Comparative Study of Sulfides from Porphyry, Skarn, and Carbonate-Replacement Mineralization at the Recsk Porphyry-Mineralized Complex, Hungary
Abstract
:1. Introduction
Geology and Mineralization of the Intrusive–Volcanic Complex at the Recsk
2. Materials and Methods
2.1. Samples
2.2. Methods
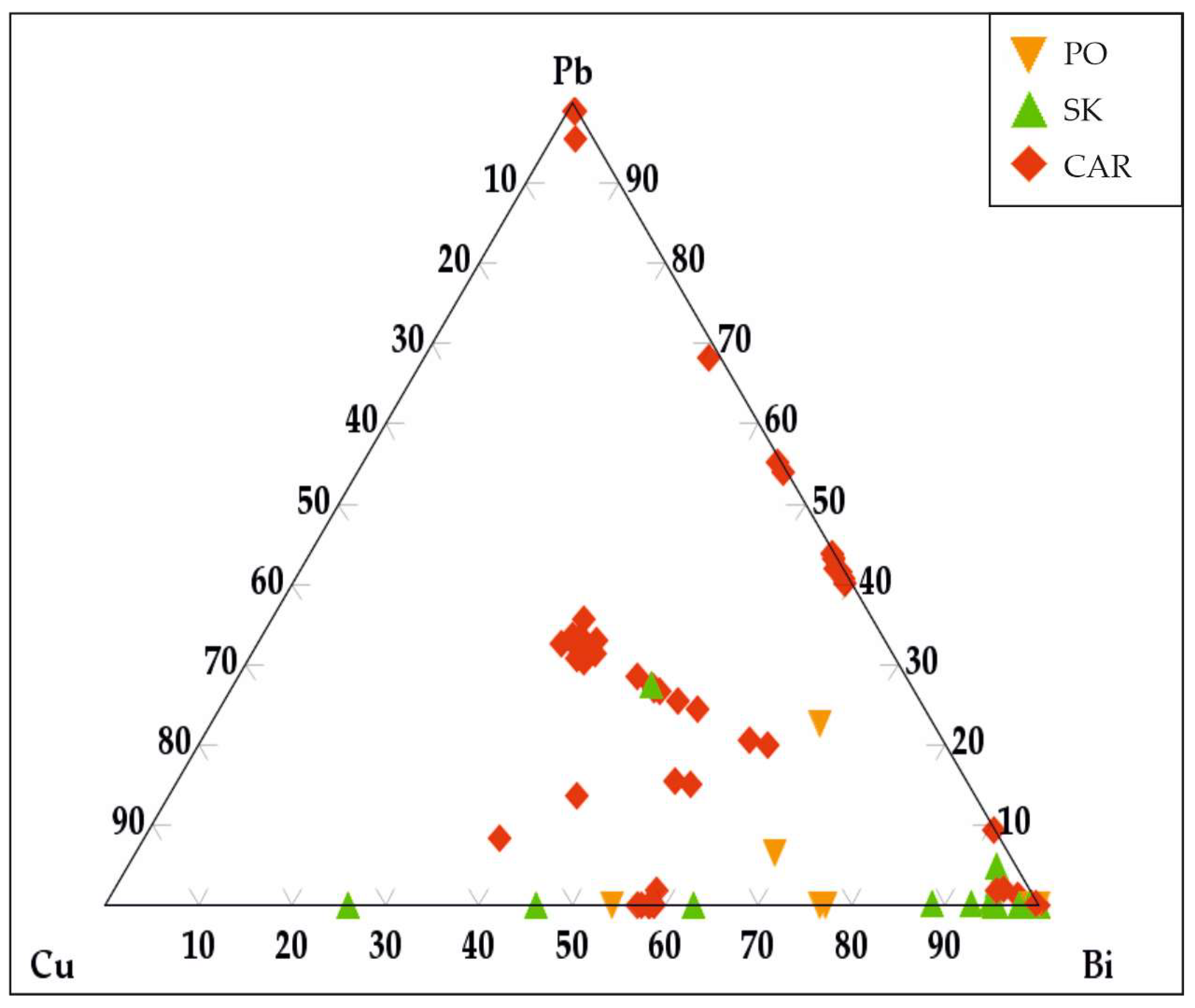
3. Results
3.1. Petrography
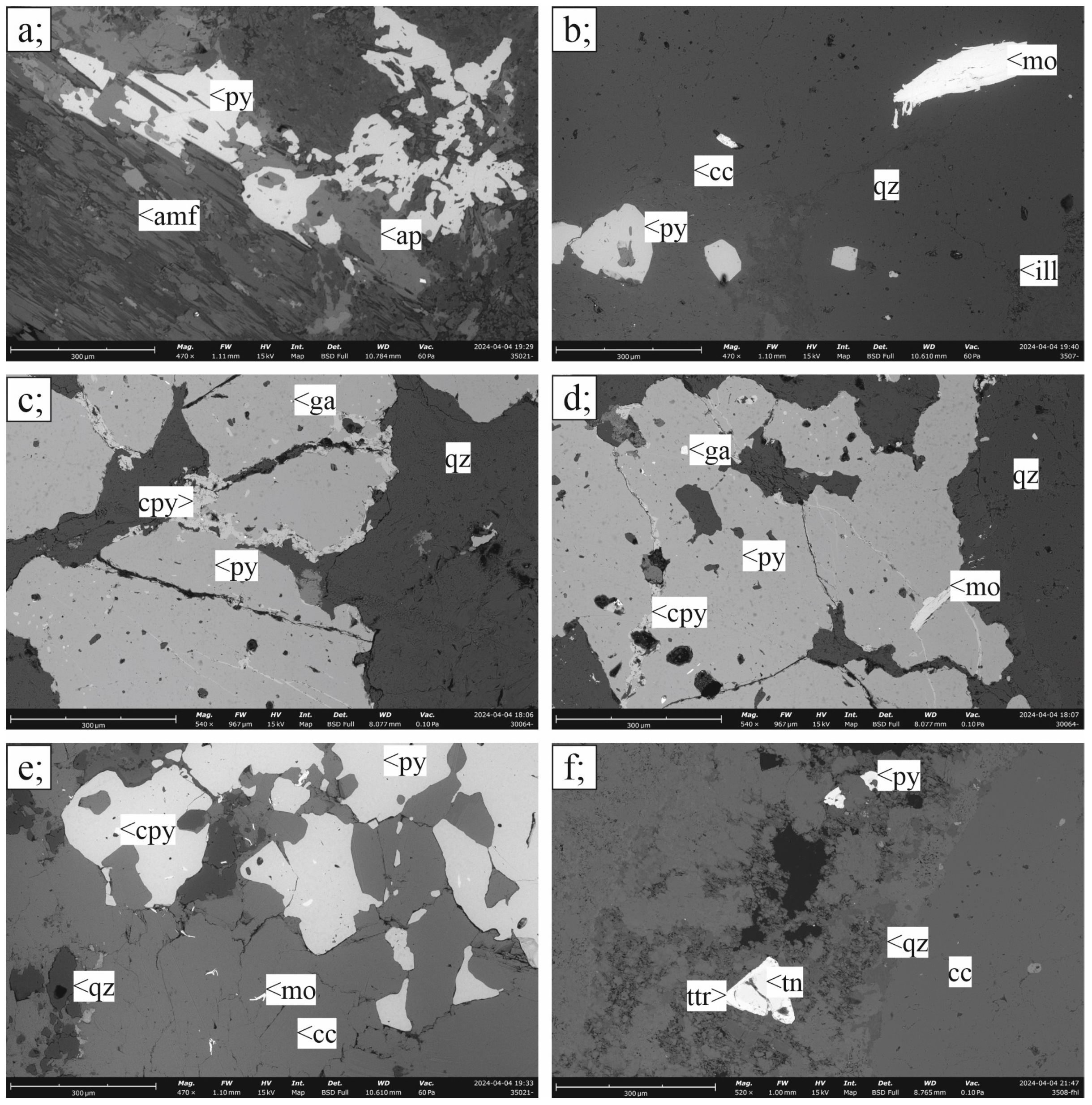
3.1.1. Porphyry Cu–(Mo)–Au Ore
3.1.2. Skarn Ores
3.1.3. Carbonate-Replacement Polymetallic Mineralization
3.2. Ore Mineralogy
3.2.1. Porphyry Mineralization
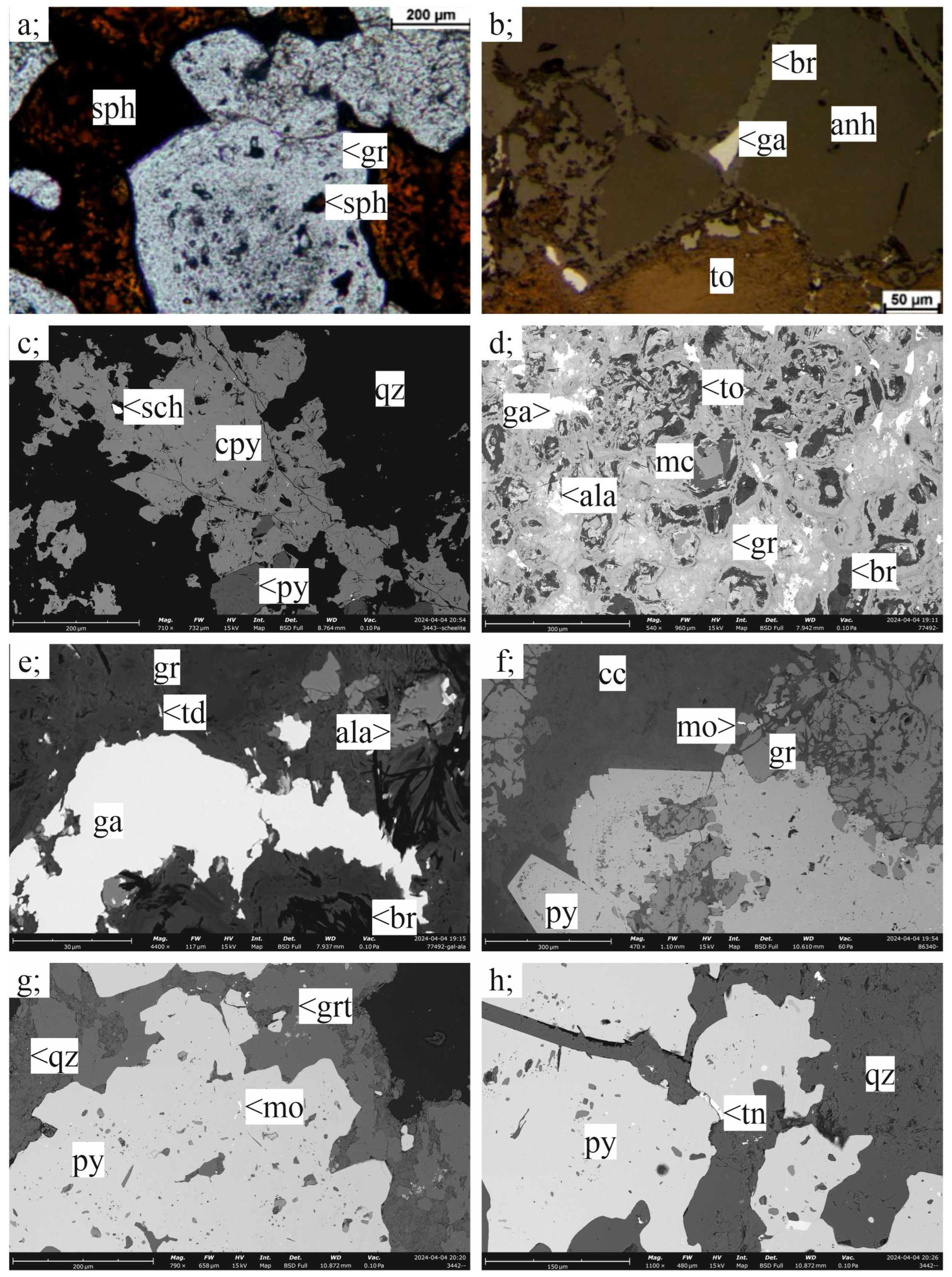
3.2.2. Skarn Mineralization
3.2.3. Carbonate-Replacement Mineralization
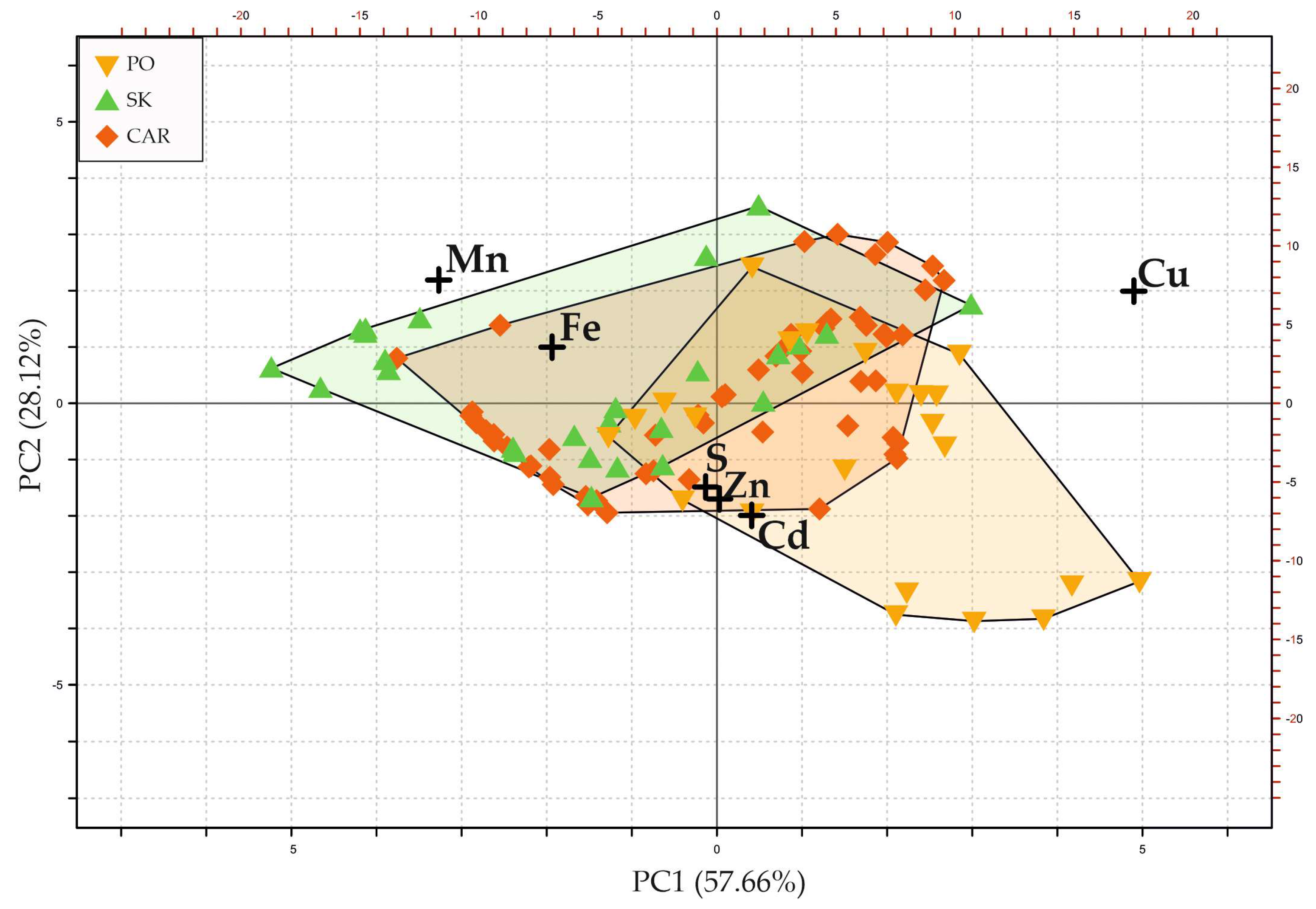
3.3. Compositions of Major Sulfide Minerals in Different Types of Mineralization
3.3.1. Molybdenite
3.3.2. Galena
3.3.3. Sphalerite
