How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes?
Abstract
:1. Introduction
2. Methods
3. Physiology of Muscle Health and the Myokines Network
3.1. Skeletal Muscle as an Endocrine Organ
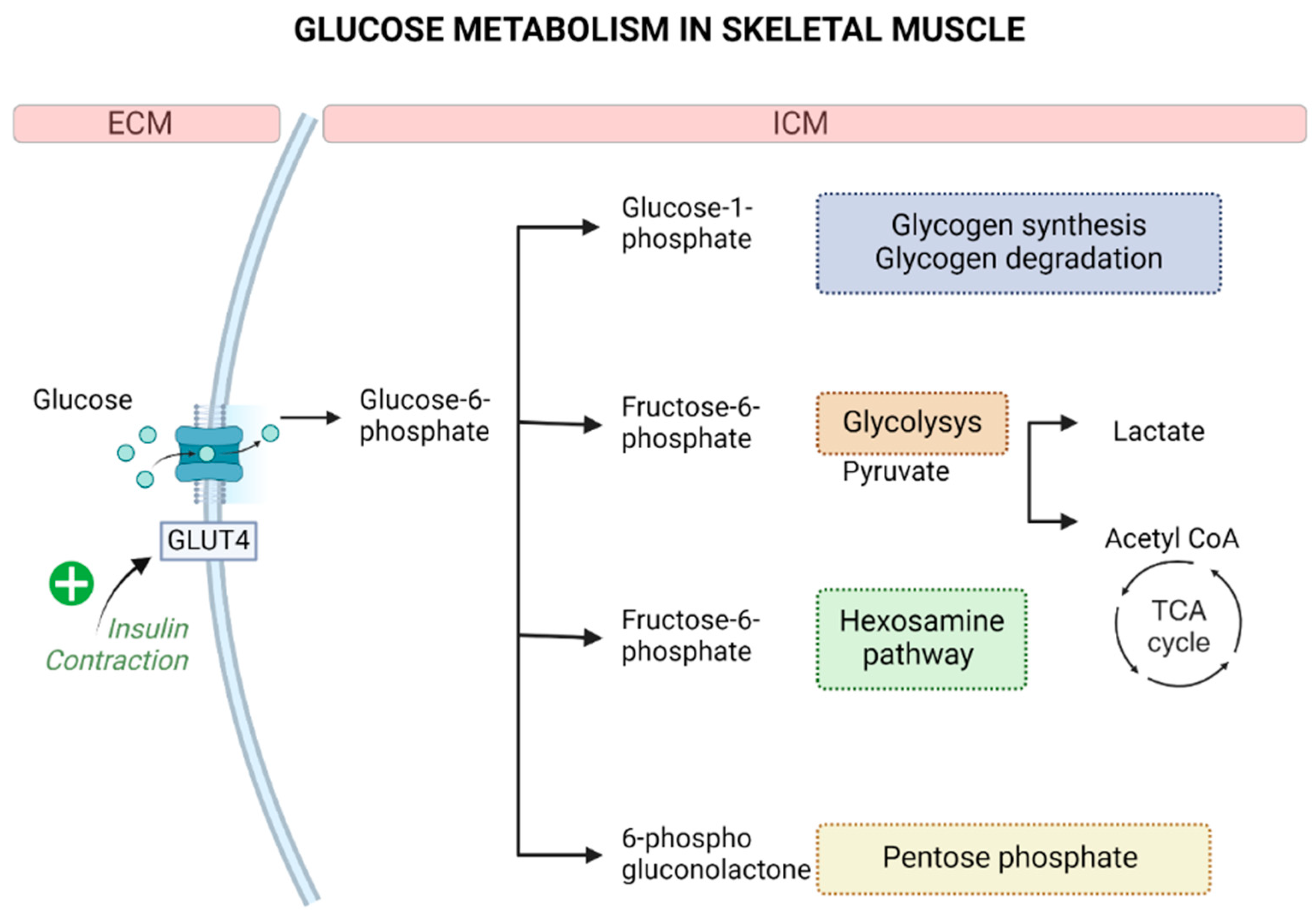
3.2. Skeletal Muscle and Glucose Metabolism
3.3. Key Myokines in the Regulation of Glucose Metabolism
4. Effects of Exercise Training on Insulin Resistance and Type 2 Diabetes in Pediatrics
| References | Exercise Type | Age (yrs) | Duration (Weeks) | Diet | Results | ||
|---|---|---|---|---|---|---|---|
| Glucidic Metabolism | Anthropometric Parameters: | Others | |||||
| Aerobic training | |||||||
| Sun et al., 2011 [155] | Aerobic | Exe (F): 13.80 ± 0.60 Exe (M): 13.38 ± 0.40 Con: 13.60 ± 0.70 | 10 | Habitual diet | No significant effect on FI and HOMA-IR | Decrease in BW | na |
| Kelly et al., 2004 [156] | Aerobic | Exe: 10.90 ± 1.99 Con: 11.00 ± 2.24 | 8 | Habitual diet | Decrease in FBG and FI | Decrease in BW | Amelioration of lipid profiles |
| Liu et al., 2018 [157] | Aerobic | Exe: 14.60 ± 0.70 Con: 14.70 ± 0.80 | 4 | 1400–1600 kcal/day | Decrease in FBG, FI, and HOMA-IR | Decrease in BW | na |
| Karacabey et al., 2009 [158] | Aerobic | Exe: 11.80 ± 0.50 Con: 11.20 ± 0.80 | 12 | Specific diet program | Decrease in FI | Decrease in BW | Decrease in cortisol levels and amelioration of lipid profile |
| McCormack et al., 2014 [159] | Aerobic | Exe:13.80 ± 2.20 Con:12.10 ± 1.20 | 8 | Habitual diet | Decrease in HOMA-IR | Decrease in BW and WC | Amelioration in body composition |
| Kim et al., 2011 [160] | Aerobic | Exe: 17.63 ± 0.49 Con na | 12 | Habitual diet | Decrease in FBG, FI and HOMA-IR | Decrease in BW | Amelioration of lipid profile |
| Leite et al., 2022 [161] | Aerobic | Exe and Con: 13.00 ± 1.90 | 12 | Nutritional guidance in Con, Habitual diet in Ex | Decrease in FI, FBG, HOMA-IR and QUICKI | Decrease in BW and BMI | Amelioration of lipid profile |
| Salahshoornezhad et al., 2022 [162] | Aerobic | Exe and Con: 10.50 ± 1.02 | 10 | na | Decrease in FBG | Decrease in BW and WC | Amelioration of lipid profile |
| Alizadeh et al., 2019 [163] | Aerobic | Exe and Con: 18.00 ± 1.5 | 6 | Habitual diet | Decrease in FBG FI and HOMA-IR | Decrease in BW | na |
| Kim et al., 2020 [147] | Aerobic | Exe and Con 15.00 ± 1.00 | 12 | Habitual diet | Decrease in FBG FI and HOMA-IR | Decrease in BW | Decrease in blood pressure levels |
| Murphy et al., 2009 [148] | Aerobic | Exe and Con: 10.21 ± 1.67 | 12 | Habitual diet | na | Decrease in BW | Decrease in blood pressure levels and lipid profile |
| Vasconcellos et al., 2015 [149] | Aerobic | Exe1: 16.60 ± 0.90 Exe2: 16.50 ± 1.20 Con: 16.90 ± 1.00 | 12 | Habitual diet | Decrease in HOMA-IR | Decrease in BW and WC | Decrease in fat mass, blood pressure, and amelioration of lipid levels |
