An Approach to Accurately Identifying Binders in Historic Mortars by the Combination of Microscopic and Microanalytical Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Analytical Background
2.2. SEM-EDS and Principal Component Analysis
2.3. Optical Microscopy—Petrography
3. Results and Discussion
3.1. SEM—EDS and Principal Component Analysis
3.2. Optical Microscopy—Petrography
4. Conclusions
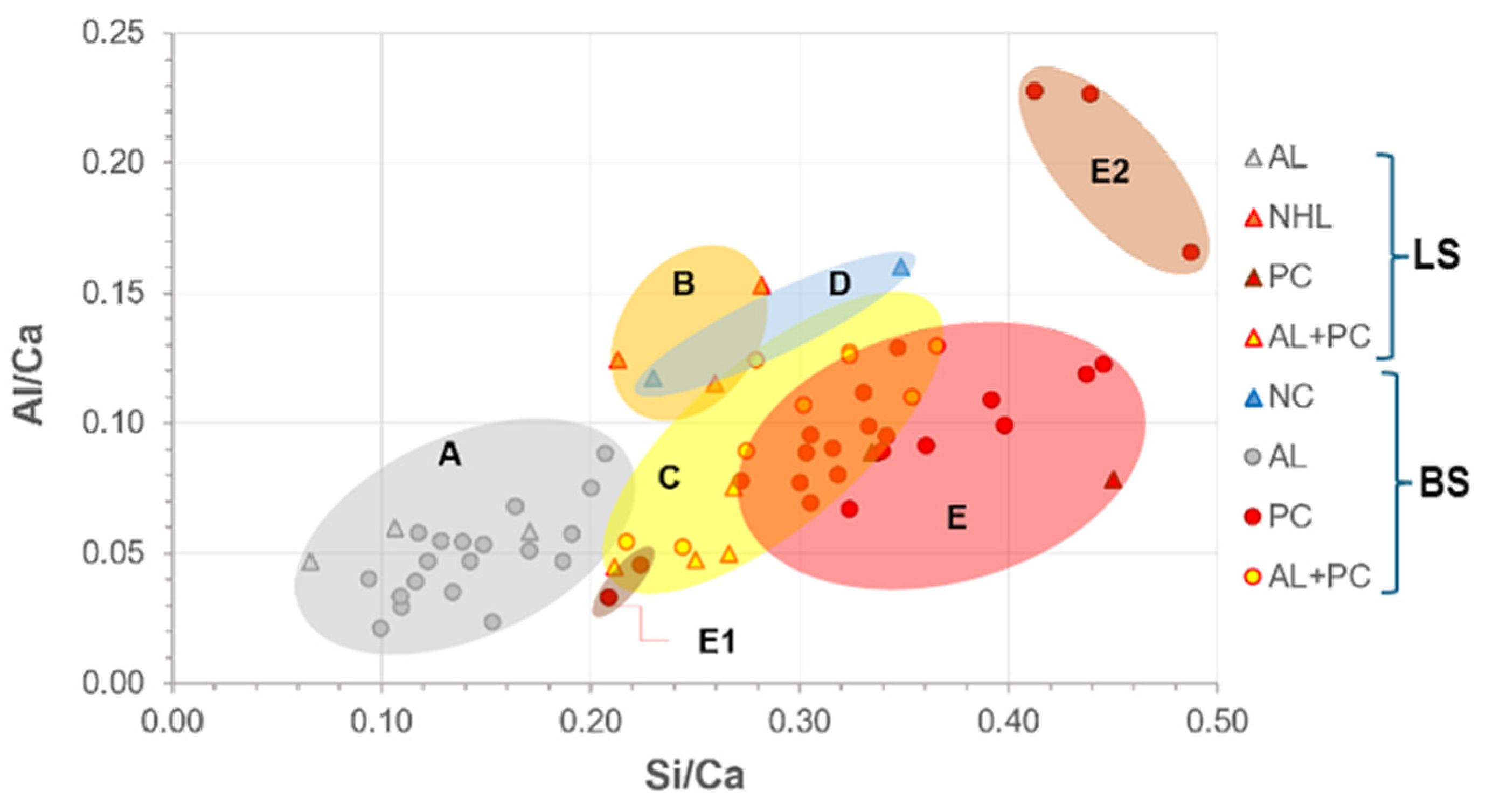
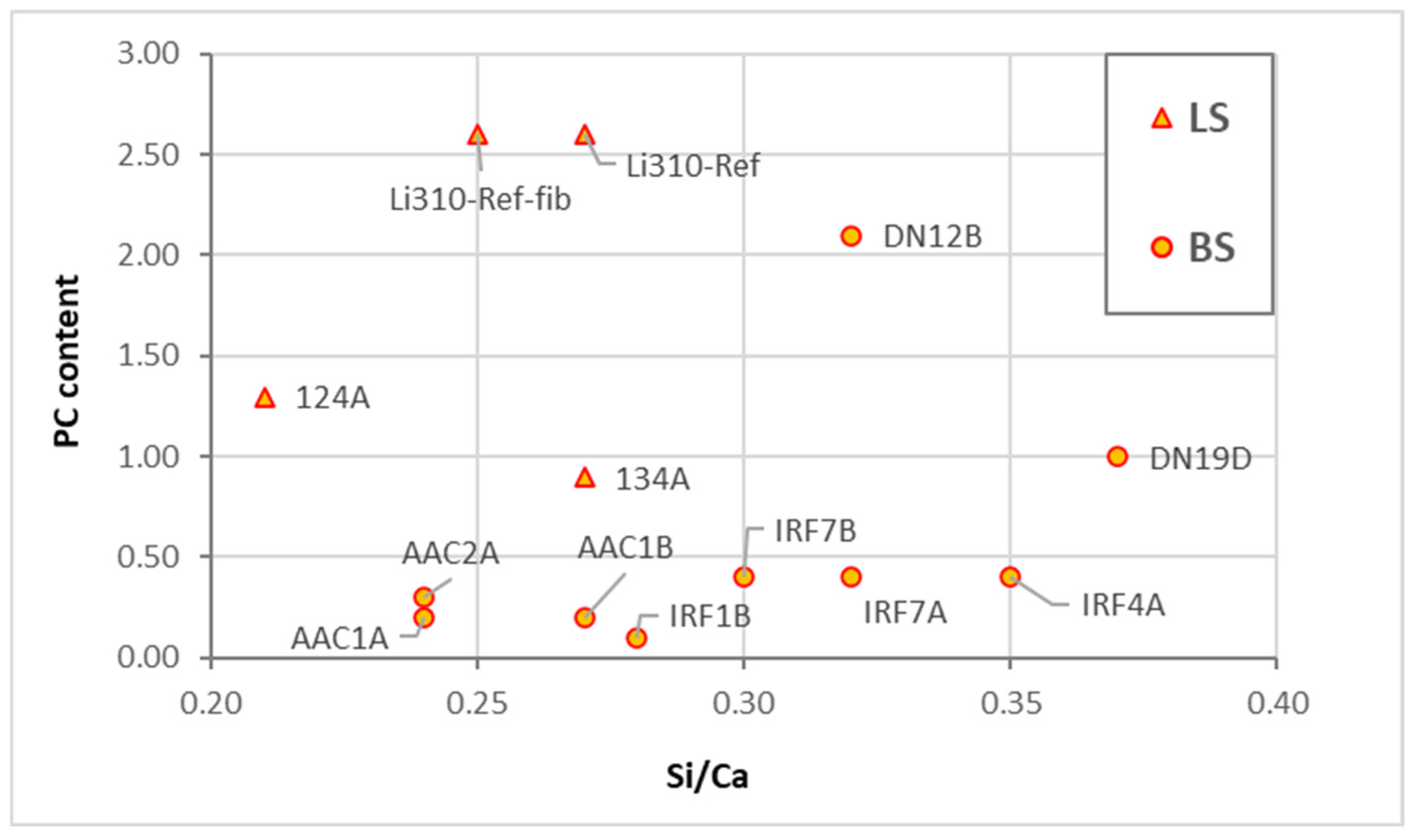
- The interval ranges for the chemical compositions of the binders were obtained with precision through EDS analyses. The following interval of elemental ratios Al/Ca and Si/Ca were established for the types of binders identified in Table 5, ensuring the accuracy of the results.