3.3.4. Kesterite
3.3.5. Tetrahedrite-Group Minerals
3.3.6. Bismuth Sulfosalts
4. Discussion
4.1. Molybdenite
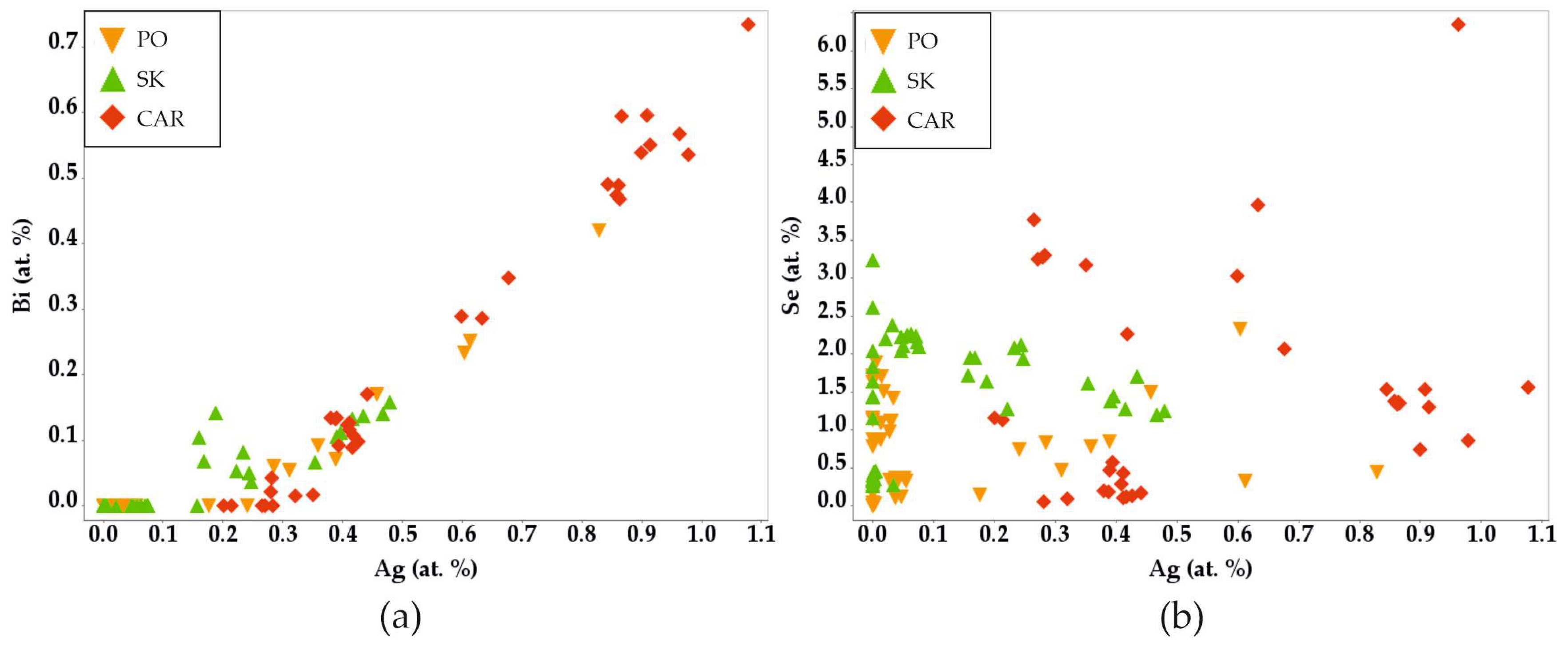
4.2. Galena
4.3. Sphalerite
4.4. Tetrahedrite-Group Minerals
4.5. Sulfosalts
4.6. Behavior of Selenum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John, D.A.; Taylor, R.D. By-Products of Porphyry Copper and Molybdenum Deposits. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Reviews in Economic Geology; Society of Economic Geologists: Littleton, CO, USA, 2016; Volume 18, Chapter 7; pp. 137–164. ISBN 9781629490922. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B. Copper Demand, Supply, and Associated Energy Use to 2050. Glob. Environ. Chang. 2016, 39, 305–315. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Bonnet, C.; Simoën, M.; Carcanague, S. Copper at the Crossroads: Assessment of the Interactions between Low-Carbon Energy Transition and Supply Limitations. Resour. Conserv. Recycl. 2020, 163, 105072. [Google Scholar] [CrossRef] [PubMed]
- Cseh-Németh, J. Deep-seated base metal ore occurrence of Recsk: Geological pattern of ore accumulation. Bull. Hung. Geol. Soc. 1975, 105, 692–708. (In Hungarian) [Google Scholar]
- Csillag, J. Rocks transformed upon magmatic effect in the Recsk area. Bull. Hung. Geol. Soc. 1975, 105, 646–671. (In Hungarian) [Google Scholar]
- Csongrády, J. Characterization of the deep-seated base metal ore mineralization of Recsk on the basis of ore-microscopic analyses. Bull. Hung. Geol. Soc. 1975, 105, 672–691. (In Hungarian) [Google Scholar]
- Baksa, C. Genetic Aspects of the Recsk Mineralized Complex, Hungary. In Geology and Metallogeny of Copper Deposits; Conference Paper; Springer: Berlin/Heidelberg, Germany, 1986; pp. 280–290. [Google Scholar] [CrossRef]
- Gatter, I.; Molnár, F.; Földessy, J.; Zelenka, T.; Kiss, J.; Szebényi, G. High-and low-sulfidation epithermal mineralization of the Màtra Mountains, Northeast Hungary. In Epithermal Mineralization of the Western Carpathians; Molnár, F., Lexa, J., Hedenquist, J., Eds.; Society of Economic Geologists Guidebook Series; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 31, pp. 155–179. [Google Scholar]
- Zelenka, T.; Szebényi, G. Exploration history of the base metal deposit at Recsk deep levels. In Mineral Exploration in Hungary during the XXth Century; Miskolc-Rudabánya; Szakáll, S., Morvai, G., Eds.; 2002; pp. 169–198. Available online: https://library.hungaricana.hu/hu/collection/muze_szak_asva_kozlemenyek/ (accessed on 20 September 2024).
