The Radioactive Rare Metal Mineralization in the World-Class Sn-Nb-Ta-U-Th-REE-Deposit Madeira (Pitinga, Amazonas State, Brazil): With Special Reference to the Complex Alteration of Pyrochlore-Group Minerals
Abstract
:1. Introduction
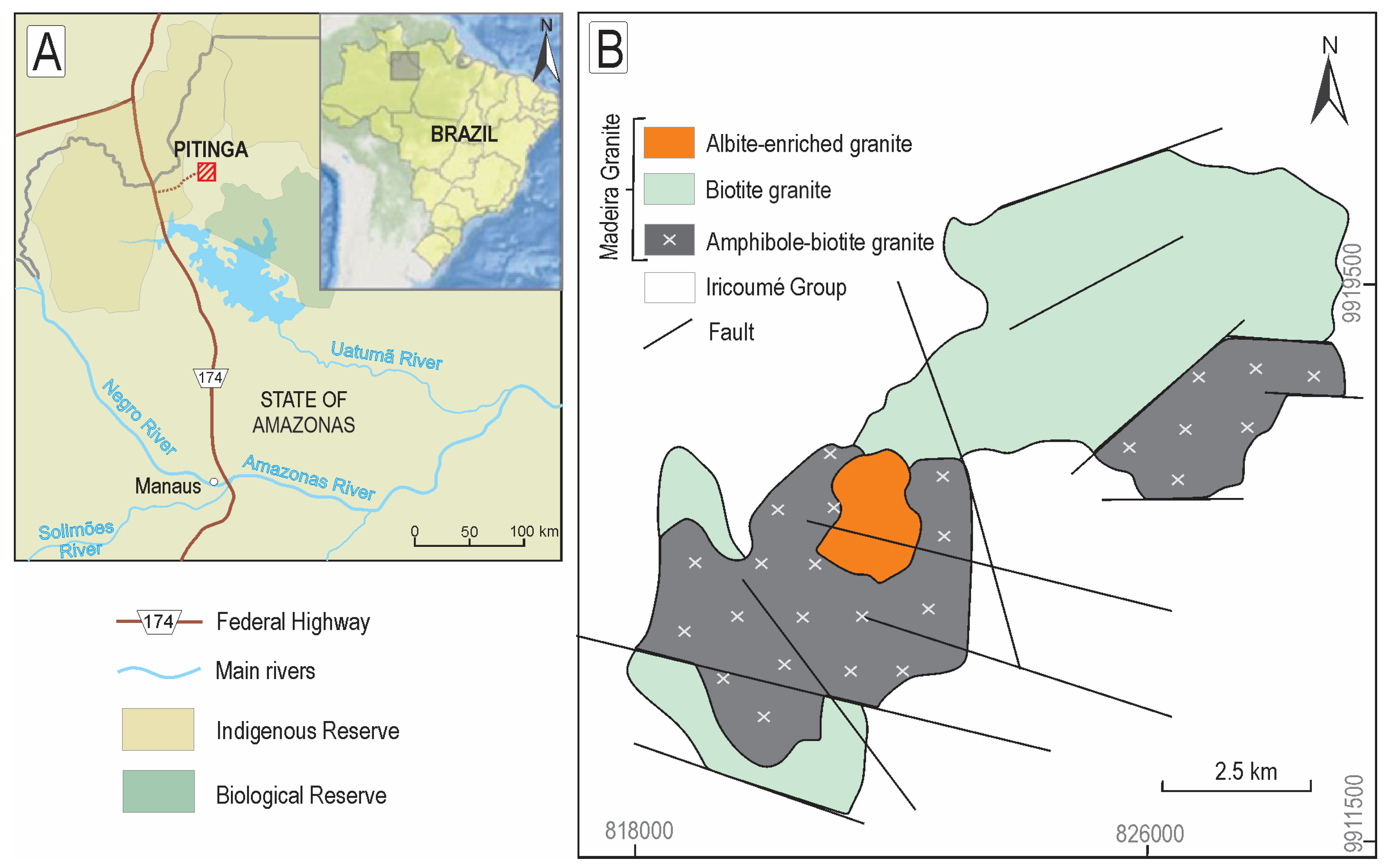
2. Geological Setting
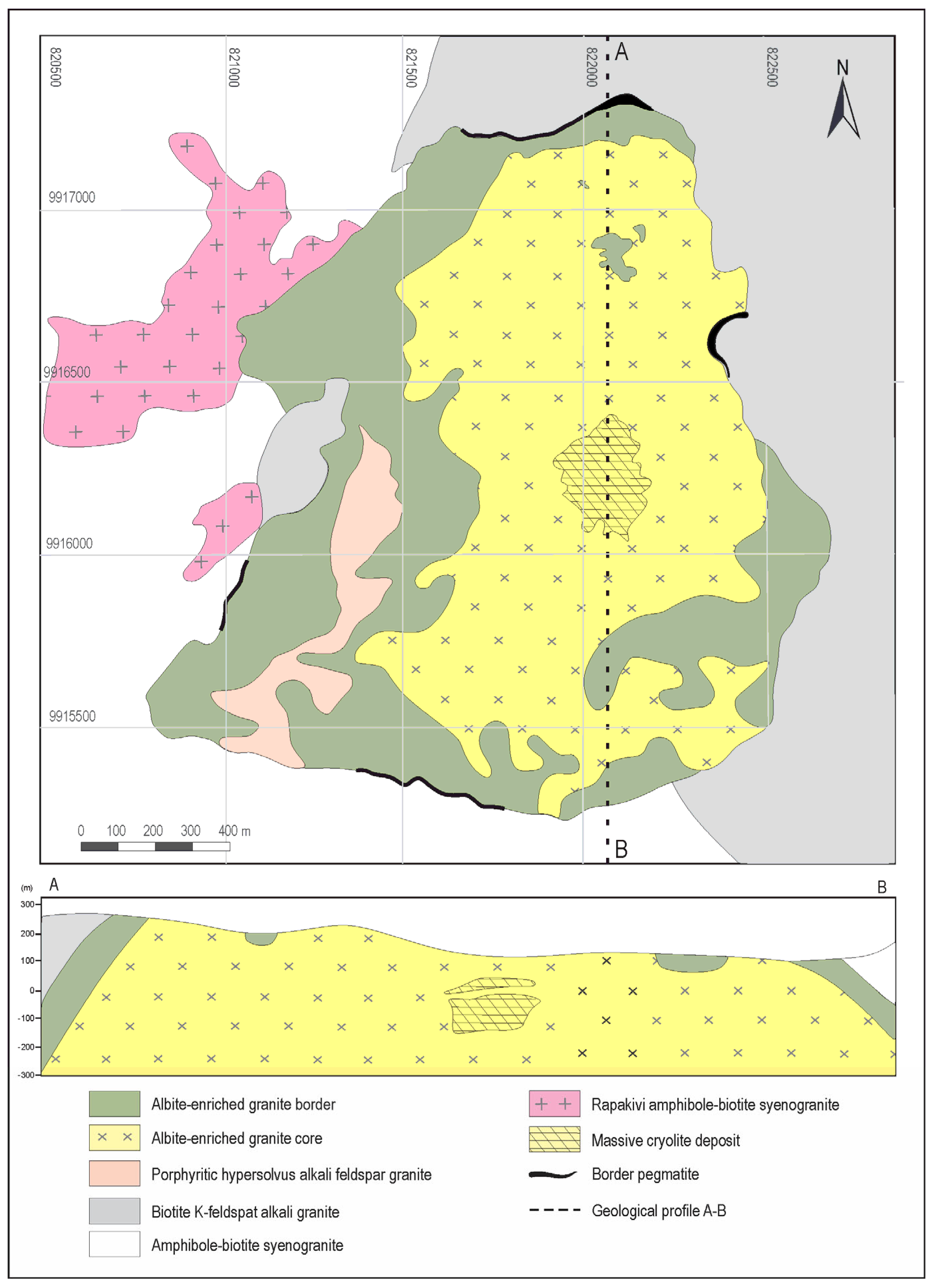
- (1)
- Approximately 50-cm thick pods and bands of the pegmatitic AEG (rarely, up to 10 m thick) [42]. They have almost the same minerals as the AGC but with much larger grain sizes. Polylithionite, riebeckite, xenotime, and thorite are much more abundant than in the AGC.
- (2)
- Border pegmatites (BPEG) that are at the contact between the AGB and the older facies (Figure 2). They are characterized by the increased sizes and amounts of K feldspar; quartz and zircon; advanced alterations of K-feldspar and biotite; and local enrichments in fluorite, polylithionite, thorite, and secondary hematite [43].
- (3)
- Pegmatite veins which are not mappable occur more commonly in the central, northern, and northwest parts of the AGC, and they have thicknesses ranging from a few centimeters to 2 m. They are heterogeneous and more commonly porphyritic. The phenocrystals may be of quartz, K-feldspar, xenotime, thorite, cryolite, polylithionite, and riebeckite. The matrix is composed of albite, quartz, K-feldspar, polylithionite, cryolite, and riebeckite; the accessory minerals are zircon, cassiterite, pyrochlore, columbite, galena, sphalerite, hematite, gagarinite, and genthelvite [44].