| Al/Ca | Si/Ca | |||
|---|---|---|---|---|
| Binder Type | Min. | Max. | Min. | Max. |
| Air lime | 0.02 | 0.09 | 0.09 | 0.20 |
| Natural hydraulic lime | 0.12 | 0.15 | 0.21 | 0.28 |
| Natural cement | 0.12 | 0.16 | 0.23 | 0.35 |
| Blended air lime and Portland cement (including ordinary, white, and Portland composite cement types) | 0.04 | 0.13 | 0.21 | 0.37 |
| Portland cement (including ordinary and white types) | 0.03 | 0.23 | 0.21 | 0.45 |
| Portland composite cements | 0.17 | 0.23 | 0.41 | 0.49 |
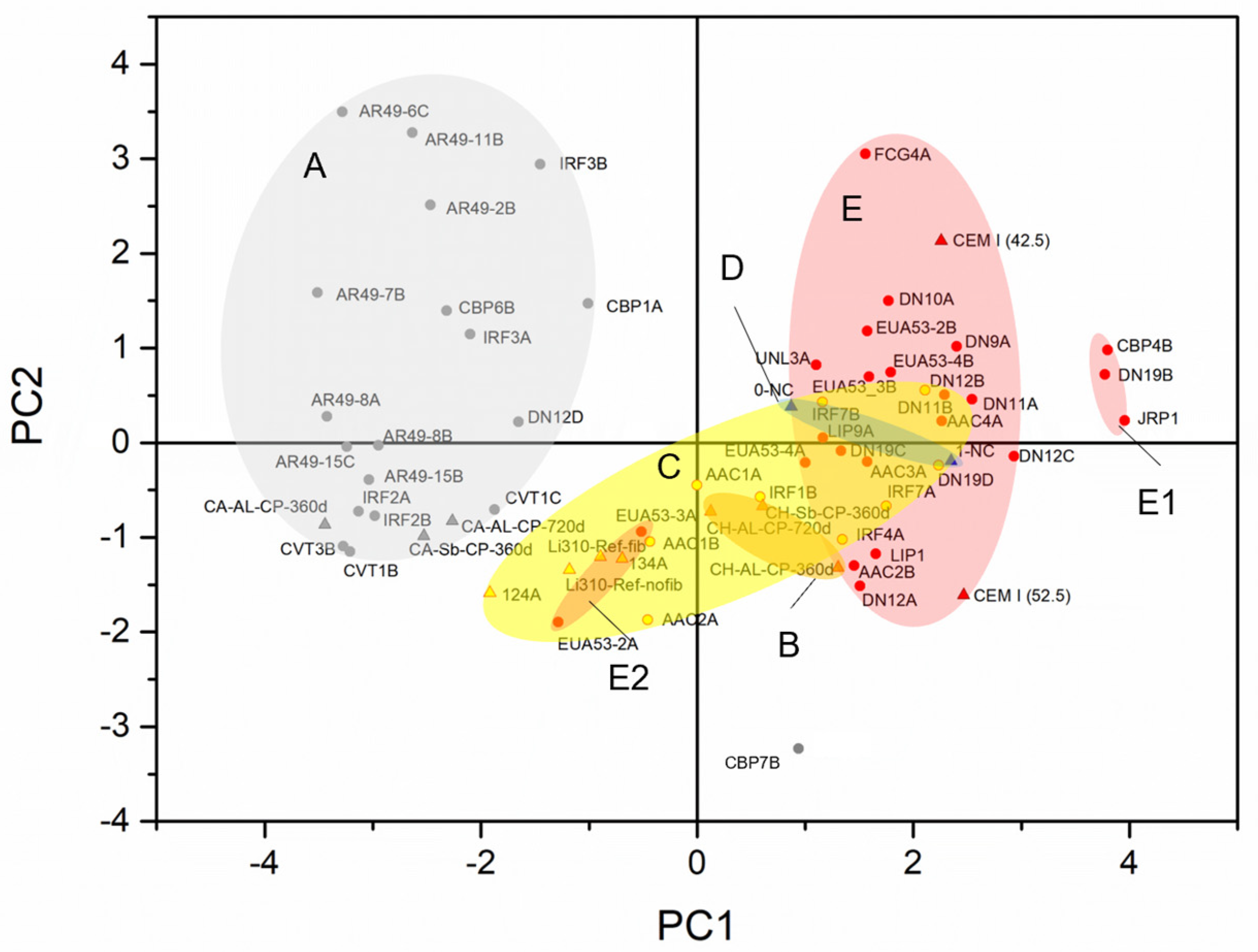
- PCA analysis did not significantly refine the clusters because the samples have similar representations of the principal components. This suggests that PCA should not be applied to samples with elements that exhibit the same weight in their chemical composition.
