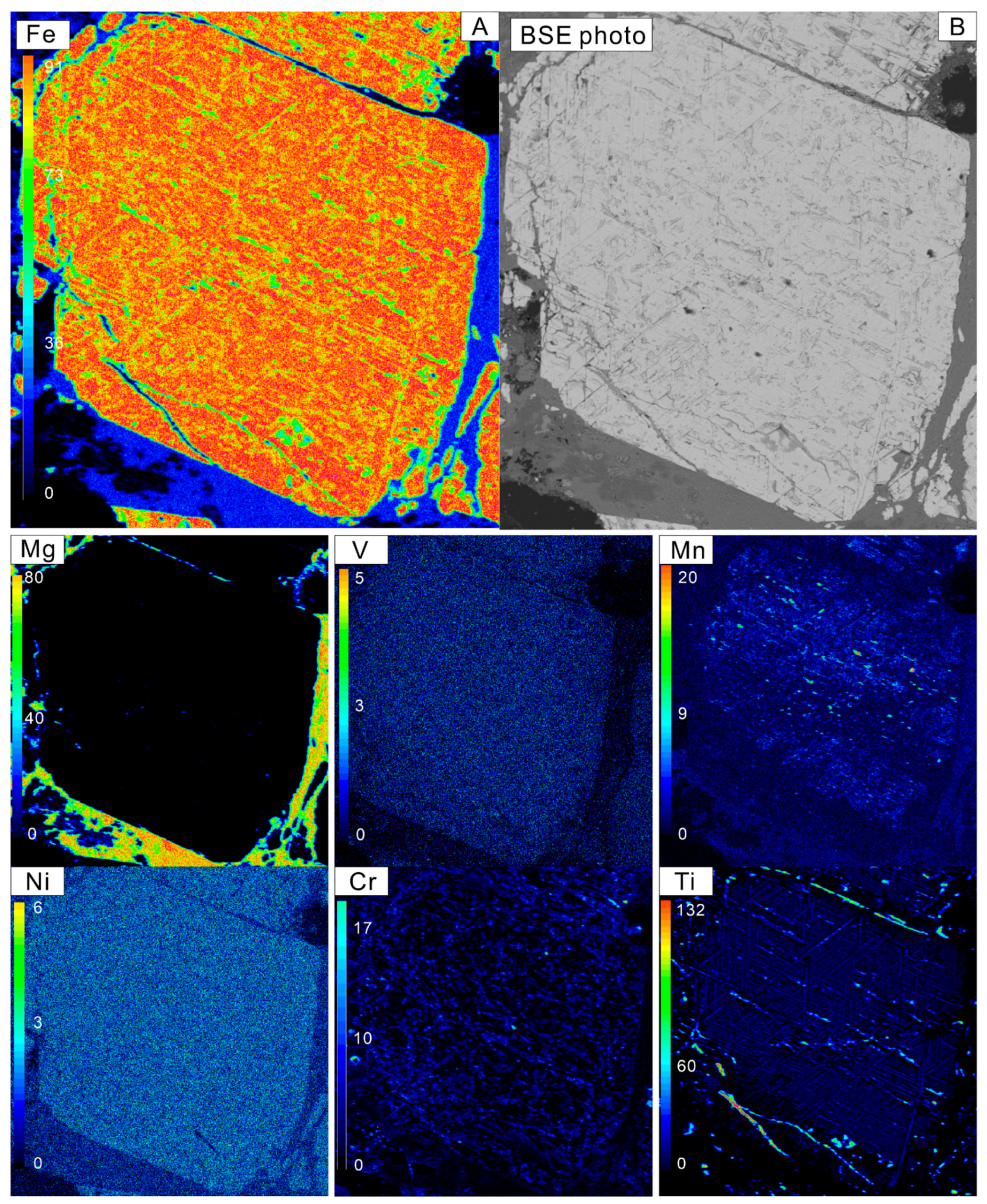
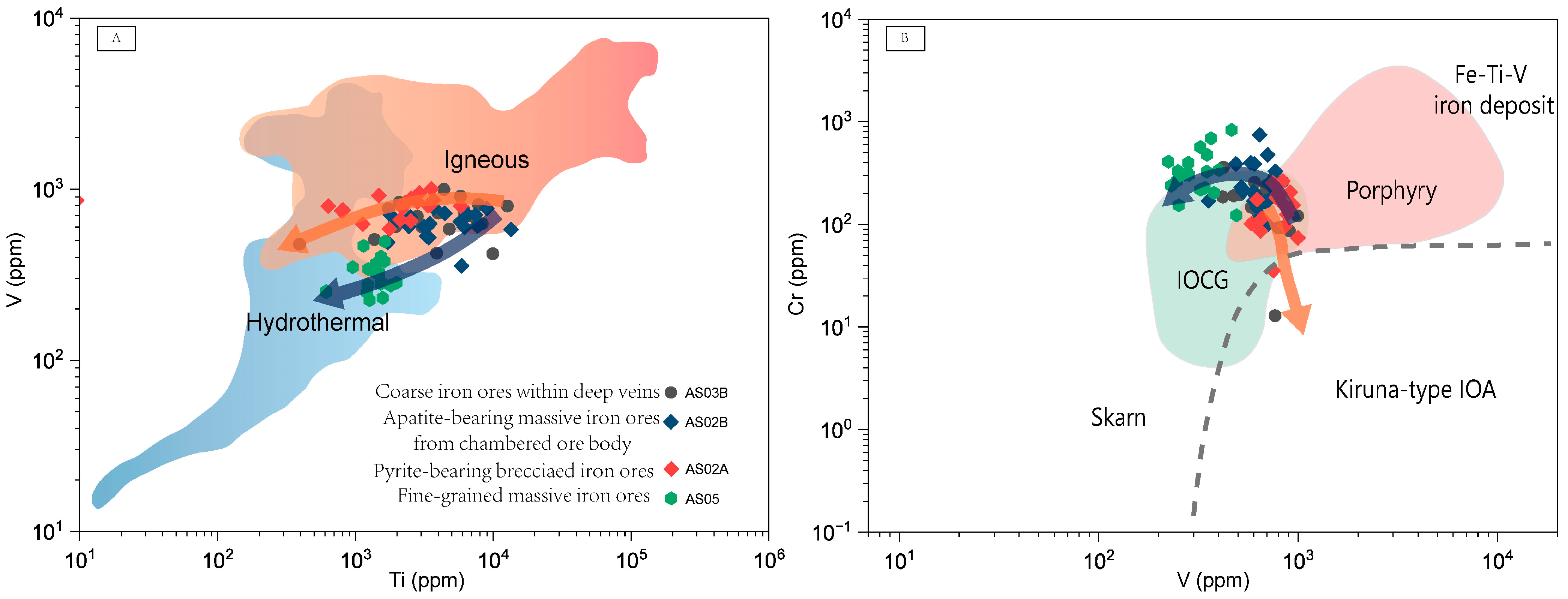

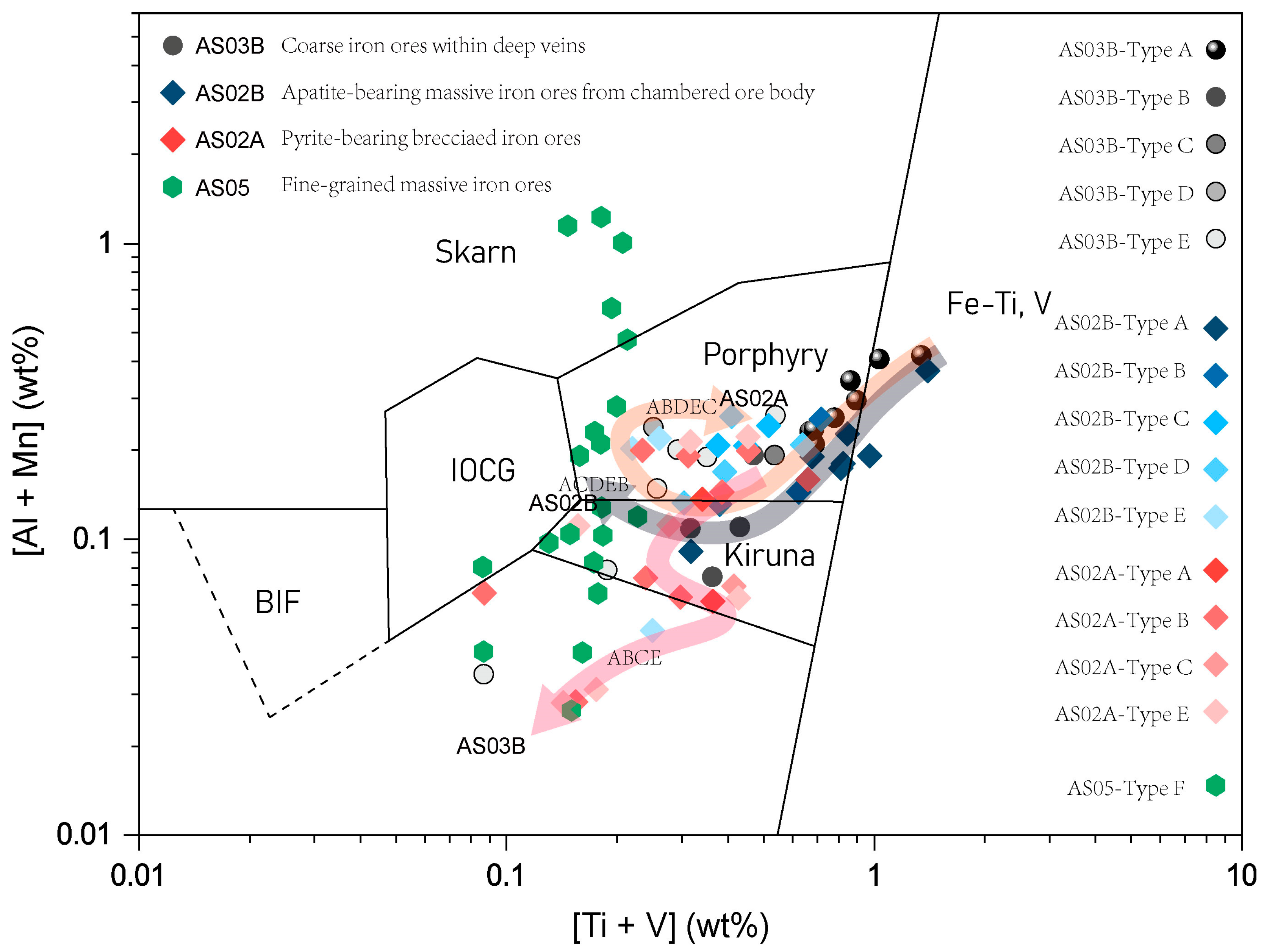

| Sample | Magnetite Grains | Iron Ore Types | Magnetite Types | SiO2 | TiO2 | Cr2O3 | Al2O3 | ZnO | NiO | Fe2O3 | FeO | MnO | V2O3 | CoO | Na2O | MgO | Total | XMg | T-min | T-max | T-every |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS02A | AS02A-MAG-1 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.03 | 0.14 | 0.01 | 0.18 | 0.03 | 0.04 | 68.25 | 30.91 | 0.08 | 0.22 | 0.08 | 0.01 | 0.03 | 100.00 | 0.00 | 431 | 452 | 441 |
| AS02A | AS02A-MAG-2 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.06 | 0.42 | 0.03 | 0.69 | 0.0050 | 0.01 | 67.18 | 31.22 | 0.04 | 0.19 | 0.05 | 0.05 | 0.06 | 100.00 | 0.00 | 479 | 499 | 489 |
| AS02A | AS02A-MAG-3 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.03 | 0.59 | 0.02 | 0.67 | 0.01 | 0.02 | 66.70 | 31.46 | 0.06 | 0.29 | 0.10 | 0.02 | 0.02 | 100.00 | 0.00 | 412 | 433 | 423 |
| AS02A | AS02A-MAG-4 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.02 | 0.62 | 0.03 | 0.69 | 0.14 | BDL | 66.65 | 31.41 | 0.02 | 0.25 | 0.12 | BDL | 0.05 | 100.00 | 0.00 | 475 | 495 | 485 |
| AS02A | AS02A-MAG-5 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.04 | 0.38 | 0.08 | 0.66 | 0.13 | 0.01 | 67.04 | 31.24 | 0.02 | 0.24 | 0.13 | BDL | 0.03 | 100.00 | 0.00 | 440 | 461 | 451 |
| AS02A | AS02A-MAG-6 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.03 | 0.48 | 0.05 | 0.51 | 0.03 | BDL | 67.09 | 31.36 | 0.01 | 0.28 | 0.13 | 0.02 | 0.01 | 100.00 | 0.00 | 351 | 373 | 362 |
| AS02A | AS02A-MAG-7 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.04 | 0.37 | 0.05 | 0.13 | BDL | 0.07 | 67.74 | 31.16 | 0.04 | 0.23 | 0.12 | BDL | 0.04 | 100.00 | 0.00 | 458 | 478 | 468 |
| AS02A | AS02A-MAG-8 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type A | 0.03 | 0.42 | 0.04 | 0.47 | 0.06 | BDL | 67.23 | 31.34 | 0.02 | 0.26 | 0.11 | BDL | 0.02 | 100.00 | 0.00 | 417 | 438 | 428 |
| AS02A | AS02A-MAG-9 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.03 | 0.58 | 0.05 | 0.24 | 0.01 | 0.04 | 67.19 | 31.47 | BDL | 0.24 | 0.13 | BDL | 0.01 | 100.00 | 0.00 | 378 | 400 | 389 |