- (4)
3. Materials and Methods
4. Results
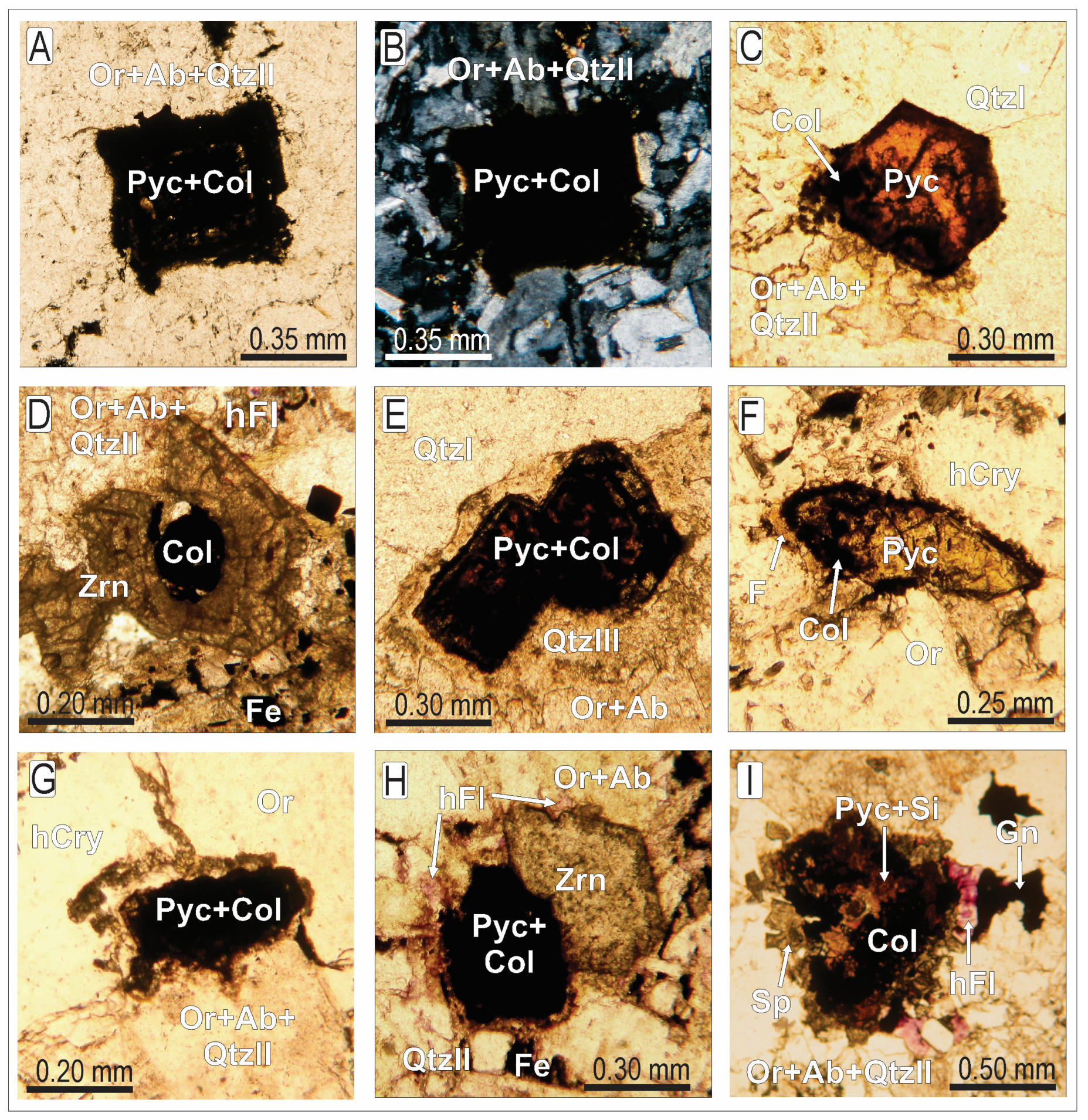
4.1. Mineralogy and Petrography
4.1.1. Pyrochlore
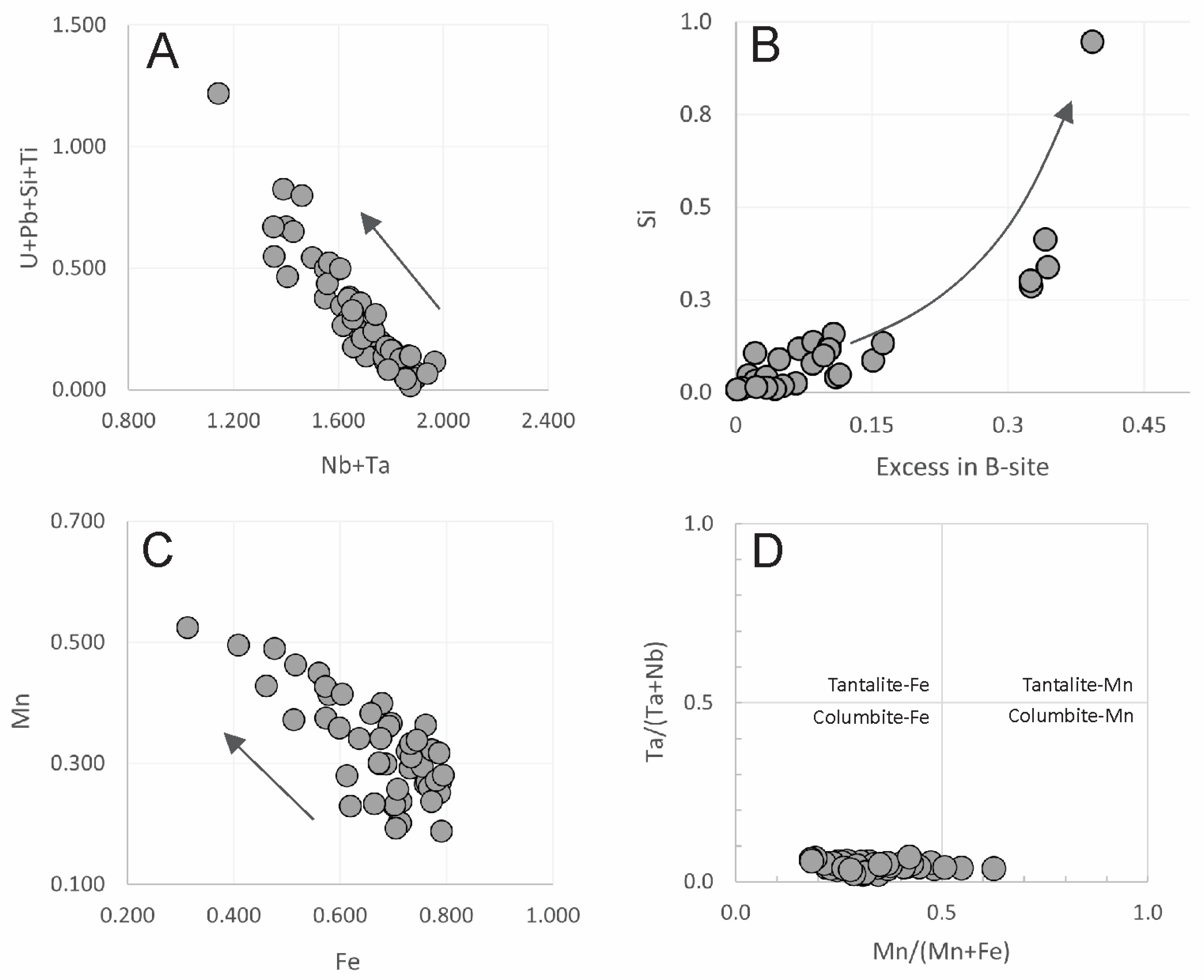
4.1.2. Columbite
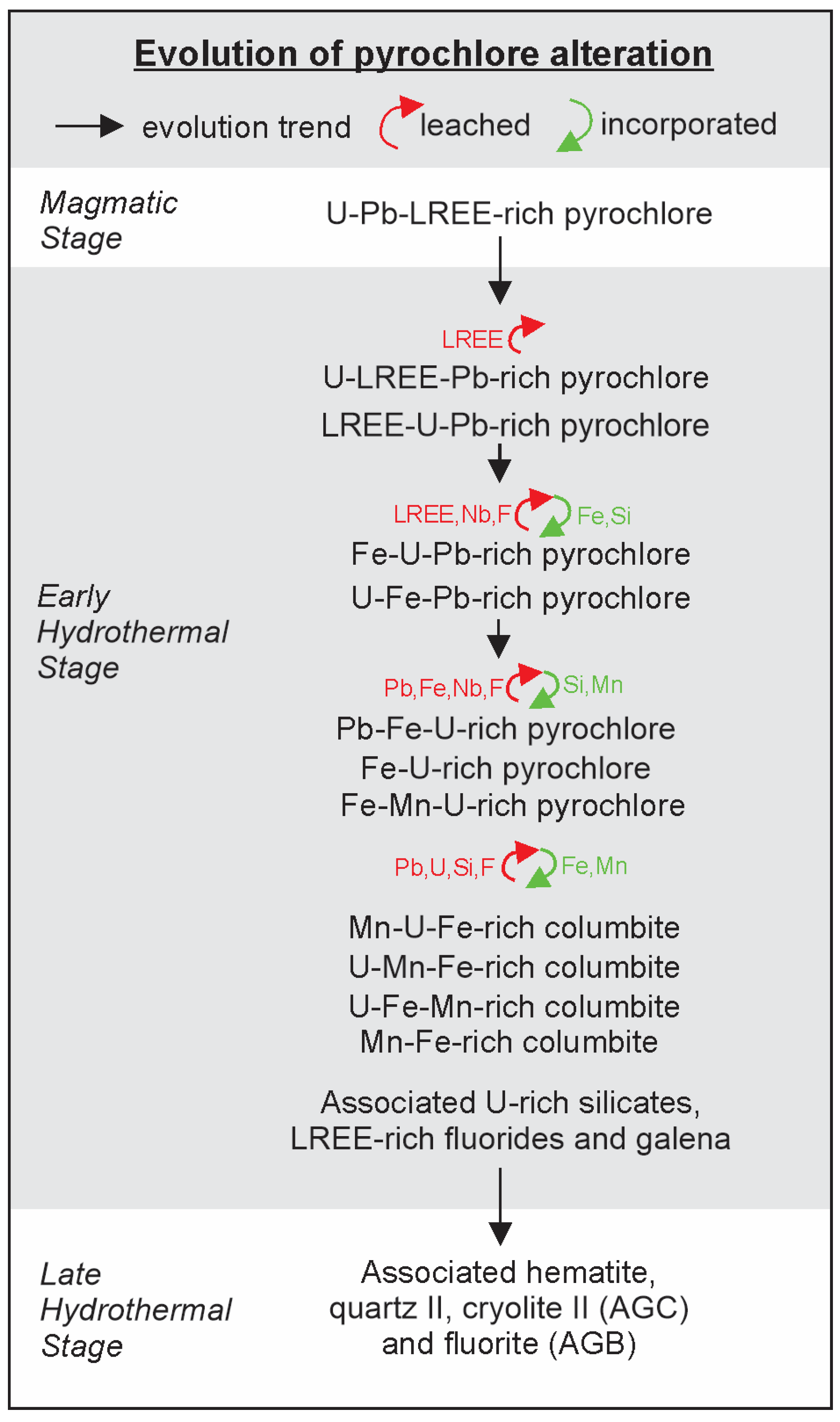
4.1.3. Miscellaneous Products of Pyrochlore Alteration
4.1.4. Late Hydrothermal Alterations
4.1.5. Pegmatites
4.2. Mineral Composition
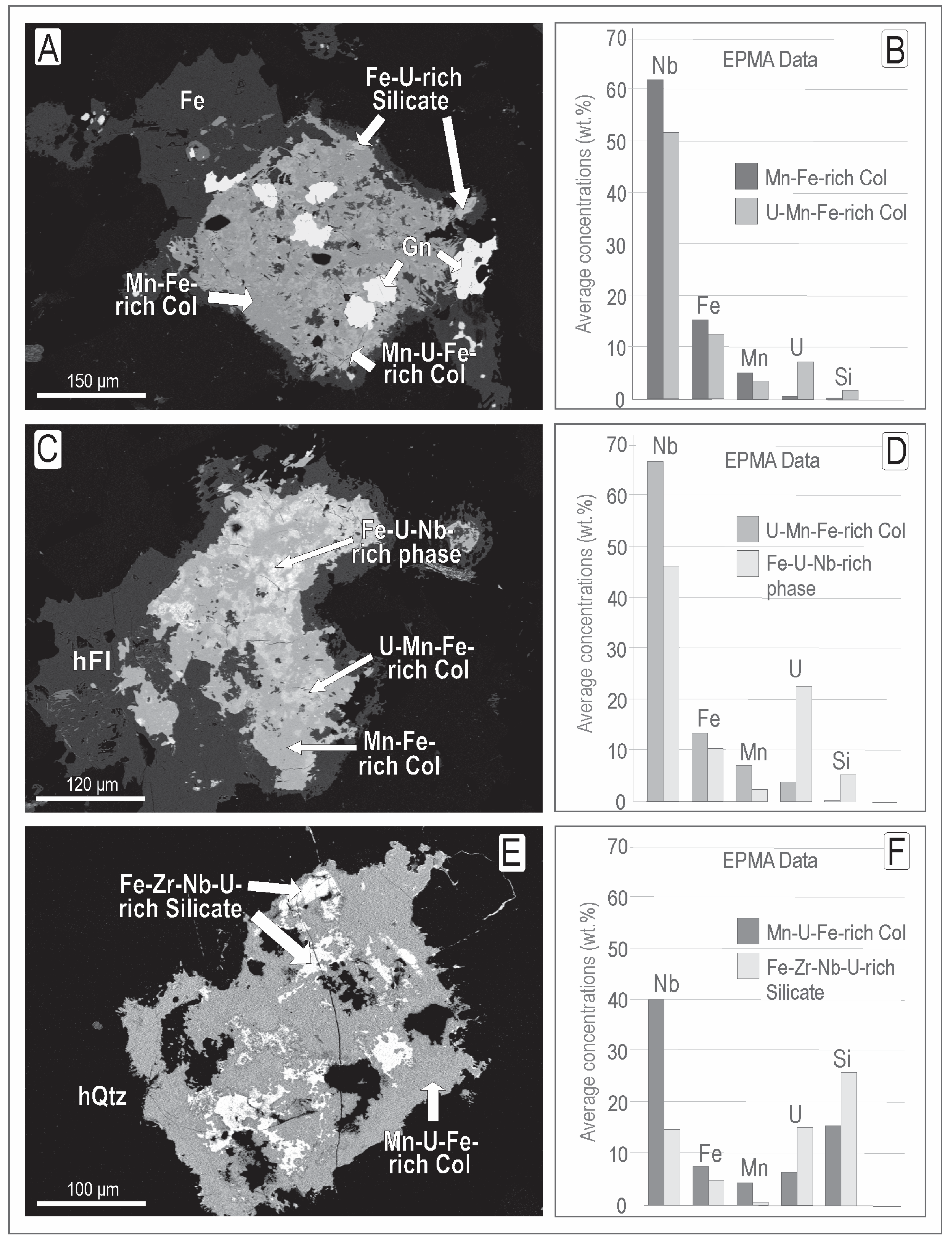
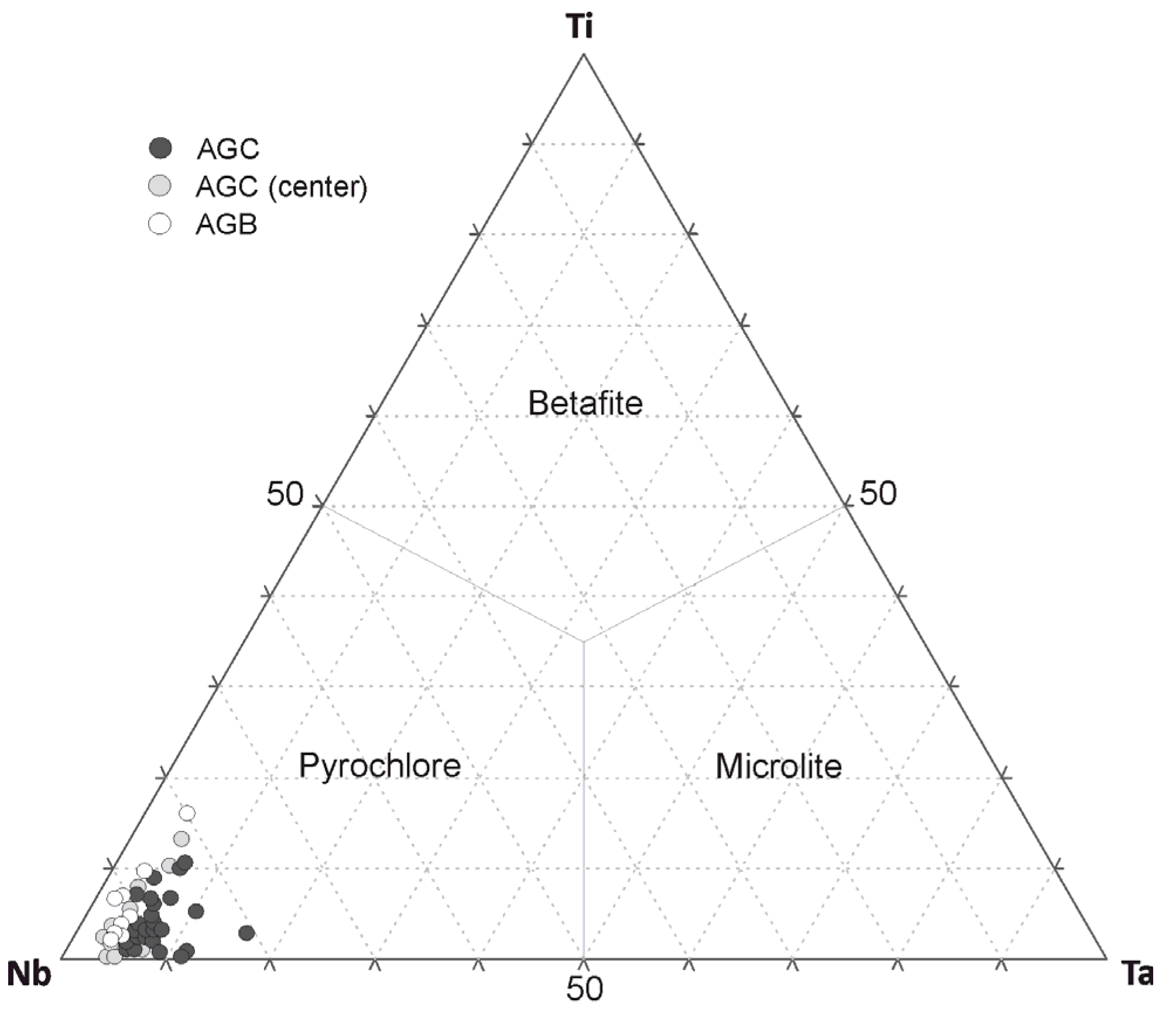
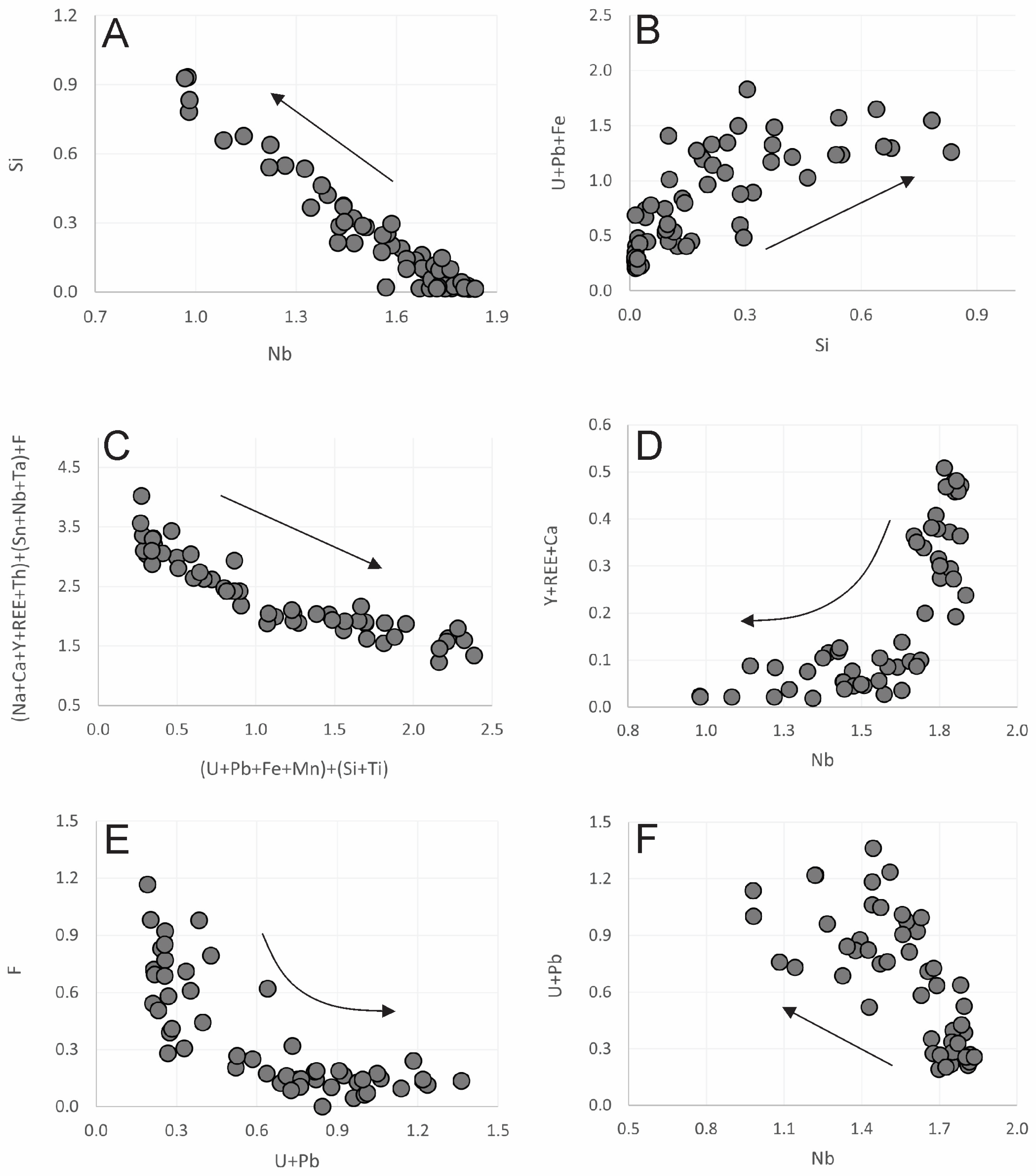
4.2.1. Pyrochlore
4.2.2. Columbite
4.2.3. Other Products of Pyrochlore Alteration
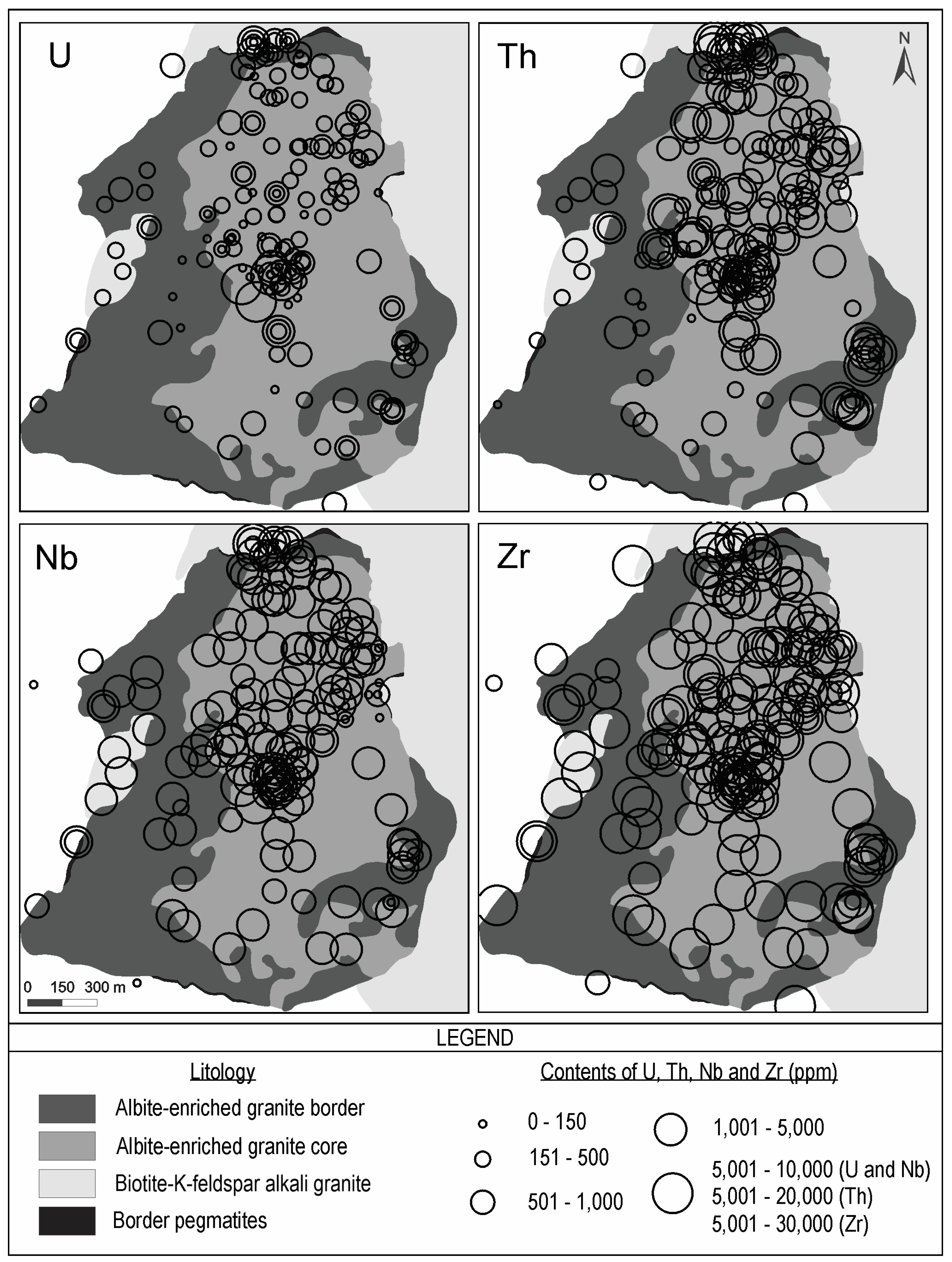
4.3. Geochemical Distribution of Uranium in the Albite-Enriched Granite and Pegmatites
5. Discussion
5.1. Primary Pyrochlore: Formation, U-Enrichment, and Distribution in the AEG
5.2. Primary Pyrochlore Hydrothermal Alteration and Its Products
5.3. Typology and Importance of U Mineralization
| Deposit and Location | Tectonic Setting | Ore Age (Ma) | Deposit Type | Host Rock/Structure (other Associated Rocks) | Ore Minerals (Minerals of Potential Interest) | Economic Parameters |
|---|---|---|---|---|---|---|
| Madeira, Pitinga, North Brazil | Guianas Shield, Amazonas craton | 1.822–1.794 | Alkaline rock-hosted | Albite granite (alkali-feldspar granite and amphibole-biotite-granite) | Pyrochlore, columbite cassiterite (thorite, xenotime, cryolite) | 164 Mt at 328 ppm UO2 (52 kt U) a |
| Rössing, Namibia | Fengcheng Mamatic Massif, Damara Orogen | 510 ± 3–429 ± 17 | Alkaline rock-hosted | Pegmatitic leucogranite-alaskite (biotite-amphibole gneiss, amphibole-biotite schist) | Uraninite, pitchblende, betafite, beta-uranophane, gummite (monazite, zircon, apatite) | 246.500 tU at 300 ppm UO2 b |
| Kvanefjeld, Ilimassauq, South Greenland | Eastern Gardar intracratonic rifting Province | 1.280–1.140 | Alkaline rock-hosted | Nepheline syenite and lujavrite (alkali granite, pulaskite, and nauajite) | Steenstrupine, monazite, eudialyte (pyrochlore, thorite, rinkite) | 673 Mt at 248 ppm U2O3 (184 kt U) c |
| Bokan Mountain, Southeast Alaska | Alexander terrane, western Canadian Cordillera | 151 ± 5 | Alkaline rock-hosted | Aegirine granite, veins and shear zones (riebeckite granite and aegirine syenite) | U-rich thorite, uraninite, U-rich thorianite, coffinite, allanite | 562 kt at 0.15–0.33 wt% UO2 (635 t U) d |
| Ghurayyah, Hijaz region, Saudi Arabia | Northwestern Arabian Shield | 620–530 | Alkaline rock-hosted | Leucocratic microgranite | Uraninite (monazite, thorite, pyrochlore, columbite, cassiterite, xenotime) | 440 Mt at 117 ppm UO2 (635 t U) e |
| Morro do Ferro, Brazil | Poços de Caldas plateau | 83–64 | Carbonatite-hosted | Lateritic profile (magnetite dyke and syenitic rocks) | Uranothorite (fluorcarbonates) | 100 t U; 110–120 ppm UO2 f |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyser, K.; Cuney, M. Geology and Geochemistry of Uranium and Thorium Deposits; Short Course Series; Mineralogical Association of Canada: Québec, QC, Canada, 2015; Volume 46, p. 345. [Google Scholar]
- Berning, J.; Cooke, R.; Hiemstra, S.A.; Hoffman, U. The Rössing uranium deposit, South West Africa. Econ. Geol. 1976, 71, 351–368. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Johnson, C. Geometallurgy of Australian uranium deposits. Ore Geol. Rev. 2014, 56, 25–44. [Google Scholar] [CrossRef]
- Cuney, M. Felsic magmatism and uranium deposits. Bull. Société Géologique Fr. 2014, 185, 75–92. [Google Scholar] [CrossRef]
- Lottering, M.J.; Lorenzen, L.; Phala, N.S.; Smit, J.T.; Schalkwyk, G.A.C. Mineralogy and uranium leaching response of low grade South African ores. Miner. Eng. 2008, 21, 16–22. [Google Scholar] [CrossRef]
- Kassab, W.A. Comparative study for leching processes of uranium, copper and cadmium from gibbsite ore material of Talet Seleim, Southwestern, Sinai, Egypt. J. Radioanal. Nucl. Chem. 2023, 332, 273–287. [Google Scholar] [CrossRef]
- Cuney, M. Evolution of uranium fractionation processes through time: Driving the secular variation of uranium deposit types. Econ. Geol. 2010, 105, 449–465. [Google Scholar] [CrossRef]
- Cuney, M.; Kyser, K. Recent and Not-so-Recent Developments in Uranium Deposits and Implications for Exploration; Short Course Series; Mineralogical Association of Canada: Québec, QC, Canada, 2008; Volume 39, p. 257. [Google Scholar]
- Krauskopf, K.B. Introduction to Geochemistry; McGraw-Hill: New York, NY, USA, 1967; p. 721. [Google Scholar]
- Pointer, C.M. Remobilisation of Uranium and Thorium by Ore-Forming Fluids: A Mineralogical Study. Ph.D. Thesis, University of Aston, Birmingham, UK, 1987; p. 311. [Google Scholar]
- Dill, H.G. Pegmatites and aplites: Their genetic and applied ore geology. Ore Geol. Rev. 2015, 69, 417–561. [Google Scholar] [CrossRef]
- IAEA, International Atomic Energy Agency. Annual Report. 2020. p. 191. Available online: https://www.iaea.org/sites/default/files/publications/reports/2020/gc65-5.pdf (accessed on 10 December 2022).
- Hogarth, D.D.; Williams, C.T.; Jones, P. Primary zoning in pyrochlore group minerals from carbonatites. Mineral. Mag. 2000, 64, 683–697. [Google Scholar] [CrossRef]
- Atencio, D.; Andrade, M.B.; Christy, A.G.; Gieré, R.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature, Can. Mineral 2010, 48, 673–698. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Wahl, R.; Cohen, A. Mineralogy and genesis of pyrochlore apatite from The Good Hope Carbonatite, Ontario: A potential Nb deposit. Mineral. Mag. 2020, 84, 81–91. [Google Scholar] [CrossRef]