| Boer et al., 2014 [164] | Aerobic | Exe1: 18.00 ± 3.20 Exe2: 16.70 ± 3.60 Con: 17.40 ± 2.40 | 15 | Habitual diet | Decrease in FBG, FI, and HOMA-IR | Decrease in BW | Decrease in body fat, blood pressure levels and amelioration of lipid profile |
| Resistance training | |||||||
| Benson et al., 2008[165] | Resistance | Exe and Con: 12.20 ± 1.3 | 8 | Habitual diet | Decrease in FBG, FI | Decrease in WC | Decrease in body fat |
| Kelly et al., 2019 [166] | Resistance | Exe: 15.29 ± 0.95 Con: 15.58 ± 0.99 | 16 | Habitual diet | Decrease in FBG | na | na |
| Lee et al., 2013 [167] | Aerobic vs. Resistance | Exe1: 14.60 ± 1.90 Exe2: 14.80 ± 1.90 Con: 15.00 ± 2.20 | 12 | Habitual diet | Decrease in FI and HOMA-IR | na | Decrease in visceral fat |
| Lee et al., 2010 [168] | Aerobic vs. Resistance | Exe1: 15.20 ± 1.90 Exe2: 14.60 ± 1.50 Con: 14.80 ± 1.40 | 12 | Habitual diet | Decrease in FI | Decrease in BW | na |
| Rasooli et al., 2021 [169] | Resistance | Exe and Con: 14.00–17.00 | 8 | Habitual diet | Decrease in FI and FBG | Decrease in BW | na |
| Seo et al., 2012 [150] | Resistance | Exe: 14.70 ± 1.51 Con: 14.60 ± 3.03 | 8 | Balanced diet | Amelioration of IR | Decrease in BW | Amelioration of lipid profile |
| Combined training | |||||||
| Chae et al., 2010 [170] | Combined (Aerobic and Resistance) | Exe: 10.60 ± 3.80 Con: 10.40 ± 3.10 | 12 | <1800–2000 kcal/day | na | Decrease in BW | Improvement of body composition and serum lipid profiles |
| Vissers et al., 2008 [171] | Combined (Aerobic and Resista ce) | Exe (F): 17.50 ± 1.30 Exe (M): 18.10 ± 1.30 Con (F): 17.10 ± 1.10 Con (M): 17.50 ± 1.40 | 24 | Counseling by a dietitian | Decrease in FBG | Decrease in BW and WC | na |
| Wong et al., 2018 [172] | Combined (Aerobic and Resistance) | Exe: 15.20 ± 1.20 Con: 15.30 ± 1.10 | 12 | Balanced diet | Decrease in FI and FBG | Decrease in BW | Decrease in body fat |
| Sefat et al., 2019 [173] | Combined (Aerobic and Resistance) | Exe: 12.40 ± 1.71 Con: 11.80 ± 2.20 | 8 | Habitual diet | Decrease in FI, FBG, and HOMA-IR | Decrease in BW | na |
| Son et al., 2017 [174] | Combined (Aerobic and Resistance) | Exe and Con: 15.00 ± 4.47 | 12 | Habitual diet | Decrease in FI, FBG, and HOMA-IR | Decrease in BW and WC | na |
| Zehsaz et al., 2016 [175] | Combined (Aerobic and Resistance) | Exe: 10.80 ± 0.90 Con: 10.30 ± 0.90 | 16 | Habitual diet | Decrease in FI and HOMA-IR | Decrease in BW and WC | Decrease in body fat and amelioration of lipid profiles |
| Davis et al., 2011 [176] | Combined (Aerobic and Resistance) | Exe: 15.70 ± 1.10 Con: 15.80 ± 1.00 | 16 | Habitual diet | Decrease in FI and HOMA-IR | Decrease in WC | Decrease in body fat |
| Farpour-Lambert et al., 2009 [177] | Combined (Aerobic and Resistance) | Exe: 9.10 ± 1.40 Con 1: 8.80 ± 1.60 Con 2: 8.50 ± 1.50 | 12 | Habitual diet | na | Decrease in BW | Decrease in blood pressure levels |