| AS02A | AS02A-MAG-11 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.03 | 0.25 | 0.06 | 0.28 | 0.10 | BDL | 67.77 | 31.11 | BDL | 0.27 | 0.11 | BDL | 0.03 | 100.00 | 0.00 | 435 | 456 | 445 |
| AS02A | AS02A-MAG-10 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.03 | 0.29 | 0.03 | 0.51 | 0.05 | 0.01 | 67.56 | 31.14 | 0.08 | 0.17 | 0.09 | BDL | 0.04 | 100.00 | 0.00 | 458 | 478 | 468 |
| AS02A | AS02A-MAG-12 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.23 | 0.0016 | 0.04 | 0.07 | 0.04 | BDL | 68.07 | 31.06 | 0.06 | 0.25 | 0.14 | BDL | 0.04 | 100.00 | 0.00 | 454 | 475 | 464 |
| AS02A | AS02A-MAG-13 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.02 | 0.13 | 0.07 | 0.03 | BDL | 0.0049 | 68.33 | 31.07 | 0.03 | 0.22 | 0.09 | BDL | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS02A | AS02A-MAG-14 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type C | 0.08 | 0.56 | 0.05 | 0.26 | 0.16 | 0.07 | 67.12 | 31.28 | BDL | 0.24 | 0.13 | 0.0044 | 0.04 | 100.00 | 0.00 | 463 | 483 | 473 |
| AS02A | AS02A-MAG-15 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.07 | 0.11 | 0.05 | 0.02 | 0.09 | 0.0048 | 68.49 | 30.70 | 0.03 | 0.23 | 0.10 | 0.06 | 0.04 | 100.00 | 0.00 | 448 | 468 | 458 |
| AS02A | AS02A-MAG-16 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.07 | 0.35 | 0.03 | 0.36 | 0.06 | BDL | 71.78 | 25.72 | 0.02 | 0.19 | 0.09 | 1.30 | 0.03 | 100.00 | 0.00 | 433 | 454 | 444 |
| AS02A | AS02A-MAG-17 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type E | 0.03 | 0.19 | 0.05 | 0.12 | 0.09 | 0.02 | 68.23 | 31.00 | BDL | 0.18 | 0.07 | 0.02 | 0.0009 | 100.00 | 0.00 | 269 | 291 | 280 |
| AS02A | AS02A-MAG-18 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type B | 0.09 | 0.97 | 0.06 | 0.51 | 0.17 | BDL | 66.22 | 31.55 | 0.03 | 0.23 | 0.07 | 0.06 | 0.04 | 100.00 | 0.00 | 457 | 477 | 467 |
| AS02A | AS02A-MAG-19 | The pyrite-bearing brecciated iron ores from the brecciated zones | AS02A-Type A | 0.08 | 0.47 | 0.08 | 0.15 | 0.01 | BDL | 67.54 | 31.20 | 0.03 | 0.25 | 0.12 | 0.04 | 0.04 | 100.00 | 0.00 | 452 | 473 | 462 |