- Molnár, F. The Cu-Au-Ag-Zn-Pb ore complex at Recsk, Hungary: A uniquely preserved and explored porphyry-skarn-epithermal system in the Palaeogene magmatic belt of the Alp-Carpathian-Dinaride system. In Proceedings of the Ninth Biennial SGA Symposium, Dublin, Ireland, 20–23 August 2007; Volume 1, pp. 153–157. [Google Scholar]
- Molnár, F.; Jung, P.; Kupi, L.; Pogány, A.; Vágó, E.; Viktorik, O.; Pécskay, Z. Epithermal Zones of the Porphyry-Skarn-Epithermal Ore Complex at Recsk. In Recsk and Lahóca—Geology of the Paleogene Ore Complex; Földessy, J., Hartai, É., Eds.; Publications of the University of Miskolc, Series A, Mining; University of Miskolc: Miskolc, Hungary, 2008; Volume 73, pp. 99–128. [Google Scholar]
- Földessy, J.; Hartai, É. Recsk and Lahóca: Geology of the Paleogene Ore Complex; Publications of the Univeristy of Miskolc, Series A, Mining; University of Miskolc: Miskolc, Hungary, 2008; Volume 73. [Google Scholar]
- Takács, Á.; Molnár, F.; Turi, J.; Mogessie, A.; Menzies, J.C. Ore Mineralogy and Fluid Inclusion Constraints on the Temporal and Spatial Evolution of a High-Sulfidation Epithermal Cu-Au-Ag Deposit in the Recsk Ore Complex, Hungary. Econ. Geol. 2017, 112, 1461–1481. [Google Scholar] [CrossRef]
- Altenberger, F.; Raith, J.G.; Bakker, R.J.; Zarasvandi, A. The Chah-Mesi Epithermal Cu-Pb-Zn-(Ag-Au) Deposit and Its Link to the Meiduk Porphyry Copper Deposit, SE Iran: Evidence from Sulfosalt Chemistry and Fluid Inclusions. Ore Geol. Rev. 2022, 142, 104732. [Google Scholar] [CrossRef]
- Cooke, D.R.; Agnew, P.; Hollings, P.; Baker, M.; Chang, Z.; Wilkinson, J.J.; White, N.C.; Zhang, L.; Thompson, J.; Gemmell, J.B.; et al. Porphyry indicator minerals (PIMS) and porphyry vectoring and fertility tools (PVFTS)–indicators of mineralization styles and recorders of hypogene geochemical dispersion halos. In Proceedings of the Decennial Mineral Exploration Conferences, Toronto, ON, Canada, 22–25 October 2017; pp. 457–470. [Google Scholar]
- Cooke, D.R.; Agnew, P.; Hollings, P.; Baker, M.; Chang, Z.; Wilkinson, J.J.; Ahmed, A.; White, N.C.; Zhang, L.; Thompson, J.; et al. Recent Advances in the Application of Mineral Chemistry to Exploration for Porphyry Copper–Gold–Molybdenum Deposits: Detecting the Geochemical Fingerprints and Footprints of Hypogene Mineralization and Alteration. Geochem. Explor. Environ. Anal. 2020, 20, 176–188. [Google Scholar] [CrossRef]
- Sykora, S.; Cooke, D.R.; Meffre, S.; Stephanov, A.S.; Gardner, K.; Scott, R.; Selley, D.; Harris, A.C. Evolution of Pyrite Trace Element Compositions from Porphyry-Style and Epithermal Conditions at the Lihir Gold Deposit: Implications for Ore Genesis and Mineral Processing. Econ. Geol. 2018, 113, 193–208. [Google Scholar] [CrossRef]
- Börner, F.; Keith, M.; Smith, D.J.; Barry, T.L.; Neumann, T.; Klemd, R. Fingerprinting Fluid Evolution by Trace Elements in Epithermal Pyrite, Vatukoula Au-Te Deposit, Fiji. Ore Geol. Rev. 2021, 137, 104314. [Google Scholar] [CrossRef]
- Cave, B.; Lilly, R.; Barovich, K. Textural and Geochemical Analysis of Chalcopyrite, Galena and Sphalerite across the Mount Isa Cu to Pb-Zn Transition: Implications for a Zoned Cu-Pb-Zn System. Ore Geol. Rev. 2020, 124, 103647. [Google Scholar] [CrossRef]
- Cioacǎ, M.E.; Munteanu, M.; Qi, L.; Costin, G. Trace Element Concentrations in Porphyry Copper Deposits from Metaliferi Mountains, Romania: A Reconnaissance Study. Ore Geol. Rev. 2014, 63, 22–39. [Google Scholar] [CrossRef]
- Keith, M.; Haase, K.M.; Chivas, A.R.; Klemd, R. Phase Separation and Fluid Mixing Revealed by Trace Element Signatures in Pyrite from Porphyry Systems. Geochim. Cosmochim. Acta 2022, 329, 185–205. [Google Scholar] [CrossRef]