| Lopes et al., 2016 [178] | Combined (Aerobic and Resistance) | Exe: 14.60 ± 1.15 Con: 14.40 ± 1.16 | 12 | Habitual diet | Decrease in FBG, FI, and HOMA-IR | Decrease in BW | na |
| Davis et al., 2009 [179] | Resistance Combined (Aerobic and Resistance) | Exe1: 15.70 ± 1.20 Exe2: 14.80 ± 1.00 Con: 15.3 ±0 1.10 | 16 | Nutritional education class | No significant changes in FBG and FI | Decrease in BW | Decrease in body fat |
| de Lira et al., 2017 [180] | HIIT LIT | Exe1: 14.95 ± 1.35 Exe2: 14.77 ± 0.94 Con: 14.72 ± 1.35 | 12 | Balanced diet | No significant changes in FBG FI HOMA-IR | na | Improvement of biomarkers related to non-alcoholic fatty liver disease |
| Meng et al., 2022 [181] | HIIT MICT | Exe1: 11.4 ± 0.80 Exe2: 11.2 ± 0.70 Con: 11.0 ± 0.70 | 12 | Habitual diet | Decrease in FI and HOMA-IR | Decrease in BMI | na |
| Plavsic et al., 2020 [182] | HIIT | Exe: 16.20 ± 1.30 Con: 15.50 ± 1.50 | 12 | 1500–1700 kcal/day | No significant changes in metabolic parameters | Decrease in BW | na |
| Abassi et al., 2020 [183] | HIIT MIIT | Exe1: 16.10 ± 0.99 Exe2: 16.50 ± 1.07 Con: 16.90 ± 1.64 | 12 | Habitual diet | Decrease in FI and HOMA-IR | Decrease in BW | Decrease in body fat |
| Racil et al., 2016 [184] | HIIT Combined (Plyometric exercise and HIIT) | Exe1: 16.60 ± 0.90 Exe2: 16.50 ± 1.20 Con: 16.90 ± 1.00 | 12 | Habitual diet | Decrease in FBG, FI, and HOMA-IR | Decrease in BW | Decrease in blood pressure levels |
| Dias et al., 2018 [185] | HIIT MICT | Exe1: 12.40 ± 1.90 Exe2: 11.90 ± 2.40 Con: 11.8 ± 2.40 | 12 | Balanced diet | No significant reductions in metabolic parameters | na | No significant reductions in metabolic parameters |
| Racil et al., 2013 [186] | HIIT MIIT | Exe1: 15.60 ± 0.70 Exe2: 16.30 ± 0.52 Con: 15.90 ± 1.20 | 12 | Habitual diet | Decrease in FI and HOMA-IR | Decrease in BW | Amelioration of lipid profile |
| Meyer et al., 2006 [187] | Swimming and Aqua aerobic training + Sports games + Walking (combined) | Exe and Con: 14.70 ± 2.20 | 24 | Habitual diet | Decrease in FI and HOMA-IR | na | Decrease in body fat, blood pressure levels, CV risk, and amelioration of lipid profile |
| Others | |||||||
| De Souza et al., 2022 [188] | Karate training | Exe and Con: 12.00–17.00 | 12 | Nutritional plan | Decrease in FBG | na | Amelioration of lipid profile and heart rate |
5. Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The Underappreciated Role of Muscle in Health and Disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Lombardi, G. Exercise-Dependent Modulation of Bone Metabolism and Bone Endocrine Function: New Findings and Therapeutic Perspectives. J. Sci. Sport Exerc. 2019, 1, 20–28. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.-Y.; Moon, S.; Park, D.-H.; Kwak, H.-B.; Kang, J.-H. Roles of Myokines in Exercise-Induced Improvement of Neuropsychiatric Function. Pflug. Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Fiore, G.; Massini, G.; Berardo, C.; Gatti, A.; Baldassarre, P.; Bianchi, A.; et al. The Effect of Healthy Lifestyle Strategies on the Management of Insulin Resistance in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2022, 14, 4692. [Google Scholar] [CrossRef]