| AS02B | AS02B-MAG-1 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type C | 0.07 | 0.74 | 0.07 | 0.53 | BDL | 0.01 | 66.51 | 31.58 | 0.13 | 0.21 | 0.04 | BDL | 0.10 | 100.00 | 0.00 | 520 | 538 | 529 |
| AS02B | AS02B-MAG-2 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.09 | 1.31 | 0.05 | 0.58 | 0.05 | 0.08 | 65.35 | 32.08 | 0.09 | 0.18 | 0.07 | BDL | 0.07 | 100.00 | 0.00 | 492 | 511 | 501 |
| AS02B | AS02B-MAG-3 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type D | 0.06 | 0.41 | 0.11 | 0.43 | BDL | BDL | 67.28 | 31.34 | 0.02 | 0.18 | 0.08 | BDL | 0.08 | 100.00 | 0.00 | 507 | 526 | 517 |
| AS02B | AS02B-MAG-4 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type C | 0.13 | 0.53 | 0.05 | 0.53 | 0.08 | BDL | 66.99 | 31.06 | 0.09 | 0.18 | 0.17 | 0.04 | 0.15 | 100.00 | 0.00 | 554 | 572 | 563 |
| AS02B | AS02B-MAG-5 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.07 | 0.42 | 0.05 | 0.30 | BDL | BDL | 67.41 | 31.42 | 0.01 | 0.20 | 0.09 | BDL | 0.02 | 100.00 | 0.00 | 417 | 438 | 427 |
| AS02B | AS02B-MAG-6 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type D | 0.04 | 0.58 | 0.04 | 0.71 | 0.17 | BDL | 66.76 | 31.19 | 0.09 | 0.18 | 0.11 | BDL | 0.13 | 100.00 | 0.00 | 546 | 564 | 555 |
| AS02B | AS02B-MAG-7 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.05 | 1.49 | 0.10 | 0.51 | 0.11 | 0.04 | 65.14 | 32.08 | 0.07 | 0.23 | 0.12 | 0.02 | 0.04 | 100.00 | 0.00 | 455 | 476 | 466 |
| AS02B | AS02B-MAG-8 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.05 | 2.24 | 0.12 | 0.94 | 0.02 | BDL | 63.70 | 31.49 | 0.16 | 0.17 | 0.13 | 0.01 | 0.98 | 100.00 | 0.02 | 755 | 763 | 759 |
| AS02B | AS02B-MAG-9 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type D | 0.25 | 0.96 | 0.22 | 0.72 | 0.05 | BDL | 65.38 | 31.89 | 0.02 | 0.19 | 0.10 | BDL | 0.21 | 100.00 | 0.00 | 586 | 602 | 594 |
| AS02B | AS02B-MAG-10 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.07 | 1.24 | 0.14 | 0.56 | 0.14 | BDL | 65.42 | 32.08 | 0.03 | 0.21 | 0.06 | BDL | 0.05 | 100.00 | 0.00 | 471 | 492 | 482 |
| AS02B | AS02B-MAG-11 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.18 | 1.27 | 0.06 | 0.63 | 0.08 | BDL | 65.20 | 32.23 | 0.02 | 0.18 | 0.05 | 0.01 | 0.11 | 100.00 | 0.00 | 529 | 548 | 539 |