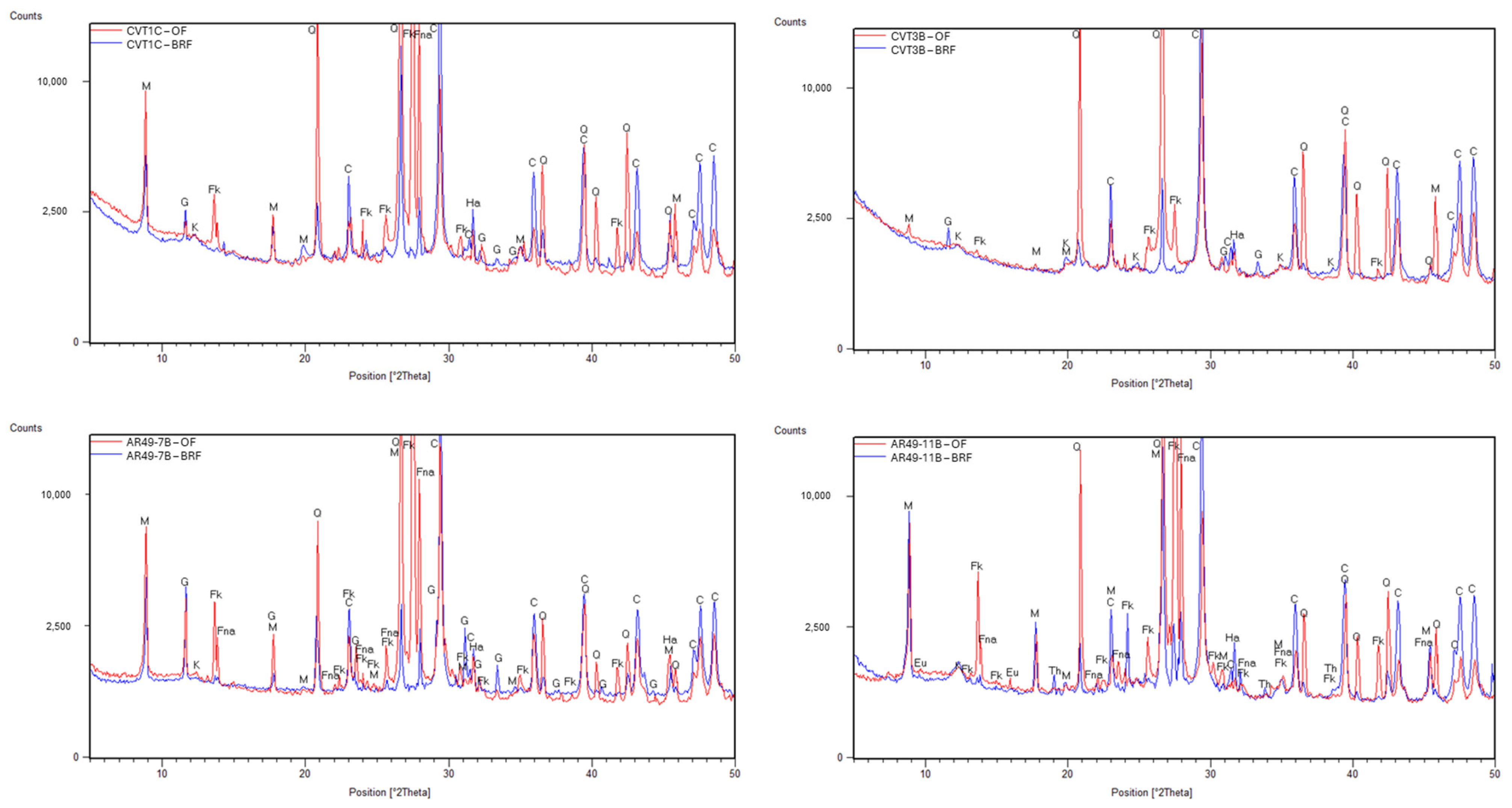
- When dealing with overlapping grouped chemical results, optical microscopy is a critical step in identifying the key compounds of the binder. When consistently applied by a skilled petrographer, this practice ensures the accuracy and reliability of the results.
- Of the key compounds mentioned above, some stand out in helping to distinguish between historical hydraulic binders, such as: natural cement binders (calcined marl fragments, under-burned, well-burned, and over-burned marl fragments, presence of unhydrated C2S crystals and the absence (or minimal presence) of alite); NHL binders (presence of small dark inclusions (non-hydrated relicts of C2S), hydration rims around individual hydraulic phases, presence of gehlenite); and portland cement binders (large size of the unhydrated C3S crystals in historic Portland cement and ubiquity of C3S in all types of Portland cement).
- In blended mortars, the pastes’ homogeneity, or compositional variability, which may be attributable to the influence of the raw materials’ composition, varying according to their provenance, points to being influential in EDS analyses.
- Although some of the samples analyzed were manufactured in the laboratory, namely the natural hydraulic lime, Portland cement, and air lime mixed with Portland cement test specimens, the chemical results can be extrapolated to these types of binders, historically applied in other periods, since the raw materials and calcination temperatures are similar to those obtained in the past, even though today there is greater quality control in the manufacturing processes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsen, J.; Van Balen, K.; Mertens, G. Hydraulicity in historic mortars: A review. In Proceedings of the 2nd Historic Mortars Conference HMC2010 and RILEM TC 203-RHM Final Workshop, Prague, Czech Republic, 22–24 September 2010. [Google Scholar]
- Furlan, V.; Bissenger, P. Les mortiers anciens. Histoire et essai d’analyse scientifique. Rev. Suisse D’art D’archéol. 1975, 32, 2–14. [Google Scholar]
- Rispoli, C.; Montesano, G.; Verde, M.; Balassone, G.; Columbu, S.; De Bonis, A.; Di Benedetto, C.; D’Uva, F.; Esposito, R.; Graziano, S.F.; et al. The key to ancient Roman mortars hydraulicity: Ceramic fragments or volcanic materials? A lesson from the Phlegrean archaeological area (southern Italy). Constr. Build. Mater. 2024, 411, 134408. [Google Scholar] [CrossRef]
- Eramo, G.; Spalluto, L.; Laviano, R. Paving stones of the Via Traiana in Egnazia (Brindisi, 2nd A.D.): Provenance of stones [Il basolato della Via Traiana nel sito archeologico di Egnazia (Fasano, Br): Provenienza dei materiali lapidei]. Rend. Online Soc. Geol. Ital. 2008, 3, 357–358. [Google Scholar]
- Lezzerini, M.; Legnaioli, S.; Lorenzetti, G.; Palleschi, V.; Tamponi, M. Characterization of historical mortars from the bell tower of St. Nicholas church (Pisa, Italy). Constr. Build. Mater. 2014, 69, 203–212. [Google Scholar] [CrossRef]
- Miriello, D.; Bloise, A.; Crisci, G.M.; De Luca, R.; De Nigris, B.; Martellone, A.; Osanna, M.; Pace, R.; Pecci, A.; Ruggieri, N. New compositional data on ancient mortars and plasters from Pompeii (Campania—Southern Italy): Archaeometric results and considerations about their time evolution. Mater. Charact. 2018, 146, 189–203. [Google Scholar]
- Lezzerini, M.; Spampinato, M.; Sutter, A.; Montevecchi, N.; Aquino, A. Petrographic characteristics of the mortars from the Pisa’s Cathedral apse. In Proceedings of the IMEKO TC4 International Conference on Metrology for Archaeology and Cultural Heritage, MetroArchaeo, Calabria, Italy, 19–21 October 2022; pp. 459–463. [Google Scholar]
- Sitzia, F.; Beltrame, M.; Columbu, S.; Lisci, C.; Miguel, C.; Mirão, J. Ancient restoration and production technologies of Roman mortars from monuments placed in hydrogeological risk areas: A case study. Archaeol. Anthropol. Sci. 2020, 12, 147. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Ricca, M.; Rovella, N.; Comite, V.; De Buergo, M.A.; Crisci, G.M.; Barca, D. Archaeometric approach for the study of mortars from the underwater archaeological site of Baia (Naples) Italy: Preliminary results. Period. Miner. 2015, 84, 553–557. [Google Scholar] [CrossRef]
- Rispoli, C.; De Bonis, A.; Guarino, V.; Graziano, S.F.; di Benedetto, C.; Esposito, R.; Morra, V.; Cappelletti, P. The Ancient Pozzolanic Mortars of the Thermal Complex of Baia (Campi Flegrei, Italy). J. Cult. Herit. 2019, 40, 143–154. [Google Scholar] [CrossRef]
- Parker, J. A Certain Cement or Terras to Be Used in Aquatic and Other Buildings and Stucco Work. British Patent n. 2120, 27 July 1796. [Google Scholar]
- Kozłowski, R.; Hughes, D.; Weber, J. Roman cements: Key materials of the built heritage of the 19th Century. In Materials, Technologies and Practice in Historic Heritage Structures; Dan, M.B., Přikryl, R., Török, A., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Hughes, D.; Swann, S.; Gardner, A. Roman cement. J. Arch. Conserv. 2007, 13, 21–36. [Google Scholar] [CrossRef]
- CEN EN 459-1; Building Lime—Part 1: Definitions, Specifications, and Conformity Criteria. European Committee for Standardization: Brussels, Belgium, 2015.