- Brandt, C.; Pedersen, B.K. The Role of Exercise-Induced Myokines in Muscle Homeostasis and the Defense against Chronic Diseases. J. Biomed. Biotechnol. 2010, 2010, 520258. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. (1985) 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 785–809. ISBN 978-0-470-65071-4. [Google Scholar]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Raschke, S.; Eckardt, K.; Bjørklund Holven, K.; Jensen, J.; Eckel, J. Identification and Validation of Novel Contraction-Regulated Myokines Released from Primary Human Skeletal Muscle Cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef]
- Weiss, R.; Kaufman, F.R. Metabolic Complications of Childhood Obesity: Identifying and Mitigating the Risk. Diabetes Care 2008, 31 (Suppl. 2), S310–S316. [Google Scholar] [CrossRef]
- Chang, C.; Liu, W.; Zhao, X.; Li, S.; Yu, C. Effect of Supervised Exercise Intervention on Metabolic Risk Factors and Physical Fitness in Chinese Obese Children in Early Puberty. Obes. Rev. 2008, 9 (Suppl. 1), 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lin, J.E.; Blomain, E.S.; Waldman, S.A. Antiobesity Pharmacotherapy: New Drugs and Emerging Targets. Clin. Pharmacol. Ther. 2013, 95, 53–66. [Google Scholar] [CrossRef]
- Marson, E.C.; Delevatti, R.S.; Prado, A.K.G.; Netto, N.; Kruel, L.F.M. Effects of Aerobic, Resistance, and Combined Exercise Training on Insulin Resistance Markers in Overweight or Obese Children and Adolescents: A Systematic Review and Meta-Analysis. Prev. Med. 2016, 93, 211–218. [Google Scholar] [CrossRef]
- Ross, M.; Kargl, C.K.; Ferguson, R.; Gavin, T.P.; Hellsten, Y. Exercise-Induced Skeletal Muscle Angiogenesis: Impact of Age, Sex, Angiocrines and Cellular Mediators. Eur. J. Appl. Physiol. 2023, 123, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Gillen, J.B. Skeletal Muscle Mechanisms Contributing to Improved Glycemic Control Following Intense Interval Exercise and Training. Sports Med. Health Sci. 2023, 5, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kazeminasab, F.; Sharafifard, F.; Miraghajani, M.; Behzadnejad, N.; Rosenkranz, S.K. The Effects of Exercise Training on Insulin Resistance in Children and Adolescents with Overweight or Obesity: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2023, 14, 1178376. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Goldstein, M.S. Humoral Nature of the Hypoglycemic Factor of Muscular Work. Diabetes 1961, 10, 232–234. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Febbraio, M.; Saltin, B. Searching for the Exercise Factor: Is IL-6 a Candidate? J. Muscle Res. Cell Motil. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 Is a Novel Factor Mediating Glucose Homeostasis during Skeletal Muscle Contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb Perspect Med. 2017, 7, a029793. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; Raschke, S.; Knebel, B.; Scheler, M.; Irmler, M.; Passlack, W.; Muller, S.; Hanisch, F.-G.; Franz, T.; Li, X.; et al. Secretome Profiling of Primary Human Skeletal Muscle Cells. Biochim. Biophys. Acta 2014, 1844, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, M.-C.; Bigot, A.; Jensen, S.S.; Dennis, J.L.; Rogowska-Wrzesinska, A.; Lainé, J.; Gache, V.; Furling, D.; Jensen, O.N.; Voit, T.; et al. In-Depth Analysis of the Secretome Identifies Three Major Independent Secretory Pathways in Differentiating Human Myoblasts. J. Proteom. 2012, 77, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bouzakri, K.; Plomgaard, P.; Berney, T.; Donath, M.Y.; Pedersen, B.K.; Halban, P.A. Bimodal Effect on Pancreatic β-Cells of Secretory Products from Normal or Insulin-Resistant Human Skeletal Muscle. Diabetes 2011, 60, 1111–1121. [Google Scholar] [CrossRef]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased Secretion and Expression of Myostatin in Skeletal Muscle from Extremely Obese Women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef]
- Pratesi, A.; Tarantini, F.; Di Bari, M. Skeletal Muscle: An Endocrine Organ. Clin. Cases Min. Bone Metab. 2013, 10, 11–14. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of Skeletal Muscle Mass in Mice by a New TGF-Beta Superfamily Member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Palsgaard, J.; Brøns, C.; Friedrichsen, M.; Dominguez, H.; Jensen, M.; Storgaard, H.; Spohr, C.; Torp-Pedersen, C.; Borup, R.; De Meyts, P.; et al. Gene Expression in Skeletal Muscle Biopsies from People with Type 2 Diabetes and Relatives: Differential Regulation of Insulin Signaling Pathways. PLoS ONE 2009, 4, e6575. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Arounleut, P.; Kellum, E.; Cain, M.; Immel, D.; Liang, L.-F. Recombinant Myostatin (GDF-8) Propeptide Enhances the Repair and Regeneration of Both Muscle and Bone in a Model of Deep Penetrant Musculoskeletal Injury. J. Trauma. 2010, 69, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between Transforming Growth Factor-Β1, Myostatin, and Decorin. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar] [CrossRef] [PubMed]