| AS02B | AS02B-MAG-12 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type B | 0.06 | 0.98 | 0.05 | 0.49 | 0.0037 | 0.04 | 66.20 | 31.95 | 0.02 | 0.10 | 0.10 | BDL | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS02B | AS02B-MAG-13 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type A | 0.03 | 1.03 | 0.06 | 0.72 | 0.12 | 0.01 | 65.93 | 31.79 | BDL | 0.18 | 0.07 | 0.03 | 0.04 | 100.00 | 0.00 | 464 | 484 | 474 |
| AS02B | AS02B-MAG-14 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type B | 0.04 | 0.54 | 0.06 | 0.35 | 0.05 | BDL | 67.26 | 31.38 | 0.05 | 0.16 | 0.09 | 0.02 | 0.01 | 100.00 | 0.00 | 350 | 372 | 361 |
| AS02B | AS02B-MAG-15 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type C | 0.06 | 0.62 | 0.06 | 0.55 | 0.04 | BDL | 66.81 | 31.31 | 0.08 | 0.23 | 0.11 | 0.01 | 0.13 | 100.00 | 0.00 | 543 | 561 | 552 |
| AS02B | AS02B-MAG-16 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type D | 0.10 | 0.57 | 0.07 | 0.54 | 0.05 | 0.03 | 66.82 | 31.47 | 0.03 | 0.15 | 0.10 | BDL | 0.07 | 100.00 | 0.00 | 494 | 513 | 503 |
| AS02B | AS02B-MAG-17 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type E | 0.08 | 0.30 | 0.05 | 0.19 | 0.04 | BDL | 67.76 | 31.23 | BDL | 0.21 | 0.11 | 0.0022 | 0.04 | 100.00 | 0.00 | 452 | 473 | 462 |
| AS02B | AS02B-MAG-18 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type B | 0.05 | 1.08 | 0.03 | 0.46 | 0.0012 | BDL | 66.04 | 31.79 | 0.17 | 0.21 | 0.10 | 0.01 | 0.07 | 100.00 | 0.00 | 495 | 514 | 505 |
| AS02B | AS02B-MAG-19 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type E | 0.10 | 0.28 | 0.11 | 0.46 | 0.06 | 0.02 | 67.49 | 31.04 | 0.10 | 0.14 | 0.10 | 0.02 | 0.07 | 100.00 | 0.00 | 497 | 516 | 506 |
| AS02B | AS02B-MAG-20 | The apatite-bearing massive iron ores from the chambered ore body | AS02B-Type E | 0.08 | 0.33 | 0.03 | 0.72 | 0.03 | BDL | 67.18 | 31.20 | 0.04 | 0.19 | 0.13 | 0.02 | 0.06 | 100.00 | 0.00 | 487 | 506 | 496 |
| AS03B | AS03B-MAG-1 | The coarse iron ores within deep veins | AS03B-Type E | 0.05 | 0.23 | 0.06 | 0.27 | 0.02 | BDL | 68.02 | 30.96 | 0.01 | 0.15 | 0.13 | 0.04 | 0.05 | 100.00 | 0.00 | 467 | 487 | 477 |