- Kullerud, G. The FeS-ZnS system—A geological thermometer. Nor. Geol. Tiddskrift 1953, 32, 62–147. [Google Scholar]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, Germanium, Indium, and Other Trace and Minor Elements in Sphalerite as a Function of Deposit Type—A Meta-Analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Barton, I.F.; Rathkopf, C.A.; Barton, M.D. Rhenium in Molybdenite: A Database Approach to Identifying Geochemical Controls on the Distribution of a Critical Element. Min. Met. Explor. 2020, 37, 21–37. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Kelson, C.R.; Guerin, R.; Kalleske, N.; Danyushevsky, L. Trace Element Heterogeneity in Molybdenite Fingerprints Stages of Mineralization. Chem. Geol. 2013, 347, 175–189. [Google Scholar] [CrossRef]
- Terada, K.; Osaki, S.; Ishihara, S.; Kiba, T. Distribution of Rhenium in Molybdenites from Japan. Geochem. J. 1971, 4, 123–141. [Google Scholar] [CrossRef]
- Marushchenko, L.I.; Baksheev, I.A.; Nagornaya, E.V.; Chitalin, A.F.; Nikolaev, Y.N.; Vlasov, E.A. Compositional Evolution of the Tetrahedrite Solid Solution in Porphyry-Epithermal System: A Case Study of the Baimka Cu-Mo-Au Trend, Chukchi Peninsula, Russia. Ore Geol. Rev. 2018, 103, 21–37. [Google Scholar] [CrossRef]
- Sack, R.O. Fahlore Thermochemistry: Gaps inside the (Cu,Ag)10(Fe,Zn)2(Sb,As)4S13 Cube. Petrology 2017, 25, 498–515. [Google Scholar] [CrossRef]
- Benedek, K. Paleogene Igneous Activity along the Easternmost Segment of the Periadriatic-Balaton Lineament. Acta Geol. Hung. 2002, 45, 359–371. [Google Scholar] [CrossRef]
- Exner, C. Die Geologische Position der Magmatite des Periadriatischen Lineamentes; Geologische Bundesanstalt: Vienna, Austria, 1976; pp. 3–64. [Google Scholar]
- Földessy, J.; Zelenka, T.; Benedek, K.; Pécskay, Z.; Mádai, F. The Recsk Paleogene magmatism in a regional context. In Recsk and Lahóca—Geology of the Paleogene Ore Complex; Földessy, J., Hartai, É., Eds.; Publications of the University of Miskolc, Series A, Mining; University of Miskolc: Miskolc, Hungary, 2008; Volume 73, pp. 7–20. [Google Scholar]
- Raith, J.G.; Hutter, F.; Altenberger, F.; Weilbold, J.; Auer, C.; Krause, J.; Berndt, J.; Neinavaie, H. Polymetallic Tungsten Skarn Mineralisation Related to the Periadriatic Intrusion at Lienzer Schlossberg, East Tyrol, Austria. Austrian J. Earth Sci. 2024, 117, 87–112. [Google Scholar] [CrossRef]
- Arató, R.; Dunkl, I.; Takács, Á.; Szebényi, G.; Gerdes, A.; Von Eynatten, H. Thermal Evolution in the Exhumed Basement of a Stratovolcano: Case Study of the Miocene Mátra Volcano, Pannonian Basin. J. Geol. Soc. 2018, 175, 820–835. [Google Scholar] [CrossRef]
- Zelenka, T.; Markó, B. Comparative results of exploratory shaft-sinking and tunnel-driving and exploratory deep drilling at the Recsk ore deposit. Bull. Hung. Geol. Soc. 1979, 109, 469–477. (In Hungarian) [Google Scholar]
- Baksa, C. The genetic framework of the Recsk ore genesis. Acta Mineroligica-Petrogr. Szeged. 1983, XXVI/I, 87–97. [Google Scholar]
- Kovács, S.; Gecse, Z.; Pelikán, P.; Zelenka, T.; Szebényi, G.; Szabó, I. Upper Triassic conodonts from deep boreholes of the Recsk–Darnó area: New data on the geology of its pre-Cenozoic basement. Bull. Hung. Geol. Soc. 2013, 143, 29–46. (In Hungarian) [Google Scholar]
- Molnár, F.; Gatter, I.; Zelenka, T.; Pécskay, Z.; Bernadett, B. Metallogeny of Paleogene and Neogene Volcanic Belts in Hungary. In Proceedings of the 7th Biannual SGA Meeting, Balkema, Rotterdam, The Netherlands, 24–28 August 2003; Volume 2, pp. 1205–1208. [Google Scholar]
- Földessy, J.; Seres-Hartai, É.; Szebényi, G. Distribution of Gold Mineralization in the Recsk Ore Complex, NE Hungary. Acta Geol. Hung. 2004, 47, 247–258. [Google Scholar] [CrossRef]
- Singer, D.A.; Berger, V.I.; Moring, B.C. Porphyry Copper Deposits of the World: Database and Grade and Tonnage Models; USGS Reports; USGS: Reston, VA, USA, 2008. [Google Scholar]
- Török, K.; Gyuricza, G.; Szeiler, R.B.; Füged, U.; Gál, N.; Gáspár, E.; Gulyás, Á.; Horváth, Z.; Kerékgyártó, T.; Korbély, B.; et al. Complex Vulnerability and Loadability Assessment Report to Recsk II. Copper Ore Proposed Area for Concession; Hungarian Office for Mining and Geology: Budapest, Hungary, 2016. Available online: https://mbfsz.gov.hu/sites/default/files/file/2018/07/06/recsk_ii_eng.pdf (accessed on 20 September 2024).