- Dey, M.; Bhattacharjee, S.; Chakrabarty, A.; Mitchell, R.H.; Pal, S.; Sen, A.K. Compositional variation and genesis of pyrochlore, belkovite and biotite from the Sevattur carbonatite complex, India. Mineral. Mag. 2021, 85, 588–606. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Pyrochlore subgroup. Am. Mineral. 1995, 80, 732–743. [Google Scholar] [CrossRef]
- Hadlich, I.W.; Bastos Neto, A.C.; Botelho, N.F.; Pereira, V.P. The thorite mineralizations in the Madeira Sn-Nb-Ta world-class deposit (Pitinga, Brazil). Ore Geol. 2019, 105, 445–466. [Google Scholar] [CrossRef]
- Minuzzi, O.R.R.; Bastos Neto, A.C.; Pereira, V.P.; Nunes, L. A columbitização do pirocloro do albita granito na mina de Pitinga (AM): Relações com a mineralização de criolita. Rev. Bras. Geoc 2006, 35, 123–137. [Google Scholar] [CrossRef]
- Bastos Neto, A.C.; Pereira, V.P.; Ronchi, L.H.; Lima, E.F.; Frantz, J.C. The world-class Sn, Nb, Ta, F (T, REE, Li) deposit and the massive cryolite associated with the albite-enriched facies of the Madeira A-type granite, Pitinga Mining District, Amazonas State, Brazil. Can. Mineral 2009, 47, 1329–1357. [Google Scholar] [CrossRef]
- Lenharo, S.L.R. Evolução Magmática e Modelo Metalogenético dos Granitos Mineralizados da Região de Pitinga, Amazonas, Brasil. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1998; p. 290. [Google Scholar]
- Costi, H.T. Petrologia de Granitos Alcalinos com alto Flúor Mineralizados em Metais Raros: O Exemplo do Albita-granito da Mina Pitinga, Amazonas, Brasil. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 2000; p. 345. [Google Scholar]
- Tang, N.; Gritsenko, Y.; Kimura, K.; Bhattacharjee, S.; Sakai, A.; Fu, M.; Takeda, H.; Man, H.; Sugawara, K.; Matsumoto, Y.; et al. Spin-orbital liquid state and liquid-gas metamagnetic transition on a pyrochlore lattice. Nat. Phys. 2022, 19, 92–98. [Google Scholar] [CrossRef]
- Costi, H.T.; Dall’agnol, R.; Pichavant, M.; Ramo, O.T. The peralkaline tin-mineralized Madeira cryolite albite-rich granite of Pitinga, Amazonian Craton, Brazil: Petrography, mineralogy and crystallization processes. Can. Mineral. 2009, 47, 1301–1327. [Google Scholar] [CrossRef]
- Almeida, F.F.M.; Hasui, Y.; Brito Neves, B.B.; Fuck, R.A. Brazilian structural Provinces: An introduction. Earth Sci. Rev. 1981, 17, 1–29. [Google Scholar] [CrossRef]
- Santos, J.O.S.; Hartmann, L.A.; Gaudete, H.E.; Groves, D.I.; McNaughton, N.J.; Fletcher, L.R.A. New understanding of the Provinces of Amazon Craton based on Integration of Field Mapping and U-Pb and Sm-Nd geochronology. Gondwana Res. 2000, 3, 453–488. [Google Scholar] [CrossRef]
- Veiga, J.P., Jr.; Nunes, A.C.B.; Fernandes, A.S.; Amaral, J.E.; Pessoa, M.R.; Cruz, S.A.S. Projeto Sulfetos de Uatumã; Relatório Final; DNPM/CPRM: Manaus, Brazil, 1979. [Google Scholar]
- Ferron, J.M.T.M.; Bastos Neto, A.C.; Lima, E.F.; Costi, H.T.; Moura, C.A.V.; Prado, M.; Galarza, M.A. Geologia e cronologia Pb-Pb de rochas graníticas e vulcânicas ácidas a intermediárias paleoproterozóicas da Província de Pitinga, Cráton Amazônico. Rev. Bras. Geocienc. 2006, 36, 499–512. [Google Scholar] [CrossRef]
- Pierosan, R.; Lima, E.F.; Nardi, L.V.S.; Campos, C.P.; Bastos Neto, A.C.; Ferron, J.M.T.M.; Prado, M. Paleoproterozoic (~1.88 Ga) felsic volcanism of the Iricoumé Group in the Pitinga Mining District area, Amazonian Craton, Brazil: Insights in ancient volcanic processes from field and petrological data. An. Acad. Bras. Ciênc 2011, 83, 921–937. [Google Scholar] [CrossRef]
- Pierosan, R.; Lima, E.F.; Nardi, L.V.S.; Bastos Neto, A.C.; Campos, C.P.; Ferron, J.M.T.M.; Prado, M. Geochemistry of Paleoproterozoic volcanic rocks of the Iricoumé Group, Pitinga Mining District, Amazonian craton, Brazil. Intern. Geol. Rev. 2011, 53, 946–976. [Google Scholar] [CrossRef]
- Simões, M.S.S.; Almeida, M.E.; Souza, A.G.H.; Silva, B.D.P.B.; Rocha, P.G. Characterization of the volcanic and hypabyssal rocks of the Paleoproterozoic Iricoumé Group in the Pitinga region and Balbina Lake area, Amazonian craton, Brazil: Petrographic distinguishing features and emplacement conditions. J. Volcan. Geotherm. Res. 2014, 286, 138–147. [Google Scholar] [CrossRef]
- Horbe, M.A.; Horbe, A.C.; Costi, H.T.; Teixeira, J.T. Geochemical characteristics of cryolite-tin-bearing granites from the Pitinga mine, northwestern Brazil—A review. J. Geochem. Exp. 1991, 40, 227–249. [Google Scholar] [CrossRef]
- Lenharo, S.L.R.; Pollard, P.J.; Born, H. Petrology and textural evolution of granites associated with tin and rare-metals mineralization at the Pitinga mine, Amazonas, Brazil. Lithos 2003, 66, 37–61. [Google Scholar] [CrossRef]
- Costi, H.T.; Borges, R.M.; Dall’agnol, R. Depósitos de estanho da mina Pitinga, estado do Amazonas. In Caracterização de Depósitos Minerais em Distritos Mineiros da Amazônia; Marini, O.J., Queiroz, E.T., Ramos, B.W., Eds.; DNPM-CT/Mineral-ADIMB: Brasília, Brazil, 2005; pp. 391–475. [Google Scholar]
- Costi, H.T.; Dall’agnoll, R.; Moura, C.A.V. Geology and Pb-Pb Geochronology of Paleoproterozoic volcanic and granitic rocks of Pitinga province, Amazonian craton, northern Brazil. Int. Geol. Rev. 2000, 42, 832–849. [Google Scholar] [CrossRef]
- Bastos Neto, A.C.; Ferron, T.M.M.; Chauvet, A.; Chemale, F.; Lima, E.F.; Barbanson, L.; Costa, C.F.M. U-Pb dating of the Madeira Suite and structural control of the albite-enriched granite at Pitinga (Amazônia, Brazil): Evolution of the A-type magmatism and implications for the genesis of the Madeira Sn-Ta-Nb (REE, cryolite) world-class deposit. Precambrian Res. 2014, 243, 181–196. [Google Scholar] [CrossRef]