- Coutinho, A.S. Fabrico e Propriedades do Betão; Laboratório Nacional de Engenharia Civil (LNEC): Lisboa, Portugal, 1988; Volume 1. [Google Scholar]
- Weber, J.; Köberle, T.; Pintér, F. Methods of microscopy to identify and characterise hydraulic binders in historic mortars—A methodological approach. In Historic Mortars; Hughes, J., Válek, J., Groot, C., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Callebaut, K.; Elsen, J.; Van Balen, K.; Viaene, W. Nineteenth century hydraulic restoration mortars in the Saint Michael’s Church (Leuven, Belgium): Natural hydraulic lime or cement? Cem. Concr. Res. 2001, 31, 397–403. [Google Scholar] [CrossRef]
- Gadermayr, N.; Pintér, F.; Weber, J. Identification of 19th century roman cements by the phase composition of clinker residues in historic mortars. In Proceedings of the 12th International Congress on the Deterioration and Conservation of Stone, New York, NY, USA, 22–25 October 2012. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry; Academic Press: New York, NY, USA, 1990. [Google Scholar]
- Veiga, M.R.; Aguiar, J.; Santos Silva, A.; Carvalho, F. Conservação e Renovação de Revestimentos de Paredes de Edifícios Antigos; LNEC: Lisboa, Portugal, 2004; p. 126. [Google Scholar]
- Veiga, M.; Velosa, A.; Magalhães, A. Evaluation of mechanical compatibility of renders to apply on old walls based on a restrained shrinkage test. Mater. Struct. 2006, 40, 1115–1126. [Google Scholar]
- Almeida, L.; Santos Silva, A.; Veiga, R.; Mirão, J. Composition of renders and plasters of award-winning buildings in Lisbon (Portugal): A contribution to the knowledge of binders used in the 20th Century. Int. J. Arch. Herit. 2023, 1–31. [Google Scholar] [CrossRef]
- Mayo, C.M.; Ramírez-Casas, J.; Sanz, D.; Ezquerra, A.N.; Rosel, J.R. Methodology of identification of natural and historic Portland cements. Application and study in mortars of Madrid and Barcelona. In Proceedings of the 5th Historic Mortars Conference, Pamplona, Spain, 19–21 June 2019. [Google Scholar]
- Middendorf, B.; Hughes, J.J.; Callebaut, K.; Baronio, G.; Papayianni, I. Investigative methods for the characterisation of historic mortars—Part 2: Chemical characterisation. Mater. Struct. 2005, 38, 771–780. [Google Scholar] [CrossRef]
- Arliguie, G.; Hornain, H. Grandubé: Grandeurs Associées à la Durabilité des Bétons, 1st ed.; Presses Ecole Nationale Ponts Chaussees: Paris, France, 2007; p. 438. [Google Scholar]
- Lindqvist, J.E.; Sandström, M. Recommendations of RILEM TC 167-COM: Characterization of old mortars. Quantitative analysis of historical mortars using optical microscopy. Mater. Struct. 2000, 167, 612–617. [Google Scholar] [CrossRef]
- Walsh, J.J. Petrography: Distinguishing Natural Cement from Other Binders in Historical Masonry Construction Using Forensic Microscopy Techniques. J. ASTM Intern. 2007, 4, 1. [Google Scholar]
- Pavia, S.; Caro, S. Petrographic microscope investigation of mortar and ceramic technologies for the conservation of the built heritage. In Proceedings of the SPIE O3A: Optics for Arts, Architecture, and Archaeology, Munich, Germany, 16 July 2007; Volume 6618. [Google Scholar]
- Mertens, G.; Madau, P.; Durinck, D.; Blanpain, B.; Elsen, J. Quantitative mineralogical analysis of hydraulic limes by X-ray diffraction. Cem. Concr. Res. 2007, 37, 1524–1530. [Google Scholar] [CrossRef]
- Santos, A.R.; Veiga, M.R.; Santos Silva, A.; Brito, J.; Álvarez, J.I. Evolution of the microstructure of lime based mortars and influence on the mechanical behaviour: The role of the aggregates. Constr. Build. Mater. 2018, 187, 907–922. [Google Scholar] [CrossRef]
- Santos, A.R.; Veiga, M.R.; Santos Silva, A.; Brito, J. Impact of aggregates on fresh mortars’ properties. In Proceedings of the 5th Historic Mortars Conference, Pamplona, Spain, 19–21 June 2019. [Google Scholar]
- Pederneiras, C.M. Improving Cracking Performance of Mortars with Selected Recycled Fibres for Non-Structural Uses. Ph.D. Thesis, Civil Engineering, Instituto Superior Técnico, University of Lisbon, Lisbon, Portugal, 2021. [Google Scholar]