- Földessy, J.; Szebényi, G. The mineralizations of the the Recsk deeps and Lahóca—Short geological overview. In Recsk and Lahóca—Geology of the Paleogene Ore Complex; Földessy, J., Hartai, É., Eds.; Publications of the University of Miskolc, Series A, Mining; University of Miskolc: Miskolc, Hungary, 2008; Volume 73, pp. 85–98. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2003; Available online: https://www.r-project.org (accessed on 20 September 2024).
- Sillitoe, R.H. Porphyry Copper Systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Ramdohr, P. Ore Minerals and Their Intergrowths, 2nd ed.; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Barton, P.B.; Bethke, P.M. Chalcopyrite Disease in Sphalerite: Pathology and Epidemiology. Am. Mineral. 1987, 72, 451–467. [Google Scholar]
- Foord, E.E.; Shawe, D.R. The Pb-Bi-Ag-Cu-(Hg) chemsitry of galena and some associated sulfosalts: A review and some new data from Colorado, California and Pennsylvania. Can. Mineral. 1989, 27, 363–382. [Google Scholar]
- Blackburn, W.H.; Schwendeman, J.F. Trace Element Substitution in Galena. Can. Miner. 1977, 15, 365–373. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and Minor Elements in Sphalerite: A LA-ICPMS Study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, S.; Karup-Møller, S.; et al. Sulfosalt Systematics: A Review. Report of the Sulfosalt Sub-Committee of the IMA Commission on Ore Mineralogy. Eur. J. Mineral. 2008, 20, 7–62. [Google Scholar] [CrossRef]
- Pasava, J.; Svojtka, M.; Veselovský, F.; Ďurišová, J.; Ackerman, L.; Pour, O.; Drábek, M.; Halodová, P.; Haluzová, E. Laser Ablation ICPMS Study of Trace Element Chemistry in Molybdenite Coupled with Scanning Electron Microscopy (SEM)—An Important Tool for Identification of Different Types of Mineralization. Ore Geol. Rev. 2016, 72, 874–895. [Google Scholar] [CrossRef]
- Renock, D.; Becker, U. A First Principles Study of Coupled Substitution in Galena. Ore Geol. Rev. 2011, 42, 71–83. [Google Scholar] [CrossRef]
- George, L.; Cook, N.J.; Cristiana, C.; Wade, B.P. Trace and Minor Elements in Galena: A Reconnaissance LA-ICP-MS Study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- Craig, J.R. Phase Relations and Mineral Assemblages in the Ag-Bi-Pb-S System. Miner. Depos. 1967, 1, 278–306. [Google Scholar] [CrossRef]
- Yin, S.; Wirth, R.; He, H.; Ma, C.; Pan, J.; Xing, J.; Xu, J.; Fu, J.; Zhang, X.N. Replacement of Magnetite by Hematite in Hydrothermal Systems: A Refined Redox-Independent Model. Earth Planet. Sci. Lett. 2022, 577, 117282. [Google Scholar] [CrossRef]
- Bortnikov, N.S.; Dobrovol’skaya, M.G.; Genkin, A.D.; Naumov, V.B.; Shapenko, V.V. Sphalerite-Galena Geothermometers: Distribution of Cadmium, Manganese, and the Fractionation of Sulfur Isotopes. Econ. Geol. 1995, 90, 155–180. [Google Scholar] [CrossRef]
- Ebel, D.S.; Sack, R. Arsenic-Silver Incompatibility in Fahlore. Mineral. Mag. 1991, 55, 521–528. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.T.; Holwell, D.A.; Dye, M.D. A Review of Te and Se Systematics in Hydrothermal Pyrite from Precious Metal Deposits: Insights into Ore-Forming Processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]






| Drillcore | Spl. Nr. | Coordinates (WGS 84) | Mineralogy | Mineralization Type | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Ore Minerals | Gangue Minerals | |||
| Rm-30 | 63 | 47.9349 | 20.0694 | −290.3 | py, cpy, fh, ga, sphs | fs, qz, ag, | PO |
| Rm-30 | 64 | 47.9349 | 20.0694 | −400.6 | py, ga, sph, sphs, mo | qz, fs, ag, cc, dol | PO-CAR |