- Horbe, M.A.; Horbe, A.C.; Teixeira, J.T.; Costi, H.T. Granito Madeira: Petrologia, petroquímica e mineralizações. SBG Simp. Geol. Amaz. 1985, 2, 284–320. [Google Scholar]
- Minuzzi, O.R.R. Gênese e Evolução da Mineralização de Criolita, Pirocloro e Columbita da Subfacies Albita Granito de Núcleo, Mina Pitinga, Amazonas, Brasil. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2005; p. 249. [Google Scholar]
- Bastos Neto, A.C.; Pereira, V.P.; Lima, E.F.; Ferron, J.M.; Minuzzi, O.; Prado, M.; Ronchi, L.H.; Flores, J.A.; Frantz, J.C.; Pires, A.; et al. A jazida de criolita da Mina Pititnga (Amazonas). In Caracterização de Depósitos Minerais em Distritos Mineiros da Amazônia; Marini, O.J., Queiroz, E.T., Ramos, B.W., Eds.; DNPM-CT/Mineral-ADIMB: Brasília, Brazil, 2005; pp. 481–547. [Google Scholar]
- Pires, A.C. Xenotima, Gagarinita, Fluocerita e Waimirita da Mina Pitinga (AM): Mineralogia e Avaliação Preliminar do Potencial do Albita Granito Para Exploração de Elementos Terras Raras e ítrio. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2010; p. 201. [Google Scholar]
- Nardi, L.V.S.; Formoso, M.L.L.; Jarvis, K.; Oliveira, L.; Bastos Neto, A.C.; Fontana, E. REE, Y, Nb, U and Th contents and tetrad effect in zircon from a magmatic-hydrothermal F-rich system of Sn-rare metal-cryolite mineralized granites from the Pitinga Mine, Amazonia, Brazil. J. S. Am. Earth Sci. 2012, 33, 34–42. [Google Scholar] [CrossRef]
- Stolnik, D. Caracterização da Xenotima na fácies Pegmatítica do Albita Granito de Núcleo, Pitinga (AM); Monografia; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2015; p. 67. [Google Scholar]
- Lengler, H.F. Pegmatitos do albita Granito Madeira: Avaliação do Minério para Fins de Beneficiamento; Monografia; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 2016; p. 118. [Google Scholar]
- Paludo, C.M.; Bastos Neto, A.C.; Pereira, V.P.; Botelho, N.F. Mineralogia e geoquímica de pegmatites ricos em ETR, F e metais alcalinos associados à facies albita granito no depósito de Sn-Nb-Ta-(F, ETR, U, Th) Madeira (mina Pitinga, AM, Brasil). Pesq. Geocienc. 2018, 45, 1–28. [Google Scholar]
- Minuzzi, O.R.R.; Bastos Neto, A.C.; Pereira, V.P.; Flores, J.A.A. The massive cryolite deposit and the disseminated ore of cryolite from the Pitinga mine (Amazon, Brazil). Rev. Bras. Geoc. 2006, 36, 104–123. [Google Scholar] [CrossRef]
- Bastos Neto, A.C.; Pereira, V.P.; Pires, A.C.; Barbanson, L.; Chauvet, A. Fluorine-rich xenotime from the Nb-Ta-Sn Madeira world-class deposit associated with the albite-enriched granite at Pitinga, Amazonia, Brazil. Can. Mineral. 2012, 50, 1019–1032. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D.; Rhede, D.; Seifert, W.; Rickers, K.; Förster, H.J.; Heinrich, W.; Davidson, P. The transition from peraluminous to peralkaline granitic melts: Evidence from melt inclusions and accessory minerals. Lithos 2006, 91, 137–149. [Google Scholar] [CrossRef]
- Martin, R.F. A-type granites of crustal origin ultimately result from open-system fenitization-type reactions in an extensional environmental. Lithos 2006, 91, 125–136. [Google Scholar] [CrossRef]
- Dolejs, D.; Baker, D.R. Liquidus equilibria in the system K2O-Na2O-Al2O3-SiO2-F2O to 100 MPa 2: Differentiation paths of fluorosilicic magmas in hydrous systems. J. Petrol. 2007, 48, 807–828. [Google Scholar] [CrossRef]
- Ercit, T.S. The geochemistry and crystal chemistry of columbite-group minerals from granitic pegmatites southwestern Grenville Province, Canadian Shield. Can. Mineral. 1994, 32, 421–438. [Google Scholar]
- Hogarth, D.D. Classification and nomenclature of the pyrochlore group. Am. Mineral. 1977, 62, 403–410. [Google Scholar]
- Lumpkin, G.R.; Mariano, A.N. Natural occurrence and stability of pyrochlore in carbonatites, related hydrothermal systems, and weathering environments. In The Scientific Basis for Nuclear Waste Management; Murphy, W.M., Knecht, D.A., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1996; pp. 831–838. [Google Scholar]
- Johan, Z.; Johan, V. Accessory minerals of the Cínovec (Zinnwald) granite cupola, Czech Republic: Indicators of petrogenetic evolution. Mineral. Petrol. 2004, 83, 113–150. [Google Scholar] [CrossRef]
- Johan, V.; Johan, Z. Accessory minerals of the Cinovec (Zinnwald) granite cupola, Czech Republic, part 1: Nb-, Ta and Ti-bearing oxides. Mineral. Petrol. 1994, 51, 323–343. [Google Scholar] [CrossRef]
- Ercit, T.S.; Wise, M.A.; Černý, P. Compositional and structural systematics of the columbite group. Am. Mineral. 1995, 80, 613–619. [Google Scholar] [CrossRef]
- Wise, M.A.; Černý, P.; Falster, A.U. Scandium substitution in Columbite-group minerals and ixiolite. Can. Mineral. 1998, 36, 673–680. [Google Scholar]
- Burke, E.A.J. Tidying up mineral names: An IMA-CNMNC scheme for suffixes, hyphens and diacritical marks. Mineral Rec. 2008, 39, 131–135. [Google Scholar]
- Mücke, A.; Struntz, H. Petscheckite and liandratite, two new pegmatite minerals from Madagascar. Am. Mineral. 1978, 63, 941–946. [Google Scholar]
- Mücke, A.; Keck, E. Untersuchungen an Columbiten (Fe,Mn)(Nb,Ta)2O6 und mit Columbit verwachsenen Neufund Petscheckit U(Fe,Mn)(Nb,Ta)2O8 aus dem Pegmatite von Hagendorf-Süd/Oberpfalz. Aufschluss 2008, 59, 373–392. [Google Scholar]
- Wall, F.; Williams, C.T.; Woollley, A.R. Pyrochlore from weathered carbonatite at Lueshe, Zaire. Mineral. Mag. 1996, 60, 731–750. [Google Scholar] [CrossRef]