- Famya, C.; Brough, A.R.; Taylor, H.F.W. The C-S-H gel of Portland cement mortars: Part I. The interpretation of energy-dispersive X-ray microanalyses from scanning electron microscopy, with some observations on C-S-H, AFm and Aft phase compositions. Cem. Concr. Res. 2003, 3, 1389–1398. [Google Scholar]
- Balksten, K.; Nitz, B.; Hughes, J.J.; Lindqvist, J.E. Petrography of historic mortar materials: Polarising light microscopy as a method for characterising lime-based mortars. In Proceedings of the 5th Historic Mortars Conference, Universidad de Navarra, Pamplona, Spain, 19–21 June 2019. [Google Scholar]
- Elsen, J.; Mertens, G.; Snellings, R. Portland cement and other calcareous hydraulic binders: History, production and mineralogy. Europ. Mineral. Uni. Notes Miner. 2011, 9, 441–479. [Google Scholar] [CrossRef]
- Vicat, L.J. Recherches Expérimentales sur les Chaux de Construction, les Bétons et les Mortiers Ordinaires; Goujon: Paris, France, 1818. [Google Scholar]
- Eckel, E. Cements, Limes and Plasters. Their Materials, Manufacture and Properties; Routledge, Taylor & Francis Group: New York, NY, USA, 2015. [Google Scholar]
- Mertens, G.; Elsen, J.; Laduron, D.; Brulet, R. Mineralogy of the calcium-silicate phases present in ancient mortars from Tournai. Archeometrie 2006, 30, 61–65. [Google Scholar]
- Mertens, G.; Lindqvist, J.E.; Sommain, D.; Elsen, J. Calcareous Hydraulic Binders from a Historical Perspective. In Proceedings of the 1st Historical Mortars Conference, Characterization, Diagnosis, Conservation, Repair and Compatibility, Lisbon, Portugal, 24–28 September 2008; pp. 1–15. [Google Scholar]
- Pecchioni, E.; Fratini, F.; Cantisani, E. Atlas of the Ancient Mortars in Thin Section Under Optical Microscope, 2nd ed.; Nardini: Florence, Italy, 2020; p. 78. [Google Scholar]
- Bugini, R.; Toniolo, L. La presenza di grumi bianchi nelle malte antiche: Ipotesi sull’origine. Arkos 1990, 12, 4–8. [Google Scholar]
- Cantisani, E.; Fratini, F.; Pecchioni, E. Optical and Electronic Microscope for Minero-Petrographic and Microchemical Studies of Lime Binders of Ancient Mortars. Minerals 2022, 12, 41. [Google Scholar] [CrossRef]
- Hughes, J.; Cuthbert, S. The petrography and microstructure of medieval lime mortars from the west of Scotland: Implications for the formulation of repair and replacement mortars. Mater. Struct. 2000, 33, 594–600. [Google Scholar]
- Pavia, S.; Fitzgerald, B.; Howard, R. Evaluation of properties of magnesian lime mortar. In Structural Studies, Repair, and Maintenance of Heritage Architecture IX; Brebbia, C.A., Torpiano, A., Eds.; Transactions on the Built Environment; WIT Press: Southampton, UK, 2005; Volume 83, pp. 375–384. [Google Scholar]
- Leslie, A.B.; Hughes, J.J. Binder microstructure in lime mortars: Implications for the interpretation of analysis results. Quart. J. Eng. Geol. 2002, 35, 257–263. [Google Scholar]
- Leslie, A.B.; Gibbons, P. Mortar analysis and repair specification in the conservation of Scottish historic buildings. In Proceedings of the International RILEM Workshop on Historic Mortars: Characteristics and Tests; Bartos, P., Groot, C., Hughes, J.J., Eds.; RILEM: Paisley, UK, 2000; pp. 273–280. [Google Scholar]
- Furlan, V.; Pancella, R. Examen microscopique en lumière rèfléchie de ciments, bétons et mortiers. Chantiers 1982, 13, 25–30. [Google Scholar]
- Gödicke-Dettmering, T.; Strübel, G. Mineralogische und technologische Eigenschaften von hydraulischen Kalken als Bindemittel für Restaurierungsmörtel in der Denkmalpflege. Giessener Geol. Schr. 1996, 56, 131–154. [Google Scholar]
- Pecchioni, E.; Fratini, F.; Cantisani, C. Le Malte Antiche e Moderne tra Tradizione e Innovazione, 2nd ed.; Pàtron: Bologna, Italy, 2018; p. 231. [Google Scholar]
- Moropoulu, A.; Cakmak, A.S.; Biscontin, G.; Bakolas, A.; Zendri, E. Advanced Byzantine cement based composites resisting earthquake stress: The crushed brick/lime mortars of Justinian’s Hagia Sophia. Constr. Build. Mater. 2002, 16, 543–552. [Google Scholar]