| AS03B | AS03B-MAG-2 | The coarse iron ores within deep veins | AS03B-Type E | 0.03 | 0.33 | 0.08 | 0.33 | 0.05 | BDL | 67.62 | 31.19 | 0.08 | 0.18 | 0.13 | BDL | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS03B | AS03B-MAG-4 | The coarse iron ores within deep veins | AS03B-Type E | 0.08 | 0.07 | 0.06 | 0.06 | 0.06 | BDL | 68.39 | 31.01 | 0.02 | 0.14 | 0.10 | BDL | 0.01 | 100.00 | 0.00 | 393 | 415 | 404 |
| AS03B | AS03B-MAG-5 | The coarse iron ores within deep veins | AS03B-Type E | 0.04 | 0.35 | 0.08 | 0.50 | BDL | BDL | 67.37 | 31.07 | 0.09 | 0.25 | 0.11 | BDL | 0.15 | 100.00 | 0.00 | 553 | 571 | 562 |
| AS03B | AS03B-MAG-6 | The coarse iron ores within deep veins | AS03B-Type A | 0.03 | 1.03 | 0.06 | 0.66 | BDL | 0.04 | 66.05 | 31.55 | 0.05 | 0.21 | 0.10 | 0.03 | 0.19 | 100.00 | 0.00 | 575 | 592 | 583 |
| AS03B | AS03B-MAG-7 | The coarse iron ores within deep veins | AS03B-Type E | 0.08 | 0.47 | 0.07 | 0.46 | 0.07 | BDL | 67.12 | 31.27 | 0.09 | 0.20 | 0.11 | 0.01 | 0.05 | 100.00 | 0.00 | 475 | 495 | 485 |
| AS03B | AS03B-MAG-8 | The coarse iron ores within deep veins | AS03B-Type A | 0.06 | 0.96 | 0.03 | 0.60 | 0.12 | BDL | 66.06 | 31.65 | 0.09 | 0.27 | 0.05 | 0.02 | 0.09 | 100.00 | 0.00 | 517 | 536 | 527 |
| AS03B | AS03B-MAG-8RM | The coarse iron ores within deep veins | AS03B-Type B | 0.01 | 0.66 | 0.06 | 0.56 | 0.03 | BDL | 66.86 | 31.18 | 0.06 | 0.21 | 0.10 | BDL | 0.27 | 100.00 | 0.01 | 608 | 624 | 616 |
| AS03B | AS03B-MAG-9 | The coarse iron ores within deep veins | AS03B-Type A | 0.07 | 1.39 | 0.04 | 0.76 | 0.0037 | BDL | 65.07 | 32.14 | 0.12 | 0.18 | 0.10 | BDL | 0.13 | 100.00 | 0.00 | 541 | 559 | 550 |
| AS03B | AS03B-MAG-10 | The coarse iron ores within deep veins | AS03B-Type E | 0.08 | 0.73 | 0.04 | 0.49 | 0.04 | 0.02 | 66.57 | 31.19 | 0.17 | 0.29 | 0.15 | BDL | 0.23 | 100.00 | 0.00 | 592 | 608 | 600 |
| AS03B | AS03B-MAG-10RM | The coarse iron ores within deep veins | AS03B-Type A | 0.06 | 1.19 | 0.03 | 0.72 | 0.11 | 0.03 | 65.69 | 31.56 | 0.09 | 0.20 | 0.09 | 0.05 | 0.18 | 100.00 | 0.00 | 572 | 589 | 580 |
| AS03B | AS03B-MAG-11 | The coarse iron ores within deep veins | AS03B-Type A | 0.04 | 2.11 | 0.03 | 0.75 | 0.01 | 0.02 | 63.90 | 32.07 | 0.28 | 0.23 | 0.10 | BDL | 0.46 | 100.00 | 0.01 | 665 | 678 | 671 |