- Dill, H.G. An overview of the pegmatitic landscape from the pole to the equator–Applied geomorphology and ore guides. Ore Geol. Rev. 2017, 91, 795–823. [Google Scholar] [CrossRef]
- Dill, H.G.; Gerdes, A.; Weber, B. Age and mineralogy of supergene uranium minerals-tools to unravel geomorphological and palaeohydrological processes in granitic terrains (Bohemian Massif, SE Germany). Geomorphology 2010, 117, 44–65. [Google Scholar] [CrossRef]
- Killeen, P.G. Gamma ray spectrometric methods in uranium explorations: Application and interpretation. Econ. Geol. 1979, 31, 163–229. [Google Scholar]
- Bea, F. Residence of REE, Y, Th and U in granites and crustal protoliths: Implications for the chemistry of crustal melts. J. Petrol. 1996, 37, 521–552. [Google Scholar] [CrossRef]
- Ogunleye, P.O.; Garba, I.; Ike, E.C. Factors contributing to enrichment and crystallization of niobium in pyrochlore in the Kaffo albite arfvedsonite granite, Ririwai Complex, Younger Granites province of Nigeria. J. Afr. Earth Sci. 2006, 44, 372–382. [Google Scholar] [CrossRef]
- Linnen, R.L.; Keppler, H. Columbite solubility in granitic melts: Consequences for the enrichment and fractionation of Nb and Ta in the Earth’s crust. Contrib. Mineral. Petrol. 1997, 128, 213–227. [Google Scholar] [CrossRef]
- Redkin, A.F.; Borodulin, G.P. Pyrochlores as indicators of the uranium-bearing potential of magmatic melts. Geochemistry 2009, 432, 787–790. [Google Scholar] [CrossRef]
- Whalen, J.B.; Currie, K.L.; Chappell, B.W. A-type granites: Geochemical characteristics, discrimination and petrogenesis. Contrib. Miner. Petrol. 1987, 95, 407–419. [Google Scholar]
- Giovannini, A.L.; Bastos Neto, A.C.; Porto, C.G.; Pereira, V.P.; Takehara, L.; Barbanson, L.; Bastos, P.H.S. Mineralogy and geochemistry of laterites from the Morro dos Seis Lagos Nb (Ti, REE) deposit (Amazonas, Brazil). Ore Geol. Rev. 2017, 88, 461–480. [Google Scholar]
- Lottermoser, B.G.; England, B.M. Compositional variation in pyrochlores from the Mt. Weld carbonatite laterite, Western Australia. Mineral. Petrol. 1988, 38, 37–51. [Google Scholar] [CrossRef]
- Cordeiro, P.F.O.; Brod, J.A.; Palmieri, M.; Oliveira, C.G.; Barbosa, E.S.R.; Santos, R.V.; Gaspar, J.C.; Assis, L.C. The Catalão I niobium deposit, central Brazil: Resources, geology and pyrochlore chemistry. Ore Geol. Rev. 2011, 41, 112–121. [Google Scholar]
- Roberts, S.K.; Bourcier, W.L.; Shaw, H.F. Aqueous dissolution kinetics of pyrochlore, zirconolite and brannerite at 25, 50, and 75 °C. Radiochim. Acta 2000, 88, 539–546. [Google Scholar]
- Xu, H.F.; Wang, Y.F.; Zhao, P.H.; Bourcier, W.L.; Konynenburg, R.V.; Shaw, H.F. Investigation of pyrochlore-based U-bearing ceramic nuclear waste: Uranium leaching test and TEM observation. Environ. Sci. Technol. 2004, 38, 1480–1486. [Google Scholar]
- Pöml, P.; Geisler, T.; Cobos-Sabaté, J.; Wiss, T.; Raison, P.E.; Schmid-Beurmann, P.; Deschanels, X.; Jégou, C.; Heimink, J.; Putnis, A. The mechanism of the hydrothermal alteration of cerium- and plutonium-doped zirconolite. J. Nucl. Mater. 2011, 410, 10–23. [Google Scholar] [CrossRef]
- Seifert, W.; Kämpf, H.; Wasternack, J. Compositional variation in apatite, phlogopite and other accessory minerals of the ultramafic Delitzsch complex, Germany: Implication for cooling history of carbonatites. Lithos 2000, 53, 81–100. [Google Scholar] [CrossRef]
- Bambi, A.C.J.M.; Costanzo, A.; Gonçalves, A.O.; Melgarejo, J.C. Tracing the chemical evolution of primary pyrochlore from plutonic to volcanic carbonatites: The role of fluorine. Mineral. Mag. 2012, 76, 377–392. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Bismayer, U. Compositional variation in pyrochlores of Amba Dongar carbonatite complex, Gujarat. J. Geol. Soc. India 2010, 75, 495–502. [Google Scholar] [CrossRef]
- Starikova, A.E.; Bazarova, E.P.; Savel’eva, V.B.; Sklyarov, E.V.; Khromova, E.A.; Kanakin, S.V. Pyrochlore-group minerals in the granite-hosted Katugin rare-metal deposit, Transbaikalia, Russia. Minerals 2019, 9, 490. [Google Scholar] [CrossRef]
- Wu, B.; Hu, Y.Q.; Bonnetti, C.; Xu, C.; Wang, R.C.; Zhang, Z.S.; Li, Z.Y.; Yin, R. Hydrothermal alteration of pyrochlore group minerals from the Miaoya carbonatite complex, central China and its implications for Nb mineralization. Ore Geol. Rev. 2021, 132, 1040–1059. [Google Scholar] [CrossRef]
- Geisler, T.; Pöml, P.; Stephan, T.; Janssen, A.; Putnis, A. Experimental observation of an interface-controlled pseudomorphic replacement reaction in a natural crystalline pyrochlore. Am. Mineral. 2005, 90, 1683–1687. [Google Scholar] [CrossRef]
- Geisler, T.; Seydoux-Guilaume, A.M.; Pöml, P.; Golla-Schindler, U.; Berndt, J.; Wirth, R.; Pollok, K.; Janssen, A.; Putnis, A. Experimental hydrothermal alteration of crystalline and radiation-damaged pyrochlore. J. Nucl. Mater. 2005, 344, 17–23. [Google Scholar] [CrossRef]
- Van Wambeke, L. A Study of Some Niobium-Bearing Minerals of the Lueshe Carbonatite Deposit (Kivu, Republic of Congo); Report of European Atomic Energy Community-Euratom, EUR 2110.e; Joint Nuclear Research Center: Ispra, Italy, 1965. [Google Scholar]
- Nasraoui, M.; Bilal, E. Pyrochlores from the Lueshe carbonatite complex (Democratic Republic of Congo): A geochemical record of different alteration stages. J. Asian Earth Sci. 2000, 18, 237–251. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals: Microlite subgroup. Am. Mineral. 1992, 77, 179–188. [Google Scholar]