- Ingham, J. Geomaterials under the Microscope: A Colour Guide; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 192. [Google Scholar]
- Mayo, C.; Sanz, D.; Pineda, J.I. Metodología simplificada de identificación mediante MOP de las cales hidráulicas y los cementos naturales. In Proceedings of the VI Jornadas FICAL—Forum Iberico de la Cal, Pamplona, Spain, 28–30 May 2018. [Google Scholar]
- Jepsen, B.B.; Christensen, P. Petrographic examination of hardened concrete. Bull. Intern. Assoc. Engin. Geol. 1989, 39, 99–103. [Google Scholar]
- Poole, A.; Sims, I. Concrete Petrography. A Handbook of Investigative Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Pavía, S. A Petrographic study of the technology of hydraulic mortars at masonry bridges, harbours and mill ponds. In Proceedings of the Concrete Research and Bridge Infrastructure Symposium, Galway, Ireland, 4–5 December 2008; pp. 253–264. [Google Scholar]


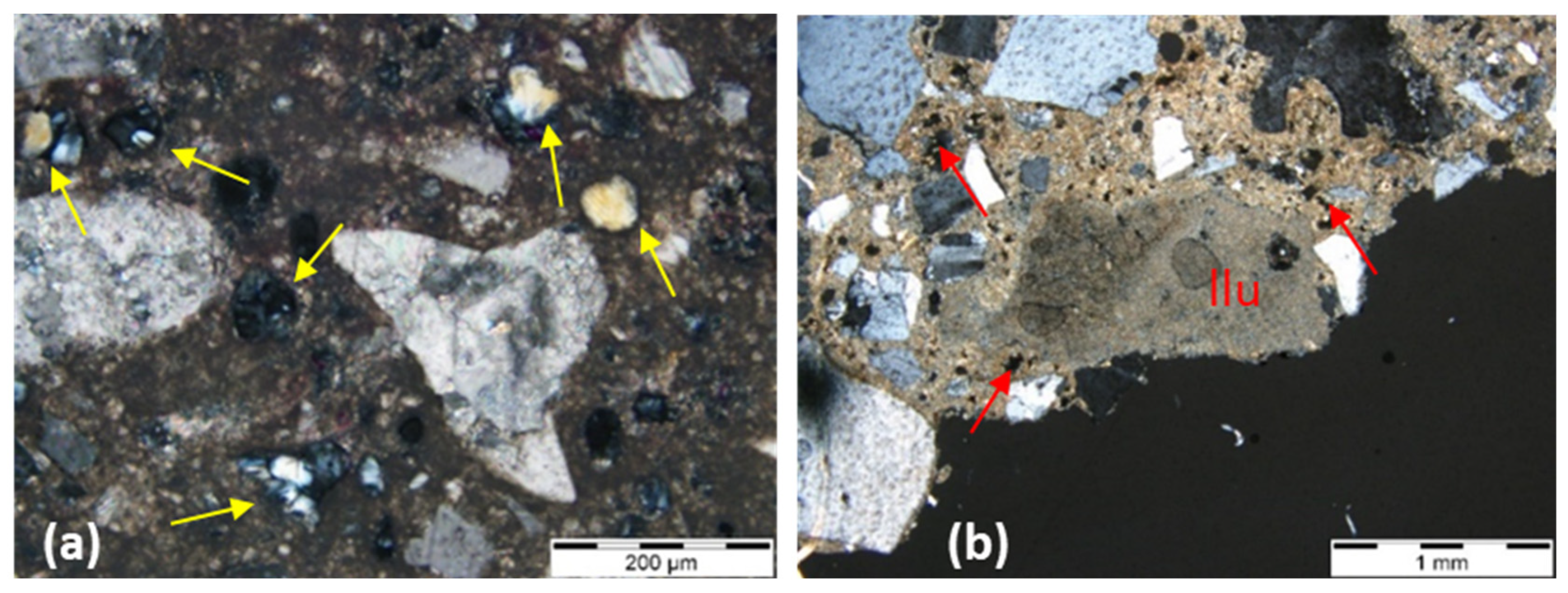
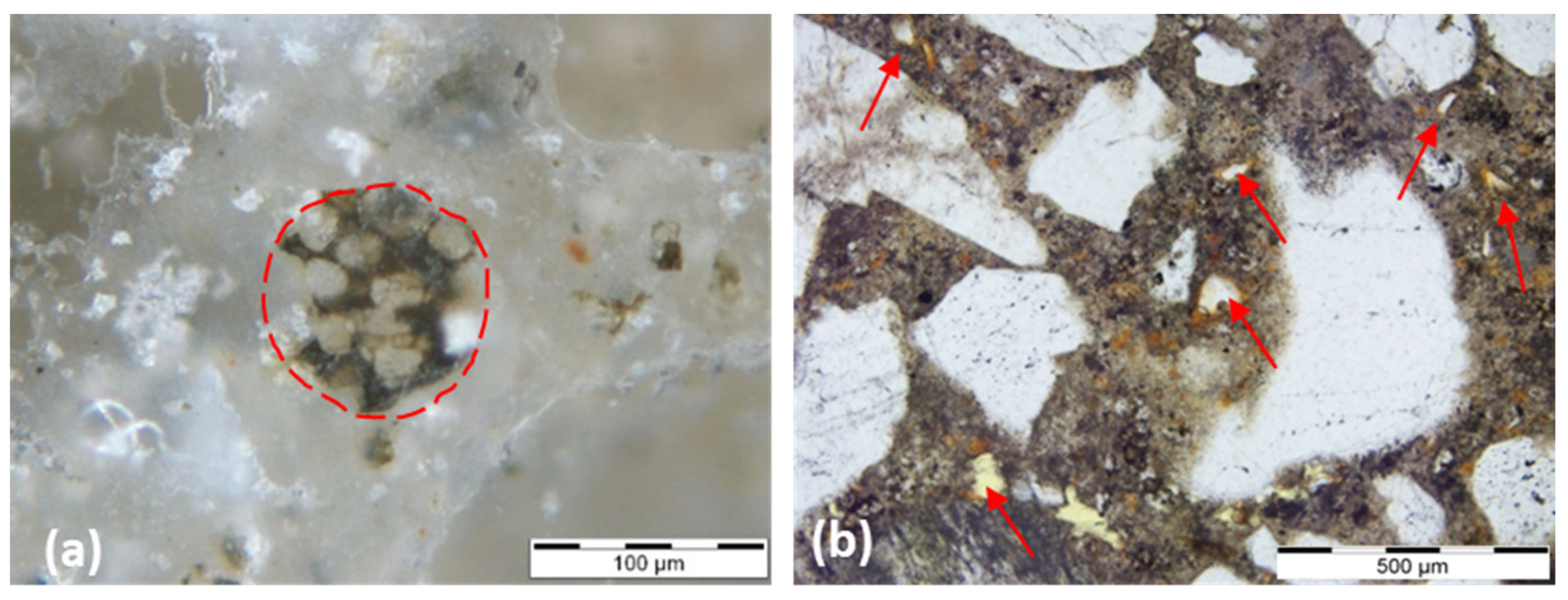
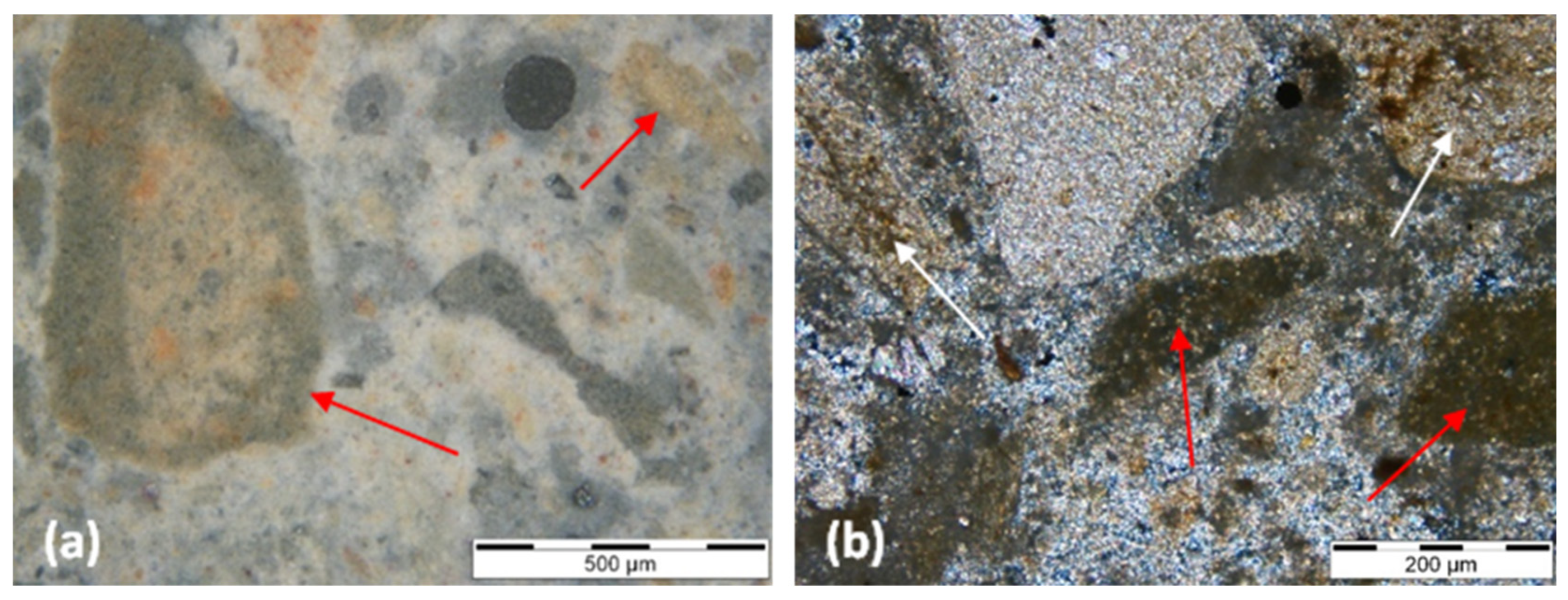
| Sample ID | Construction Period | Case Study/Building | Type of Building | Binder Type | Binder to Aggregate Ratio by Weight (b:a) |
|---|---|---|---|---|---|
| CVT1B | 1902–1903 | CVT (1903)/ Ventura Terra building (Lisbon) | Residencial | AL | 1:5.4 |
| CVT1C | AL | 1:7.8 | |||
| CVT3B | AL | 1:4.3 | |||
| AR49-2B | 1920–1923 | AR49 (1923)/ Luiz Rau building (Lisbon) | Residential | AL | (c) |
| AR49-6C | AL | 1:5.8 | |||
| AR49-7B | AL | 1:3 | |||
| AR49-8A | AL | 1:2.9 | |||
| AR49-8B | AL | 1:11.2 | |||
| AR49-11B | AL | 1:6.7 | |||
| AR49-15B | AL | 1:7.1 | |||
| AR49-15C | AL | 1:7.9 | |||
| IRF1B | 1934–1938 | IRF (1938)/Nossa Senhora de Fátima church (Lisbon) | Church | AL+OPC | 1:0.1:7 |
| IRF2A | AL | 1:4.3 | |||
| IRF2B | AL | 1:9.9 | |||
| IRF3A | AL | 1:4.2 | |||
| IRF3B | AL | 1:8 | |||
| IRF4A | AL+OPC | 1:0.4:5.4 | |||
| IRF7A | AL+OPC | 1:0.4:3.8 | |||
| IRF7B | AL+OPC | 1:0.4:5.3 | |||
| CBP1A | 1938–1939 | CBP (1939)/Bernardo da Maia house (Lisbon) | Residential | AL | 1:8.4 |
| CBP4B (d) | OPC | 1:20.3 | |||
| CBP6B | AL | 1:8.7 | |||
| CBP7B | AL | 1:11.2 | |||
| DN9A | 1936–1940 | DN (1940)/Diário de Notícias building (Lisbon) | Office and service | OPC | 1:6.1 |
| DN10A | OPC | 1:7 | |||
| DN11A | OPC | 1:7.4 | |||
| DN11B | OPC | 1:4.2 | |||
| DN12A | OPC | 1:12.9 | |||
| DN12B | AL+OPC | 1:2.1:15.1 | |||
| DN12C | OPC | 1:4.2 | |||
| DN12D | AL | 1:4.3 | |||
| DN19B | PCC | 1:25.2 (a) | |||
| DN19C | OPC | 1:8.9 | |||
| DN19D | AL+PCC | 1:1:6 (a) | |||
| AAC1A | 1942–1944 | AAC (1944)/Cristino da Silva building (Lisbon) | Residential | AL+OPC | 1:0.2:6.1 |
| AAC1B | AL+OPC | 1:0.2:7 | |||
| AAC2A (c) | AL+WPC | 1:0.3:1.0 | |||
| AAC2B | OPC | 1:24.5 | |||
| AAC3A (c) | OPC | 1:3.0 | |||
| AAC4A (c) | OPC | 1:1.9 | |||
| LIP1A | 1955–1957 | LIP (1954)/Laboratories of Pasteur Institute of Lisbon (Lisbon) | Laboratory and services | OPC | 1:7.6 |
| LIP9A | OPC | 1:6.6 | |||
| EUA53-2A (c) | 1966–1969 | EUA53 (1970)/America building (Lisbon) | Residential and commercial | WPC (e) | 1:3.7 |
| EUA53-2B | OPC | 1:6.7 | |||
| EUA53-3A (c) | WPC (e) | 1:19.1 | |||
| EUA53-3B | OPC | 1:4.9 | |||
| EUA53-4A (c) | WPC | 1:11.1 | |||
| EUA53-4B | OPC | 1:11.5 | |||
| FCG4A | 1963–1969 | FCG (1975)/Calouste Gulbenkian Foundation Headquarters and Museum (Lisbon) | Office and cultural facilities | OPC | 1:10.2 |
| JRP2A | 1984–1987 | JRP (1987)/Jacob Rodrigues Pereira Institute (Lisbon) | Educational/academic | PCC | 1:4.9 (a) |
| UNL3A | 2000–2002 | UNL (2002)/New University of Lisbon Rectory (Lisbon) | Educational/academic | OPC | 1:10.2 |
| 0-NC | 1887 | Hivernacle (Barcelona) | Greenhouse | NC | (b) |
| 1-NC | 1900 | Villarroel (Barcelona) | Residential | NC | (b) |
| Specimens ID | Mortar Formulation | Binder Type | b:a (a) |
|---|---|---|---|
| CA-Sb-CP-360d | CL90-S air lime mortar with siliceous sand, 360 days laboratory curing | AL | 1:11 |
| CA-AL-CP-360d | CL90-S air lime mortar with washed siliceous sand, 360 days laboratory curing | AL | 1:11 |