- Rue, N.; Vissing, J.; Galbo, H. Insulin Resistance and Increased Muscle Cytokine Levels in Patients with Mitochondrial Myopathy. J. Clin. Endocrinol. Metab. 2014, 99, 3757–3765. [Google Scholar] [CrossRef]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, Cytokines and Satellite Cells: What Role Do They Play in Muscle Damage and Regeneration Following Eccentric Exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Toth, K.G.; McKay, B.R.; De Lisio, M.; Little, J.P.; Tarnopolsky, M.A.; Parise, G. IL-6 Induced STAT3 Signalling Is Associated with the Proliferation of Human Muscle Satellite Cells Following Acute Muscle Damage. PLoS ONE 2011, 6, e17392. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 Treatment Increases Fatty Acid Turnover in Elderly Humans in Vivo and in Tissue Culture in Vitro. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E155–E162. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Ehses, J.A.; Hammar, E.B.; Van Lommel, L.; Quintens, R.; Martens, G.; Kerr-Conte, J.; Pattou, F.; Berney, T.; Pipeleers, D.; et al. Interleukin-6 Regulates Pancreatic Alpha-Cell Mass Expansion. Proc. Natl. Acad. Sci. USA 2008, 105, 13163–13168. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-like Peptide-1 Secretion from L Cells and Alpha Cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Mauer, J.; Chaurasia, B.; Goldau, J.; Vogt, M.C.; Ruud, J.; Nguyen, K.D.; Theurich, S.; Hausen, A.C.; Schmitz, J.; Brönneke, H.S.; et al. Signaling by IL-6 Promotes Alternative Activation of Macrophages to Limit Endotoxemia and Obesity-Associated Resistance to Insulin. Nat. Immunol. 2014, 15, 423–430. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Zhu, J.; Sun, B.; Branca, M.; Tang, Y.; Foster, W.; Xiao, X.; Huard, J. Decorin Gene Transfer Promotes Muscle Cell Differentiation and Muscle Regeneration. Mol. Ther. 2007, 15, 1616–1622. [Google Scholar] [CrossRef]
- Broholm, C.; Laye, M.J.; Brandt, C.; Vadalasetty, R.; Pilegaard, H.; Pedersen, B.K.; Scheele, C. LIF Is a Contraction-Induced Myokine Stimulating Human Myocyte Proliferation. J. Appl. Physiol. (1985) 2011, 111, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Görgens, S.W.; Hjorth, M.; Eckardt, K.; Wichert, S.; Norheim, F.; Holen, T.; Lee, S.; Langleite, T.; Birkeland, K.I.; Stadheim, H.K.; et al. The Exercise-Regulated Myokine Chitinase-3-like Protein 1 Stimulates Human Myocyte Proliferation. Acta Physiol. (Oxf.) 2016, 216, 330–345. [Google Scholar] [CrossRef]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The Myokine Decorin Is Regulated by Contraction and Involved in Muscle Hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-Type Cytokine Signalling through the Gp130/Jak/STAT Pathway. Biochem. J. 1998, 334 Pt 2, 297–314. [Google Scholar] [CrossRef]
- Metcalf, D. The Unsolved Enigmas of Leukemia Inhibitory Factor. Stem. Cells 2003, 21, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Seo, I.H.; Chung, I.; Kim, S.A.; Lee, J.O.; Lee, H.J.; Kim, S.E.; Han, J.A.; Kang, M.J.; Kim, S.J.; et al. Effect of Chitinase-3-like Protein 1 on Glucose Metabolism: In Vitro Skeletal Muscle and Human Genetic Association Study. FASEB J. 2020, 34, 13445–13460. [Google Scholar] [CrossRef]