- Uher, P.; Ondrejka, M.; Konečny, P. Magmatic and post-magmatic YREE-Th phosphate, silicate and Nb-Ta-Y-REE oxide minerals in A-type metagranite: An example from the Turčok massif, the Western Carpathians, Slovakia. Mineral. Mag. 2009, 73, 1009–1025. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Siachoque, A.; Garcia, R.; Vlach, S.R.F. Occurrence and composition of columbite-(Fe) in the reduced A-type Desemborque Pluton, Graciosa Province (S-SE Brazil). Minerals 2020, 10, 411. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Radha, A.V.; Navrotsky, A. The energetics of nanophase calcite. Geochim. Cosmochim. Acta 2011, 75, 7893–7905. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Sørensen, H. The Ilímassauq complex, South Greenland: Status of mineralogical research with new results. Geol. Greenl. Surv. Bull. 2001, 190, 167. [Google Scholar]
- Fraenkel, M.O.; Santos, R.C.; Loureiro, F.E.V.L.; Muniz, W.S. Jazida de uranio do planalto Poços de Caldas, Minas Gerais. In Principais Depósitos Minerais do Brasil; Schobbenhaus, C., Coelho, C.E.S., Eds.; DNPM/CVRD: Brasília, Brazil, 1985; pp. 89–103. [Google Scholar]
- MacKevett, E.M. Geology and ore deposits of the Bokan Mountain area, Southeastern Alaska, United States. Geol. Surv. Bull. 1936, 1154, 125. [Google Scholar]
- Balashov, Y.A. The Geochemistry of the Lovozero Alkaline Massif; The Australian National University: Canberra, Australia, 1968; p. 395, (English Translation by D.A. Brown). [Google Scholar]
- Bowden, P.; Turner, D.C. Peralkaline and associated ring-complexes in the Nigeria-Niger Province, West Africa. In The Alkaline Rocks; Sørensen, H., Ed.; John Wiley: London, UK, 1974; pp. 330–351. [Google Scholar]
- Sørensen, H.; Rose-Hansen, J.; Nielsen, B.L.; Lovborg, L.; Sørensen, E.; Lundgaard, T. The uranium deposit at Kvanefjeld, the Ilímaussaq intrusion, South Greenland: Geology, reserves, benefication. Rapp. Grøen. Geol. Und. 1974, 60, 54. [Google Scholar] [CrossRef]
- Staatz, M.H. I and L uranium and thorium vein system, Bokan Mountain, southern Alaska. Econ. Geol. 1978, 73, 512–523. [Google Scholar] [CrossRef]
- Drysdall, A.R.; Jackson, J.; Ramsay, C.R.; Douch, C.J.; Hackett, D. Rare element mineralization related to Precambrian alkali granites in the Arabian Shield. Econ. Geol. 1984, 79, 1366–1377. [Google Scholar] [CrossRef]
- Aubert, G. Les coupoles granitiques de Montebras et d’Echassières (Massif Central français) et lagenèse de leurs minéralisations en étain, lithium, tungstène et béryllium. Mémoire BRGM 1969, 46, 345. [Google Scholar]
- Cuney, M.; Marignac, C.; Weisbrod, A. The Beauvoir topaz-lepidolite albite granite (Massif Central, France); the disseminated magmatic Sn-Li-Ta-Nb-Be mineralization. Econ. Geol. 1992, 87, 1766–1794. [Google Scholar] [CrossRef]
- Kinnaird, J.A.; Bowden, P.; Ixer, R.A.; Odling, N.W.A. Mineralogy, geochemistry and mineralization of the Ririwai complex, northern Nigeria. J. Afr. Earth Sci. 1985, 3, 185–220. [Google Scholar] [CrossRef]
- Pointer, C.M.; Ashworth, J.R.; Ixer, R.A. The zircon-thorite mineral group in metasomatized granite, Ririwai, Nigeria 1: Geochemistry and metastable solid solution of thorite and coffinite. Mineral. Petrol. 1988, 38, 245–262. [Google Scholar] [CrossRef]
- Pointer, C.M.; Ashworth, J.R.; Ixer, R.A. The zircon-thorite mineral group in metasomatized granite, Ririwai, Nigeria 2: Zoning, alteration and exsolutions in zircon. Mineral. Petrol. 1988, 39, 21–37. [Google Scholar] [CrossRef]
- Förster, H.J.; Seltmann, R.; Tischendorf, G. High-fluorine, low-phosphorus A-type (post-collision) silicic magmatism in the Erzgebirge. Terra Nostra 1995, 7, 32–35. [Google Scholar]
- Förster, H.J. Composition and origin of intermediate solid solutions in the system thorite-xenotime-zircon-coffinite. Lithos 2006, 88, 35–55. [Google Scholar] [CrossRef]
- McMaster, S.A. Investigation into the Synthesis, Characterization and Uranium Extraction of the Pyrochlore Mineral Betafite. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2016; p. 244. [Google Scholar]
- Berning, J. The Rössing uranium deposit, southwest Africa/Namibia. In Mineral Deposits of Southern Africa; Anhaeusser, C.R., Maske, S., Eds.; Geological Society of South Africa: Johannesburg, Africa, 1986; pp. 1819–1832. [Google Scholar]
- Cuney, M. Preliminary results on the petrology and fluid inclusions studies of the Rössing uraniferous cannot be ruled out alaskites. Geol. Soc. S. Afr. 1980, 83, 39–45. [Google Scholar]
- Armstrong, R.L. Rb-Sr dating of the Bokan Mountain Granite Complex and its country rocks. Can. J. Earth Sci. 1985, 22, 1233–1236. [Google Scholar] [CrossRef]
- Lalande, P.G. Final Report on Preliminary Geological and Geophysical Investigations on the Ghurayyah Radioactive Granite, Kingdom of Saudi Arabia; Mineral Resources Open-File Report; Directorate General: Saudi Arabia, 1977; Volume 605, p. 3.
- Stoezer, D.B. Distribution and tectonic setting of plutonic rocks of the Arabian Shield. J. Afr. Earth Sc. 1986, 4, 31–46. [Google Scholar] [CrossRef]
- Thompson, T.B. Geology and uranium-thorium mineral deposits of the Bokan Mountain Granite Complex, Southeastern Alaska. Ore Geol. Rev. 1988, 3, 193–210. [Google Scholar] [CrossRef]
- Ulbrich, H.H.; Vlach, S.R.F.; Ulbrich, M.N.C.; Kawashita, K. Penecontemporaneous syenitic-phonolitic and basic-ultrabasic-carbonatitic rocks at the Poços de Caldas alkaline massif, SE Brazil: Geologic and geochronologic evidence. Rev. Bras. Geoc. 2002, 32, 15–26. [Google Scholar] [CrossRef]
- Energy Transition Minerals Ltd. Kvanefjeld Mineral Resources. 2015. Available online: https://etransmin.com/kvanefjeld-project/ (accessed on 13 May 2023).