| CH-AL-CP-360d | NHL 3.5 (1) mortar with washed siliceous sand, 360 days laboratory curing | NHL | 1:5.6 |
| CH-Sb-CP-360d | NHL 3.5 (1) mortar with siliceous sand, 360 days laboratory curing | NHL | 1:5.6 |
| CEM I (42.5) | Portland cement mortar (CEM I 42.5) with siliceous sand, 180 days laboratory curing | OPC | 1:3 |
| CEM I (52.5) | Portland cement type mortar (CEM I 52.5) with siliceous sand, 180 days laboratory curing | OPC | 1:3 |
| Li310-Ref-fib | Blended mortar with air lime and Portland cement (CEM I 42.5) with siliceous sand and organic fibers, 360 days laboratory curing | AL+OPC | 1:2.6:26.3 |
| Li310-Ref | Blended mortar with air lime and Portland cement (CEM I 42.5) with siliceous sand, 360 days laboratory curing | AL+OPC | 1:2.6:26.3 |
| CA-AL-CP-720d | CL90-S air lime mortar with washed siliceous sand, 720 days laboratory curing | AL | 1:11 |
| CH-AL-CP-720d | NHL 3.5 (1) mortar with washed siliceous sand, 720 days laboratory curing | NHL | 1:5.6 |
| 124A | Blended mortars with air lime and white Portland cement (CEM I 42.5) with siliceous sand, 4 years laboratory curing | AL+WPC | 1:1.3:19.7 |
| 134A | AL+WPC | 1:0.9:17.5 |
| Sample ID | Case Study | Binder Type | Al/Ca | Si/Ca | |
|---|---|---|---|---|---|
| CVT1B | Lisbon buildings | CVT (1903) | AL | 0.03 | 0.11 |
| CVT1C | AL | 0.06 | 0.19 | ||
| CVT3B | AL | 0.02 | 0.15 | ||
| AR49-2B | AR49 (1923) | AL | 0.05 | 0.13 | |
| AR49-6C | AL | 0.05 | 0.12 | ||
| AR49-7B | AL | 0.02 | 0.10 | ||
| AR49-8A | AL | 0.03 | 0.11 | ||
| AR49-8B | AL | 0.05 | 0.19 | ||
| AR49-11B | AL | 0.07 | 0.16 | ||
| AR49-15B | AL | 0.05 | 0.14 | ||
| AR49-15C | AL | 0.05 | 0.17 | ||
| IRF1B | IRF (1938) | AL+OPC | 0.12 | 0.28 | |
| IRF2A | AL | 0.04 | 0.09 | ||
| IRF2B | AL | 0.04 | 0.12 | ||
| IRF3A | AL | 0.06 | 0.12 | ||
| IRF3B | AL | 0.05 | 0.14 | ||
| IRF4A | AL+OPC | 0.11 | 0.35 | ||
| IRF7A | AL+OPC | 0.13 | 0.32 | ||
| IRF7B | AL+OPC | 0.11 | 0.30 | ||
| CBP1A | CBP (1939) | AL | 0.07 | 0.20 | |
| CBP4B | OPC | 0.23 | 0.44 | ||
| CBP6B | AL | 0.03 | 0.13 | ||
| CBP7B | AL | 0.09 | 0.21 | ||
| DN9A | DN (1940) | OPC | 0.11 | 0.39 | |
| DN10A | OPC | 0.09 | 0.34 | ||
| DN11A | OPC | 0.10 | 0.40 | ||
| DN11B | OPC | 0.10 | 0.33 | ||
| DN12A | OPC | 0.12 | 0.44 | ||
| DN12B | AL+OPC | 0.13 | 0.32 | ||
| DN12C | OPC | 0.12 | 0.45 | ||
| DN12D | AL | 0.05 | 0.15 | ||
| DN19B | PCC | 0.23 | 0.41 | ||
| DN19C | OPC | 0.07 | 0.31 | ||
| DN19D | AL+PCC | 0.13 | 0.37 | ||
| AAC1A | AAC (1944) | AL+OPC | 0.05 | 0.24 | |
| AAC1B | AL+OPC | 0.09 | 0.27 | ||
| AAC2A | AL+WPC | 0.05 | 0.22 | ||
| AAC2B | OPC | 0.09 | 0.36 | ||
| AAC3A | OPC | 0.10 | 0.31 | ||
| AAC4A | OPC | 0.09 | 0.34 | ||
| LIP1A | LIP (1954) | OPC | 0.13 | 0.35 | |
| LIP9A | OPC | 0.07 | 0.32 | ||
| EUA53-2A | EUA53 (1970) | WPC | 0.05 | 0.22 | |
| EUA53-2B | OPC | 0.09 | 0.30 | ||
| EUA53-3A | WPC | 0.03 | 0.21 | ||
| EUA53-3B | OPC | 0.08 | 0.32 | ||
| EUA53-4A | WPC | 0.08 | 0.30 | ||
| EUA53-4B | OPC | 0.09 | 0.32 | ||
| FCG4A | FCG (1975) | OPC | 0.11 | 0.33 | |
| JRP2A | JRP (1987) | PCC | 0.17 | 0.49 | |
| UNL3A | UNL (2002) | OPC | 0.08 | 0.27 | |
| CA-Sb-CP-360d | Laboratory formulated test specimens | AL | 0.06 | 0.11 | |
| CA-AL-CP-360d | AL | 0.05 | 0.07 | ||
| CH-AL-CP-360d | NHL | 0.15 | 0.26 | ||
| CH-Sb-CP-360d | NHL | 0.12 | 0.21 | ||
| CEM I (42.5) | OPC | 0.09 | 0.33 | ||
| CEM I (52.5) | OPC | 0.08 | 0.45 | ||
| Li310-Ref-fib | AL+OPC | 0.05 | 0.25 | ||
| Li310-Ref | AL+OPC | 0.05 | 0.27 | ||
| CA-AL-CP-720d | AL | 0.06 | 0.17 | ||
| CH-AL-CP-720d | NHL | 0.12 | 0.28 | ||
| 124A | AL+WPC | 0.04 | 0.21 | ||
| 134A | AL+WPC | 0.08 | 0.27 | ||
| 0-NC | Barcelona buildings | NC | 0.12 | 0.23 | |
| 1-NC | NC | 0.16 | 0.35 | ||
| Authors [by Reference] | |||||||
|---|---|---|---|---|---|---|---|
| NHL and other hydraulic lime mortars/binders | [17] | [27] | [47] | [48] | [49,50] | [40,49,51] | [51] |