| Rm-34 | 05 | 47.9373 | 20.0615 | −602.3 | py, sph, ga,cpy, sphs | qz, cc | CAR |
| Rm-34 | 06 | 47.9373 | 20.0615 | −697.3 | py, sph, sphs, fhl | qz, cc | CAR |
| Rm-34 | 07 | 47.9373 | 20.0615 | −716.3 | py, mo, sch, sph, fhl, sphs | dol, qz, | CAR |
| Rm-34 | 42 | 47.9373 | 20.0615 | −796.3 | py, sph, cpy, fhl, sphs, sch, mo | qz, grt | SK |
| Rm-34 | 43 | 47.9373 | 20.0615 | −818.3 | py, sphs, sch, cpy | qz | SK |
| Rm-34 | 44 | 47.9373 | 20.0615 | −832.8 | py, sphs, sch, cpy | fs, qz, ag | PO |
| Rm-34 | 46 | 47.9373 | 20.0615 | −961.3 | py, fhl, sphs | fs, qz, ag | PO |
| Rm-35 | 5 | 47.9359 | 20.0658 | −337.8 | py, cpy, sph, ga, sphs | fs, qz, ag | PO |
| Rm-35 | 6 | 47.9359 | 20.0658 | −334.3 | py, cpy, sph, ga | fs, qz, ag | PO |
| Rm-35 | 7 | 47.9359 | 20.0658 | −373.8 | py, mo, cpy, ga, fhl | fs, qz, ag, cc | PO |
| Rm-35 | 8 | 47.9359 | 20.0658 | −372.8 | py, mo, fhl, sph, | fs, qz, ag, cc | PO |
| Rm-35 | 13 | 47.9359 | 20.0658 | −569.3 | py, mo, cpy | fs, qz, ag, cc | PO |
| Rm-35 | 16 | 47.9359 | 20.0658 | −677.3 | py, mo, cpy | fs, qz, ag, cc | PO |
| Rm-35 | 17 | 47.9359 | 20.0658 | −682.8 | py, mo | fs, qz, ag | PO |
| Rm-35 | 21 | 47.9359 | 20.0658 | −782.3 | py, mo | fs, qz, ag | PO |
| Rm-35 | 23 | 47.9359 | 20.0658 | −786.3 | py, mo, cpy, sphs | qz, grt, px | SK |
| Rm-35 | 24 | 47.9359 | 20.0658 | −837.3 | py, mo, cpy | fs, qz, ag | PO |
| Rm-45 | 340 | 47.9329 | 20.0709 | −839.3 | py, cpy, mo, | qz, amf | SK |
| Rm-61 | 01 | 47.9420 | 20.0625 | −664.5 | py, sph, cpy, ga,sphs | qz, cc, dol, fl | CAR |
| Rm-63 | 03 | 47.9324 | 20.0604 | −506.3 | py, ga, sph, sphs | cc | CAR |
| Rm-64 | 01 | 47.9280 | 20.06202 | −233.0 | py, sph, ga, fhl, sphs | qz, cc, dol | CAR |
| Rm-70 | 01 | 47.9203 | 20.0698 | −576.9 | py, sph, ga, cpy | cc, px, ag | SK |
| Rm-70 | 16 | 47.9203 | 20.0698 | −313.3 | py, cpy, mo, ga | fs, qz, ag | PO |
| Rm-71 | 74 | 47.9172 | 20.0580 | −926.9 | py, cpy, ga, sph, sphs | cc, qz, anh | SK |
| Rm-71 | 499 | 47.9172 | 20.0580 | −907.0 | py, sph, ga, cpy, sphs | grt, qz, px, cc | SK |
| Rm-71 | 02 | 47.9203 | 20.0698 | −717.9 | py, sph, ga, cpy | px, ep, srp, cc | SK |
| Rm-73 | 49 | 47.9186 | 20.0631 | −58.3 | py, cpy, mo, sph, ga, sphs | fs, qz, ag | PO |
| Rm-77 | 492 | 47.92569 | 20.0633 | −219.3 | py, po, mt, sph, ga, to, va, ala | grt, anh, br | SK |
| Rm-82 | 516 | 47.9021 | 20.0513 | −645.2 | py, sph, ga, cpy | anh | SK |
| Rm-87 | 01 | 47.9368 | 20.0557 | −422.3 | py, sph, ga, fhl, cpy | qz, cc | CAR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biró, M.; Raith, J.G.; Feichter, M.; Hencz, M.; Kiss, G.B.; Virág, A.; Molnár, F. Comparative Study of Sulfides from Porphyry, Skarn, and Carbonate-Replacement Mineralization at the Recsk Porphyry-Mineralized Complex, Hungary. Minerals 2024, 14, 956. https://doi.org/10.3390/min14090956
Biró M, Raith JG, Feichter M, Hencz M, Kiss GB, Virág A, Molnár F. Comparative Study of Sulfides from Porphyry, Skarn, and Carbonate-Replacement Mineralization at the Recsk Porphyry-Mineralized Complex, Hungary. Minerals. 2024; 14(9):956. https://doi.org/10.3390/min14090956
Chicago/Turabian StyleBiró, Máté, Johann G. Raith, Monika Feichter, Máté Hencz, Gabriella B. Kiss, Attila Virág, and Ferenc Molnár. 2024. "Comparative Study of Sulfides from Porphyry, Skarn, and Carbonate-Replacement Mineralization at the Recsk Porphyry-Mineralized Complex, Hungary" Minerals 14, no. 9: 956. https://doi.org/10.3390/min14090956