- Staatz, M.H.; Hall, R.B.; Macke, B.D.L.; Armbrustmacher, T.J.; Brownfields, I.K. Thorium resources of selected regions in the United States. U.S. Geol. Surv. Circ. 1980, 824, 32. [Google Scholar]
- Gentile, E.; Figueiredo Filho, P.M. Minerais radioativos. In Projeto Diagnóstico; Associação Brasileira de Metalurgia e Materiais: São Paulo, Brazil, 1996. [Google Scholar]
- Bowden, P.; Kinnaird, J.A. Geology and mineralization of the Nigerian Anorogenic Ring Complexes. Geol. Jahrb. 1984, 56, 3–56. [Google Scholar]


| Facies | Core Albite-Enriched Granite | Boder Albite-Enriched Granite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | (1) | (2) | (3) | (4) 1 | (5) 1 | (6) | (7) 1 | (8) | (9) | (10) | (11) | (12) |
| Nb2O5 | 46.20 | 50.83 | 48.01 | 40.10 | 45.81 | 27.55 | 32.10 | 21.69 | 53.78 | 31.93 | 52.23 | 39.26 |
| Ta2O5 | 06.13 | 06.15 | 06.25 | 02.47 | 03.72 | 05.99 | 02.43 | 04.56 | 04.99 | 01.69 | 04.75 | 02.66 |
| SiO2 | 00.21 | 00.21 | 00.19 | 00.42 | 00.18 | 03.19 | 00.89 | 08.31 | 01.62 | 13.82 | 01.35 | 00.16 |
| SnO2 | 01.16 | 02.72 | 02.31 | 00.72 | 02.60 | 01.11 | 00.90 | 00.00 | 00.00 | b.d.l. | 00.00 | 00.08 |
| TiO2 | 01.05 | 00.47 | 00.50 | 00.92 | b.d.l. | b.d.l. | 01.17 | 00.81 | 01.43 | 01.45 | 00.90 | 02.61 |
| UO2 | 02.81 | 02.24 | 03.08 | 06.97 | 06.54 | 04.06 | 07.39 | 13.51 | 08.38 | 12.64 | 08.56 | 13.73 |
| ThO2 | 01.77 | 02.24 | 01.64 | 00.49 | 00.29 | 01.21 | 00.10 | 00.00 | 00.75 | 00.84 | 00.45 | 00.92 |
| Y2O3 | 01.00 | 00.71 | 00.78 | 00.13 | 00.25 | b.d.l. | 00.00 | 00.00 | 00.11 | 00.25 | 00.30 | 00.16 |
| La2O3 | 01.04 | 00.63 | 01.12 | 00.57 | 00.75 | 00.04 | 00.08 | 00.00 | 00.06 | 00.06 | 00.12 | 00.00 |
| Ce2O3 | 03.43 | 02.19 | 03.77 | 02.38 | 03.15 | 00.58 | 00.93 | 00.33 | 00.85 | 00.62 | 01.19 | 00.47 |
| Pr2O3 | 00.39 | 00.27 | 00.43 | 00.26 | 00.38 | b.d.l. | 00.00 | 00.00 | 00.06 | 00.18 | 00.15 | 00.06 |
| Nd2O3 | 01.60 | 01.12 | 01.75 | 00.74 | 00.92 | 00.27 | 00.35 | 00.15 | 00.52 | 00.49 | 00.65 | 00.24 |
| Sm2O3 | 00.56 | 00.43 | 00.35 | 00.13 | 00.21 | b.d.l. | 00.11 | 00.00 | 00.27 | 00.24 | 00.37 | 00.00 |
| Eu2O3 | b.d.l. | b.d.l. | 00.00 | 00.00 | 00.04 | 00.00 | 00.00 | 00.00 | b.d.l. | 00.05 | 00.00 | 00.09 |
| Gd2O3 | b.d.l. | 00.20 | 00.00 | 00.00 | 00.00 | b.d.l. | 00.00 | 00.00 | 00.06 | 00.24 | 00.00 | 00.00 |
| Dy2O3 | 00.46 | 00.13 | 00.35 | b.d.l. | 00.00 | b.d.l. | 00.00 | 00.00 | 00.08 | 00.59 | 00.10 | 00.00 |
| Ho2O3 | b.d.l. | 00.00 | 00.00 | b.d.l. | b.d.l. | 00.21 | 00.00 | 00.14 | b.d.l. | 00.20 | 00.00 | 00.00 |
| Er2O3 | 00.18 | 00.13 | 00.11 | 00.05 | 00.21 | 00.00 | 00.00 | 00.00 | 00.10 | 00.39 | 00.00 | 00.00 |
| Tm2O3 | 00.07 | 00.16 | 00.17 | 00.07 | 00.18 | 00.12 | 00.00 | 00.00 | 00.14 | 00.04 | 00.14 | 00.00 |
| Yb2O3 | 00.20 | 00.08 | 00.00 | 00.08 | 00.05 | 00.08 | 00.00 | 00.00 | 00.23 | 00.25 | 00.06 | 00.00 |
| Lu2O3 | b.d.l. | b.d.l. | 00.00 | 00.00 | 00.10 | b.d.l. | 00.00 | 00.00 | 00.12 | 00.16 | 00.17 | 00.00 |
| FeO 2 | 00.69 | 00.28 | 00.16 | 01.70 | 00.10 | 02.71 | 02.93 | 03.10 | 05.92 | 03.24 | 04.30 | 04.62 |
| CaO | 01.42 | 01.73 | 01.30 | 01.04 | 03.01 | b.d.l. | 00.20 | 00.00 | 00.30 | 00.34 | 01.83 | 00.00 |
| MnO | 00.11 | 00.19 | 00.22 | 00.23 | b.d.l. | 00.00 | 00.12 | 00.09 | 01.20 | 00.49 | 00.66 | 05.98 |
| PbO | 07.23 | 14.51 | 07.52 | 13.98 | 05.56 | 30.69 | 17.23 | 25.94 | 02.85 | 00.02 | 02.19 | 00.64 |
| Na2O | 00.76 | 00.20 | 00.30 | 00.18 | 00.70 | 00.06 | 00.61 | 00.20 | 00.49 | 00.31 | 00.42 | 00.00 |
| F | 02.73 | 02.96 | 02.73 | 00.85 | 02.79 | 00.40 | 00.23 | 00.20 | 00.45 | 00.21 | 02.50 | 00.00 |
| F=O2 | −01.15 | −01.24 | −01.15 | −00.36 | −01.17 | −00.17 | −00.10 | −00.08 | −00.19 | −00.09 | −01.05 | −00.00 |
| Total 3 | 80.08 | 89.57 | 83.03 | 74.18 | 76.41 | 78.12 | 67.67 | 78.93 | 83.94 | 69.34 | 82.33 | 71.69 |
| Structural formula based on a sum of 2 a.p.f.u. in the [6]B site | ||||||||||||
| U4+ | 0.052 | 0.038 | 0.055 | 0.154 | 0.127 | 0.105 | 0.190 | 0.301 | 0.131 | 0.189 | 0.141 | 0.296 |
| Th4+ | 0.034 | 0.039 | 0.030 | 0.011 | 0.006 | 0.032 | 0.003 | 0.012 | 0.013 | 0.008 | 0.020 | |
| Y3+ | 0.044 | 0.029 | 0.033 | 0.007 | 0.012 | 0.004 | 0.009 | 0.012 | 0.008 | |||
| La3+ | 0.032 | 0.018 | 0.033 | 0.021 | 0.024 | 0.002 | 0.003 | 0.002 | 0.002 | 0.003 | ||
| Ce3+ | 0.105 | 0.061 | 0.111 | 0.087 | 0.101 | 0.025 | 0.039 | 0.012 | 0.022 | 0.015 | 0.032 | 0.017 |
| Pr3+ | 0.012 | 0.007 | 0.012 | 0.010 | 0.012 | 0.001 | 0.005 | 0.004 | 0.002 | |||
| Nd3+ | 0.048 | 0.030 | 0.050 | 0.026 | 0.029 | 0.011 | 0.014 | 0.005 | 0.013 | 0.012 | 0.017 | 0.008 |
| Sm3+ | 0.016 | 0.011 | 0.010 | 0.005 | 0.006 | 0.004 | 0.007 | 0.006 | 0.010 | 0.003 | ||
| Eu3+ | 0.001 | 0.001 | ||||||||||
| Gd3+ | 0.005 | 0.002 | 0.005 | 0.002 | ||||||||
| Dy3+ | 0.012 | 0.003 | 0.009 | 0.002 | 0.013 | |||||||
| Ho3+ | 0.008 | 0.004 | 0.004 | |||||||||
| Er3+ | 0.005 | 0.003 | 0.003 | 0.002 | 0.006 | 0.002 | 0.008 | 0.003 | ||||
| Tm3+ | 0.002 | 0.004 | 0.004 | 0.002 | 0.005 | 0.003 | 0.001 | 0.001 | ||||
| Yb3+ | 0.005 | 0.002 | 0.002 | 0.001 | 0.003 | 0.005 | 0.005 | 0.004 | ||||
| Lu3+ | 0.003 | 0.003 | 0.003 | |||||||||
| Pb2+ | 0.162 | 0.297 | 0.163 | 0.373 | 0.131 | 0.958 | 0.537 | 0.700 | 0.054 | 0.044 | 0.017 | |
| Fe2+ | 0.048 | 0.018 | 0.011 | 0.141 | 0.008 | 0.263 | 0.283 | 0.260 | 0.349 | 0.182 | 0.267 | 0.375 |
| Mn2+ | 0.008 | 0.013 | 0.015 | 0.020 | 0.011 | 0.007 | 0.072 | 0.028 | 0.042 | 0.491 | ||
| Ca2+ | 0.127 | 0.141 | 0.112 | 0.111 | 0.282 | 0.025 | 0.023 | 0.024 | 0.146 | |||
| Na+ | 0.123 | 0.030 | 0.046 | 0.035 | 0.119 | 0.013 | 0.138 | 0.038 | 0.067 | 0.041 | 0.060 | |
| Ʃ [8]A | 0.835 | 0.750 | 0.698 | 1.006 | 0.872 | 1.423 | 1.248 | 1.329 | 0.774 | 0.565 | 0.797 | 1.238 |
| Nb5+ | 1.739 | 1.747 | 1.744 | 1.795 | 1.805 | 1.441 | 1.677 | 0.982 | 1.714 | 0.968 | 1.753 | 1.721 |
| Ta5+ | 0.139 | 0.127 | 0.137 | 0.067 | 0.088 | 0.189 | 0.076 | 0.124 | 0.096 | 0.031 | 0.096 | 0.070 |
| Si4+ | 0.017 | 0.016 | 0.016 | 0.042 | 0.016 | 0.370 | 0.104 | 0.833 | 0.115 | 0.929 | 0.100 | 0.016 |
| Sn4+ | 0.039 | 0.083 | 0.074 | 0.028 | 0.091 | 0.041 | 0.003 | |||||
| Ti4+ | 0.066 | 0.027 | 0.030 | 0.069 | 0.102 | 0.061 | 0.076 | 0.073 | 0.050 | 0.190 | ||
| Ʃ [6]B | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| O2− | 4.878 | 4.672 | 4.667 | 5.328 | 4.983 | 5.789 | 5.559 | 5.349 | 4.643 | 3.580 | 4.771 | 5.938 |
| OH− | 1.122 | 1.328 | 1.333 | 0.672 | 1.017 | 0.211 | 0.441 | 0.651 | 1.357 | 2.420 | 1.229 | 0.062 |
| ƩX | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 |
| F− | 0.721 | 0.713 | 0.695 | 0.268 | 0.771 | 0.148 | 0.085 | 0.062 | 0.101 | 0.046 | 0.588 | |
| OH− | 0.279 | 0.287 | 0.305 | 0.732 | 0.229 | 0.852 | 0.915 | 0.938 | 0.899 | 0.954 | 0.412 | 1.000 |
| ƩY | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Facies | AGC | AGB | AGB | AGB | AGB | AGB | AGC 1 | AGB |
|---|---|---|---|---|---|---|---|---|
| Crystal | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) |
| Nb2O5 | 66.74 | 68.93 | 65.61 | 66.67 | 63.63 | 51.29 | 40.31 | 57.12 |
| Ta2O5 | 03.32 | 05.87 | 05.72 | 05.17 | 04.21 | 05.10 | 03.17 | 04.64 |
| SiO2 | 00.51 | 00.15 | 00.57 | 00.18 | 01.70 | 04.60 | 15.80 | 00.68 |
| SnO2 | b.d.l. | b.d.l. | b.d.l. | 01.72 | 00.20 | b.d.l. | 00.84 | b.d.l. |
| TiO2 | 02.57 | 01.26 | 02.36 | 01.63 | 03.20 | 01.14 | 02.74 | 02.74 |
| UO2 | 01.15 | 00.61 | 03.64 | 03.48 | 05.19 | 06.34 | 06.72 | 08.82 |
| ThO2 | b.d.l. | 00.05 | 00.18 | 00.32 | 00.44 | 01.48 | 00.56 | 00.78 |
| Y2O3 | 00.12 | b.d.l. | 00.07 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.22 |
| La2O3 | b.d.l. | 00.06 | b.d.l. | b.d.l. | b.d.l. | 00.13 | b.d.l. | b.d.l. |
| Ce2O3 | 00.05 | 00.07 | 00.18 | 00.13 | 00.33 | 00.42 | 00.11 | 00.42 |
| Pr2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Nd2O3 | b.d.l. | b.d.l. | 00.05 | 00.05 | 00.10 | 00.14 | 00.03 | 00.29 |