| Large C2S and C3S phases in a small amount of a brown matrix consisting of C3A and C4AF. | Small clusters of C2S may be detected in trace quantities in hydraulic limes. | Dominant C2S grains can present striations in different directions | Presence of “hot spots” as part of the hydraulic phases that result from local higher temperatures in the lime kiln. | If reaction rims did not develop, hydraulic phases dispersed in the binder can nevertheless still be observed both as veins and as pore filling. | Presence of small dark inclusions (non-hydrated relicts of C2S) that can be easily recognised. | C3S can be formed due to an overheating of the raw materials during the production of NHL | |
| C3S can also be present in smaller quantities. | Hydration rims can be observed around individual hydraulic phases as a colourless rim. | The presence of gehlenite is a valuable indicator of the distinction between cement and natural hydraulic lime. | C2S: dark brown crystals medium-high relief, shaded contours, and sub idiomorphic habit. | ||||
| Natural cement | [16] | [23] | [27] | [13] | [52] | ||
| The NC residual nodules exhibit strong zoning which is best visible in transmitted light with parallel polars. | Presence of lumps of calcined marls in the natural cement (due to the calcination and to the deficient milling technology). | Under polarised light, the matrix of natural cements often appears spotted with dark isotropic areas broken by bright but dense carbonated regions. | High proportion of non-reactive ‘nodules’ or relicts (under-burned, well burned, and over-burned). | Under-burned, well burned, and over-burned marl fragments. | |||
| NC mortars can be identified equally well at low magnifications as their binders contain residual compounds that are larger than the clinker of historic Portland cement. | Presence of unhydrated C2S crystals. | The dispersed quartz grains and other sand-sized silicate minerals also aid in distinguishing natural cements. These tend to retain most of their original texture when burned at calcining temperatures. | The largest ‘nodules’ are of millimetre size. | NHL does not show calcined marl fragments and have a more homogeneous structure. | |||
| Absence of unhydrated C3S crystals. | Contain greater percentage of relicts than either the hydraulic limes or the Portland cements. | Lime nodules can be observed in the NHL samples, while the natural cements lack these nodules. | |||||
| OPC | [23] | [27] | [53,54,55] | ||||
| Large size of the unhydrated C3S crystals in historic Portland cements. The calcination process in vertical kilns was longer than in a modern horizontal kiln and it allowed the C2S and C3S crystals to acquire a larger size than in modern Portland cement. | Portland cement pastes are homogeneously isotropic or dark-colored where cementitious gels have formed, broken only by thin dispersed grains of calcium hydroxide that appear bright-colored. | The unhydrated cement grains are identified by the optical properties of the cement minerals, primarily C3S and C2S. | |||||
| WPC | [27] | ||||||
| The C2S phases are not surrounded by a brown-colored crystallographically indistinct ferrite phase (absence of brownish C4AF in the residual cement grains). | |||||||
| PCC | [53,54] | ||||||
| Unhydrated fly ash particles; GBFS—glassy slag particles with reddish hydration rims. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, L.; Santos Silva, A.; Veiga, R.; Mirão, J. An Approach to Accurately Identifying Binders in Historic Mortars by the Combination of Microscopic and Microanalytical Techniques. Minerals 2024, 14, 844. https://doi.org/10.3390/min14080844
Almeida L, Santos Silva A, Veiga R, Mirão J. An Approach to Accurately Identifying Binders in Historic Mortars by the Combination of Microscopic and Microanalytical Techniques. Minerals. 2024; 14(8):844. https://doi.org/10.3390/min14080844
Chicago/Turabian StyleAlmeida, Luís, António Santos Silva, Rosário Veiga, and José Mirão. 2024. "An Approach to Accurately Identifying Binders in Historic Mortars by the Combination of Microscopic and Microanalytical Techniques" Minerals 14, no. 8: 844. https://doi.org/10.3390/min14080844