| AS03B | AS03B-MAG-12 | The coarse iron ores within deep veins | AS03B-Type A | 0.03 | 1.65 | 0.05 | 0.66 | 0.12 | BDL | 64.95 | 31.67 | 0.30 | 0.12 | 0.08 | BDL | 0.37 | 100.00 | 0.01 | 641 | 655 | 648 |
| AS03B | AS03B-MAG-15 | The coarse iron ores within deep veins | AS03B-Type A | 0.02 | 1.30 | 0.03 | 0.65 | 0.01 | BDL | 65.57 | 31.47 | 0.22 | 0.24 | 0.12 | BDL | 0.37 | 100.00 | 0.01 | 640 | 654 | 647 |
| AS03B | AS03B-MAG-16 | The coarse iron ores within deep veins | AS03B-Type D | 0.03 | 0.29 | 0.0038 | 0.72 | 0.02 | BDL | 67.35 | 31.02 | 0.06 | 0.23 | 0.14 | 0.01 | 0.13 | 100.00 | 0.00 | 541 | 559 | 550 |
| AS03B | AS03B-MAG-17 | The coarse iron ores within deep veins | AS03B-Type C | 0.04 | 0.80 | 0.04 | 0.58 | 0.07 | BDL | 66.47 | 31.59 | 0.05 | 0.17 | 0.12 | BDL | 0.07 | 100.00 | 0.00 | 493 | 512 | 503 |
| AS03B | AS03B-MAG-19 | The coarse iron ores within deep veins | AS03B-Type A | 0.06 | 1.02 | 0.08 | 0.57 | BDL | 0.03 | 66.02 | 31.53 | 0.11 | 0.22 | 0.17 | 0.02 | 0.16 | 100.00 | 0.00 | 562 | 579 | 570 |
| AS03B | AS03B-MAG-20(18rm) | The coarse iron ores within deep veins | AS03B-Type B | 0.16 | 0.65 | 0.11 | 0.19 | 0.05 | BDL | 66.92 | 31.54 | 0.08 | 0.12 | 0.12 | BDL | 0.06 | 100.00 | 0.00 | 489 | 509 | 499 |
| AS03B | AS03B-MAG-21(18rm) | The coarse iron ores within deep veins | AS03B-Type B | 0.39 | 0.50 | 0.06 | 0.27 | 0.03 | BDL | 66.69 | 31.60 | 0.01 | 0.18 | 0.09 | 0.02 | 0.17 | 100.00 | 0.00 | 564 | 582 | 573 |
| AS03B | AS03B-MAG-22(18rm) | The coarse iron ores within deep veins | AS03B-Type B | 0.47 | 0.42 | 0.06 | 0.30 | BDL | BDL | 66.57 | 31.73 | 0.04 | 0.20 | 0.08 | 0.01 | 0.14 | 100.00 | 0.00 | 551 | 569 | 560 |
| AS05 | AS05-MAG-1 | The fine-grained massive iron ores | AS05-Type F | 1.86 | 0.24 | 0.17 | 0.10 | 0.05 | 0.01 | 63.89 | 33.30 | 0.26 | 0.10 | BDL | 0.02 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-2 | The fine-grained massive iron ores | AS05-Type F | 3.32 | 0.21 | 0.12 | 0.07 | BDL | BDL | 60.74 | 35.31 | 0.11 | 0.07 | BDL | 0.04 | 0.0027 | 100.00 | 0.00 | 313 | 335 | 324 |