| Sm2O3 | 00.17 | 00.10 | 00.18 | 00.14 | b.d.l. | 00.25 | b.d.l. | 00.28 |
| Eu2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.04 | b.d.l. | 00.12 |
| Gd2O3 | 00.08 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.15 |
| Dy2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.40 |
| Ho2O3 | b.d.l. | b.d.l. | 00.15 | b.d.l. | 00.13 | 00.10 | b.d.l. | 00.15 |
| Er2O3 | b.d.l. | 00.07 | 00.16 | 00.18 | 00.09 | 00.10 | b.d.l. | 00.38 |
| Tm2O3 | b.d.l. | b.d.l. | 00.16 | b.d.l. | 00.09 | 00.13 | 00.06 | 00.11 |
| Yb2O3 | b.d.l. | 00.05 | 00.15 | 00.07 | 00.07 | 00.10 | 00.06 | 00.31 |
| Lu2O3 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.07 |
| FeO 2 | 15.33 | 11.79 | 16.13 | 12.27 | 08.37 | 10.08 | 07.69 | 13.85 |
| CaO | 00.40 | 00.37 | b.d.l. | 00.25 | 00.28 | 00.99 | b.d.l. | b.d.l. |
| MnO | 06.70 | 08.72 | 04.92 | 07.28 | 10.05 | 02.25 | 04.39 | 04.99 |
| PbO | 00.81 | b.d.l. | 00.06 | 00.13 | 00.38 | 02.83 | 03.78 | 00.36 |
| Na2O | b.d.l. | 00.03 | 00.04 | 00.07 | b.d.l. | 00.76 | b.d.l. | 00.11 |
| F | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.44 | b.d.l. | b.d.l. |
| F = O2 | −00.00 | −00.00 | −00.00 | −00.00 | −00.00 | −00.18 | −00.00 | −00.00 |
| Total 3 | 97.89 | 98.03 | 99.74 | 99.73 | 98.45 | 88.50 | 86.15 | 95.71 |
| Structural formula based on 3 cations and 6 oxygens | ||||||||
| Fe2+ | 0.725 | 0.571 | 0.771 | 0.598 | 0.408 | 0.551 | 0.385 | 0.708 |
| Mn2+ | 0.321 | 0.428 | 0.238 | 0.359 | 0.496 | 0.125 | 0.223 | 0.258 |
| Ʃ [8]A | 1.046 | 0.999 | 1.009 | 0.957 | 0.903 | 0.676 | 0.607 | 0.966 |
| Nb5+ | 1.704 | 1.803 | 1.693 | 1.754 | 1.674 | 1.515 | 1.089 | 1.578 |
| Ta5+ | 0.051 | 0.093 | 0.089 | 0.082 | 0.067 | 0.091 | 0.052 | 0.077 |
| Si4+ | 0.029 | 0.008 | 0.032 | 0.010 | 0.099 | 0.301 | 0.946 | 0.042 |
| Sn4+ | 0.040 | 0.005 | 0.020 | |||||
| Ti4+ | 0.109 | 0.055 | 0.101 | 0.071 | 0.140 | 0.056 | 0.123 | 0.126 |
| U4+ | 0.014 | 0.008 | 0.046 | 0.045 | 0.067 | 0.092 | 0.089 | 0.120 |
| Th4+ | 0.001 | 0.002 | 0.004 | 0.006 | 0.022 | 0.008 | 0.011 | |
| Y3+ | 0.004 | 0.002 | 0.007 | |||||
| La3+ | 0.001 | 0.003 | ||||||
| Ce3+ | 0.001 | 0.002 | 0.004 | 0.003 | 0.007 | 0.010 | 0.002 | 0.009 |
| Pr3+ | ||||||||
| Nd3+ | 0.001 | 0.001 | 0.002 | 0.003 | 0.001 | 0.006 | ||
| Sm3+ | 0.003 | 0.002 | 0.004 | 0.003 | 0.006 | 0.006 | ||
| Eu3+ | 0.001 | 0.002 | ||||||
| Gd3+ | 0.002 | 0.003 | ||||||
| Dy3+ | 0.008 | |||||||
| Ho3+ | 0.003 | 0.002 | 0.002 | 0.003 | ||||
| Er3+ | 0.001 | 0.003 | 0.003 | 0.002 | 0.002 | 0.007 | ||
| Tm3+ | 0.003 | 0.002 | 0.003 | 0.001 | 0.002 | |||
| Yb3+ | 0.001 | 0.003 | 0.001 | 0.001 | 0.002 | 0.001 | 0.006 | |
| Lu3+ | 0.001 | |||||||
| Pb2+ | 0.012 | 0.001 | 0.002 | 0.006 | 0.050 | 0.061 | 0.006 | |
| Ca2+ | 0.025 | 0.023 | 0.016 | 0.017 | 0.069 | |||
| Na+ | 0.004 | 0.004 | 0.007 | 0.096 | 0.013 | |||
| Ʃ [8]B | 1.954 | 2.001 | 1.991 | 2.043 | 2.097 | 2.324 | 2.393 | 2.034 |
| Mn/(Mn + Fe) | 0.307 | 0.428 | 0.236 | 0.375 | 0.549 | 0.184 | 0.367 | 0.267 |
| Ta/(Ta + Nb) | 0.029 | 0.049 | 0.050 | 0.045 | 0.038 | 0.056 | 0.045 | 0.047 |
| Facies | AGB | AGC | AGB | AGC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal | (1) | (2) 1 | (3) | (4) | (5) | (6) | (7) | (8) 1 | (9) | (10) 1 | (11) |
| Nb2O5 | 53.78 | 49.58 | 46.52 | 16.23 | 13.48 | 03.54 | 00.40 | 01.13 | 06.52 | 00.09 | 02.49 |
| Ta2O5 | 04.99 | 03.03 | 04.97 | 01.98 | 00.33 | b.d.l. | b.d.l. | b.d.l. | 01.41 | b.d.l. | b.d.l. |
| P2O5 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.43 | 03.52 | 01.04 | 01.05 | 00.07 | 00.10 |
| SiO2 | 01.62 | 02.62 | 05.42 | 10.19 | 07.75 | 14.02 | 14.13 | 11.53 | 14.39 | 00.04 | 00.07 |
| SnO2 | b.d.l. | 0.79 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 00.23 | b.d.l. | b.d.l. | b.d.l. |
| TiO2 | 01.43 | 02.37 | 03.71 | 01.97 | 01.80 | 00.41 | b.d.l. | 00.15 | 01.18 | b.d.l. | 00.22 |
| UO2 | 08.38 | 13.35 | 22.94 | 19.91 | 42.38 | 34.35 | 21.21 | 17.34 | 04.48 | 03.81 | 00.65 |
| ThO2 | 00.75 | 05.19 | 01.08 | 01.48 | 08.23 | 10.00 | 04.70 | 30.39 | 11.82 | 03.13 | 11.61 |
| ZrO2 | b.d.l. | b.d.l. | b.d.l. | 00.61 | 0.848 | 00.68 | 00.18 | 02.52 | 13.32 | b.d.l. | b.d.l. |
| Y2O3 | 00.11 | 00.21 | 00.18 | 00.33 | b.d.l. | 01.07 | 10.27 | 03.51 | 01.89 | b.d.l. | 01.72 |
| La2O3 | 00.06 | 00.02 | 00.06 | 00.04 | 00.08 | 00.05 | b.d.l. | b.d.l. | b.d.l. | 09.08 | 05.82 |
| Ce2O3 | 00.85 | 00.44 | 00.47 | 00.22 | 00.85 | 00.68 | 00.02 | 00.18 | 00.23 | 26.33 | 17.03 |
| Pr2O3 | 00.06 | 00.08 | 00.05 | b.d.l. | 00.15 | 00.19 | b.d.l. | 00.09 | b.d.l. | 03.55 | 02.22 |
| Nd2O3 | 00.52 | 00.40 | 00.20 | 00.05 | 00.51 | 00.63 | 00.10 | 00.25 | 00.27 | 08.89 | 08.34 |
| Sm2O3 | 00.27 | 00.18 | 00.15 | 00.06 | 00.19 | 00.21 | b.d.l. | 00.63 | 00.24 | 00.87 | 02.94 |
| Eu2O3 | b.d.l. | 00.11 | 00.05 | b.d.l. | b.d.l. | 00.08 | 00.08 | b.d.l. | b.d.l. | 00.37 | 00.40 |
| Gd2O3 | 00.06 | 00.35 | 00.10 | b.d.l. | 00.07 | 00.08 | 00.50 | 00.77 | 00.29 | b.d.l. | 00.38 |
| Dy2O3 | 00.08 | 00.72 | 00.12 | b.d.l. | b.d.l. | b.d.l. | 02.43 | 00.23 | 00.71 | b.d.l. | 00.69 |
| Ho2O3 | b.d.l. | b.d.l. | b.d.l. | 00.10 | b.d.l. | 00.24 | 00.49 | 01.00 | 00.14 | 00.13 | 00.28 |
| Er2O3 | 00.10 | 00.54 | 00.19 | 00.11 | 00.12 | 00.21 | 02.06 | 01.64 | 00.44 | 00.30 | 00.26 |
| Tm2O3 | 00.14 | 00.09 | b.d.l. | 00.04 | b.d.l. | b.d.l. | 00.26 | 00.30 | 00.11 | b.d.l. | b.d.l. |
| Yb2O3 | 00.23 | 00.39 | 00.14 | 00.22 | 00.25 | 00.48 | 02.09 | 00.85 | 00.28 | 00.08 | 00.12 |
| Lu2O3 | 00.12 | 00.11 | b.d.l. | b.d.l. | b.d.l. | 00.13 | 00.57 | b.d.l. | 00.14 | b.d.l. | 00.11 |
| FeO 2 | 05.92 | 12.09 | 10.28 | 05.63 | 00.49 | 00.17 | 00.80 | 00.25 | 01.47 | 00.12 | 01.50 |
| CaO | 00.30 | b.d.l. | 00.28 | b.d.l. | 00.23 | b.d.l. | 00.62 | 00.34 | 00.65 | b.d.l. | 02.11 |
| MnO | 01.20 | 05.47 | 02.32 | 00.25 | 00.23 | b.d.l. | 00.13 | b.d.l. | 00.38 | 00.12 | 00.48 |
| PbO | 02.85 | 01.30 | b.d.l. | 00.58 | 00.08 | b.d.l. | 00.09 | 01.23 | 10.70 | 00.55 | 00.29 |
| Na2O | 00.49 | 00.14 | 00.44 | 00.26 | b.d.l. | b.d.l. | 00.02 | 00.04 | 00.17 | 00.12 | 00.24 |
| F | 00.45 | 00.49 | b.d.l. | b.d.l. | 01.24 | 02.97 | 03.92 | 04.54 | 02.61 | 17.31 | 10.75 |
| F = O2 | −00.19 | −00.21 | −00.00 | −00.00 | −00.52 | −01.25 | −01.65 | −01.91 | −01.10 | ||
| Total 3 | 83.94 | 101.88 | 99.22 | 61.66 | 80.13 | 69.43 | 66.94 | 81.10 | 73.99 | 74.96 | 70.82 |
| AGC | AGB | PEG | ||
|---|---|---|---|---|
| UO2 (ppm) | Min. | 40.00 | 34.00 | 20.00 |
| Max. | 1610.00 | 796.00 | 1180.00 | |
| Avg. | 322 (111) | 345 (54) | 553 | |
| ThO2 (ppm) | Min. | 70.00 | 36.10 | 1080.00 |
| Max. | 2388.00 | 2419.00 | 18,400.00 | |
| Avg. | 800 (113) | 696 (71) | 5127 (98) | |
| Th/U | Min. | 0.29 | 0.13 | 3.30 |
| Max. | 30.40 | 8.94 | 389.50 | |
| Avg. | 3.82 (110) | 1.85 (53) | 19.85 (64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadlich, I.W.; Bastos Neto, A.C.; Pereira, V.P.; Dill, H.G.; Botelho, N.F. The Radioactive Rare Metal Mineralization in the World-Class Sn-Nb-Ta-U-Th-REE-Deposit Madeira (Pitinga, Amazonas State, Brazil): With Special Reference to the Complex Alteration of Pyrochlore-Group Minerals. Minerals 2024, 14, 895. https://doi.org/10.3390/min14090895
Hadlich IW, Bastos Neto AC, Pereira VP, Dill HG, Botelho NF. The Radioactive Rare Metal Mineralization in the World-Class Sn-Nb-Ta-U-Th-REE-Deposit Madeira (Pitinga, Amazonas State, Brazil): With Special Reference to the Complex Alteration of Pyrochlore-Group Minerals. Minerals. 2024; 14(9):895. https://doi.org/10.3390/min14090895
Chicago/Turabian StyleHadlich, Ingrid W., Artur C. Bastos Neto, Vitor P. Pereira, Harald G. Dill, and Nilson F. Botelho. 2024. "The Radioactive Rare Metal Mineralization in the World-Class Sn-Nb-Ta-U-Th-REE-Deposit Madeira (Pitinga, Amazonas State, Brazil): With Special Reference to the Complex Alteration of Pyrochlore-Group Minerals" Minerals 14, no. 9: 895. https://doi.org/10.3390/min14090895