| AS05 | AS05-MAG-3 | The fine-grained massive iron ores | AS05-Type F | 2.22 | 0.10 | 0.10 | 0.01 | BDL | 0.03 | 63.56 | 33.76 | 0.10 | 0.07 | 0.01 | 0.04 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-4 | The fine-grained massive iron ores | AS05-Type F | 0.32 | 0.20 | 0.09 | BDL | 0.04 | BDL | 67.77 | 31.40 | 0.03 | 0.08 | 0.01 | 0.04 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-5 | The fine-grained massive iron ores | AS05-Type F | 2.24 | 0.25 | 0.10 | 0.11 | BDL | BDL | 62.94 | 33.46 | 0.74 | 0.12 | 0.02 | BDL | 0.01 | 100.00 | 0.00 | 374 | 396 | 385 |
| AS05 | AS05-MAG-6 | The fine-grained massive iron ores | AS05-Type F | 1.97 | 0.25 | 0.06 | 0.25 | 0.06 | 0.04 | 63.53 | 33.63 | 0.08 | 0.10 | BDL | 0.02 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-7 | The fine-grained massive iron ores | AS05-Type F | 2.52 | 0.21 | 0.07 | 0.20 | BDL | BDL | 62.41 | 34.26 | 0.18 | 0.10 | BDL | 0.03 | 0.03 | 100.00 | 0.00 | 434 | 454 | 444 |
| AS05 | AS05-MAG-8 | The fine-grained massive iron ores | AS05-Type F | 4.55 | 0.26 | 0.09 | 0.14 | 0.03 | BDL | 57.63 | 37.12 | 0.09 | 0.08 | BDL | 0.01 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-9 | The fine-grained massive iron ores | AS05-Type F | 0.19 | 0.10 | 0.04 | 0.01 | BDL | BDL | 68.34 | 31.13 | 0.05 | 0.07 | BDL | 0.04 | 0.01 | 100.00 | 0.00 | 386 | 408 | 397 |
| AS05 | AS05-MAG-10 | The fine-grained massive iron ores | AS05-Type F | 2.31 | 0.27 | 0.04 | 0.16 | BDL | BDL | 62.79 | 33.68 | 0.56 | 0.14 | 0.01 | 0.02 | 0.01 | 100.00 | 0.00 | 387 | 409 | 398 |
| AS05 | AS05-MAG-11 | The fine-grained massive iron ores | AS05-Type F | 3.13 | 0.25 | 0.09 | 0.17 | BDL | BDL | 60.92 | 35.11 | 0.21 | 0.08 | BDL | 0.02 | 0.0009 | 100.00 | 0.00 | 269 | 292 | 280 |
| AS05 | AS05-MAG-12 | The fine-grained massive iron ores | AS05-Type F | 6.98 | 0.20 | 0.08 | 0.23 | BDL | BDL | 52.18 | 38.76 | 1.40 | 0.07 | BDL | 0.06 | 0.04 | 100.00 | 0.00 | 454 | 475 | 465 |
| AS05 | AS05-MAG-13 | The fine-grained massive iron ores | AS05-Type F | 1.14 | 0.19 | 0.24 | 0.01 | 0.05 | BDL | 65.57 | 32.59 | 0.05 | 0.14 | BDL | 0.01 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-14 | The fine-grained massive iron ores | AS05-Type F | 5.47 | 0.27 | 0.06 | 0.21 | 0.03 | BDL | 55.49 | 37.96 | 0.29 | 0.11 | 0.05 | 0.05 | 0.01 | 100.00 | 0.00 | 389 | 411 | 400 |
| AS05 | AS05-MAG-15 | The fine-grained massive iron ores | AS05-Type F | 1.65 | 0.33 | 0.12 | 0.24 | BDL | BDL | 64.09 | 33.38 | 0.07 | 0.08 | 0.01 | 0.02 | 0.01 | 100.00 | 0.00 | 344 | 366 | 355 |
| AS05 | AS05-MAG-16 | The fine-grained massive iron ores | AS05-Type F | 4.17 | 0.30 | 0.09 | 0.18 | BDL | 0.04 | 58.43 | 35.43 | 1.23 | 0.08 | 0.02 | 0.02 | 0.0027 | 100.00 | 0.00 | 314 | 336 | 325 |
| AS05 | AS05-MAG-17 | The fine-grained massive iron ores | AS05-Type F | 6.14 | 0.26 | 0.07 | 0.43 | 0.05 | 0.03 | 53.73 | 37.71 | 1.44 | 0.07 | 0.01 | 0.04 | 0.02 | 100.00 | 0.00 | 423 | 444 | 434 |
| AS05 | AS05-MAG-18 | The fine-grained massive iron ores | AS05-Type F | 3.07 | 0.23 | 0.14 | 0.20 | 0.05 | BDL | 61.06 | 35.07 | 0.04 | 0.10 | BDL | 0.04 | BDL | 100.00 | 0.00 | BDL | BDL | BDL |
| AS05 | AS05-MAG-19 | The fine-grained massive iron ores | AS05-Type F | 1.20 | 0.23 | 0.20 | 0.15 | BDL | 0.02 | 65.37 | 32.63 | 0.03 | 0.11 | 0.01 | 0.04 | 0.01 | 100.00 | 0.00 | 358 | 380 | 369 |
| AS05 | AS05-MAG-20 | The fine-grained massive iron ores | AS05-Type F | 0.18 | 0.16 | 0.10 | 0.07 | 0.07 | 0.01 | 68.09 | 31.07 | 0.10 | 0.10 | 0.0024 | 0.04 | 0.0036 | 100.00 | 0.00 | 324 | 346 | 335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xu, W.; Liu, C.; Huang, D. The Ore-Forming Process of Washan Porphyrite Iron Deposits in the Ningwu District Associated with Iron Oxide Apatite (IOA) Deposits and Iron Oxide Copper Gold (IOCG) Deposits. Minerals 2024, 14, 841. https://doi.org/10.3390/min14080841
Liu Z, Xu W, Liu C, Huang D. The Ore-Forming Process of Washan Porphyrite Iron Deposits in the Ningwu District Associated with Iron Oxide Apatite (IOA) Deposits and Iron Oxide Copper Gold (IOCG) Deposits. Minerals. 2024; 14(8):841. https://doi.org/10.3390/min14080841
Chicago/Turabian StyleLiu, Zhen, Wei Xu, Chunming Liu, and Dezhi Huang. 2024. "The Ore-Forming Process of Washan Porphyrite Iron Deposits in the Ningwu District Associated with Iron Oxide Apatite (IOA) Deposits and Iron Oxide Copper Gold (IOCG) Deposits" Minerals 14, no. 8: 841. https://doi.org/10.3390/min14080841