- Görgens, S.W.; Eckardt, K.; Elsen, M.; Tennagels, N.; Eckel, J. Chitinase-3-like Protein 1 Protects Skeletal Muscle from TNFα-Induced Inflammation and Insulin Resistance. Biochem. J. 2014, 459, 479–488. [Google Scholar] [CrossRef]
- Dijk, W.; Beigneux, A.P.; Larsson, M.; Bensadoun, A.; Young, S.G.; Kersten, S. Angiopoietin-like 4 Promotes Intracellular Degradation of Lipoprotein Lipase in Adipocytes. J. Lipid Res. 2016, 57, 1670–1683. [Google Scholar] [CrossRef]
- Gray, N.E.; Lam, L.N.; Yang, K.; Zhou, A.Y.; Koliwad, S.; Wang, J.-C. Angiopoietin-like 4 (Angptl4) Protein Is a Physiological Mediator of Intracellular Lipolysis in Murine Adipocytes. J. Biol. Chem. 2012, 287, 8444–8456. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, D.; Barrandon, O.; Hadley, S.; Blum, B.; Peterson, Q.P.; Melton, D.A. Angptl4 Links α-Cell Proliferation Following Glucagon Receptor Inhibition with Adipose Tissue Triglyceride Metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 15498–15503. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Paton, C.M. A Review of Free Fatty Acid-Induced Cell Signaling, Angiopoietin-like Protein 4, and Skeletal Muscle Differentiation. Front. Physiol. 2022, 13, 987977. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Ryan, A.S. Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss. Metabolites 2020, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, B.W.; Goossens, G.H.; Jocken, J.W.; Kersten, S.; Blaak, E.E. Angiopoietin-Like Protein 4 and Postprandial Skeletal Muscle Lipid Metabolism in Overweight and Obese Prediabetics. J. Clin. Endocrinol. Metab. 2016, 101, 2332–2339. [Google Scholar] [CrossRef]
- Enoki, Y.; Nagai, T.; Hamamura, Y.; Osa, S.; Nakamura, H.; Taguchi, K.; Watanabe, H.; Maruyama, T.; Matsumoto, K. The G Protein-Coupled Receptor Ligand Apelin-13 Ameliorates Skeletal Muscle Atrophy Induced by Chronic Kidney Disease. J. Cachexia Sarcopenia Muscle 2023, 14, 553–564. [Google Scholar] [CrossRef]
- Dray, C.; Knauf, C.; Daviaud, D.; Waget, A.; Boucher, J.; Buléon, M.; Cani, P.D.; Attané, C.; Guigné, C.; Carpéné, C.; et al. Apelin Stimulates Glucose Utilization in Normal and Obese Insulin-Resistant Mice. Cell Metab. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Attané, C.; Foussal, C.; Le Gonidec, S.; Benani, A.; Daviaud, D.; Wanecq, E.; Guzmán-Ruiz, R.; Dray, C.; Bezaire, V.; Rancoule, C.; et al. Apelin Treatment Increases Complete Fatty Acid Oxidation, Mitochondrial Oxidative Capacity, and Biogenesis in Muscle of Insulin-Resistant Mice. Diabetes 2012, 61, 310–320. [Google Scholar] [CrossRef]
- Hu, G.; Wang, Z.; Zhang, R.; Sun, W.; Chen, X. The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review. Front. Physiol. 2021, 12, 632886. [Google Scholar] [CrossRef]
- Le Moal, E.; Liu, Y.; Collerette-Tremblay, J.; Dumontier, S.; Fabre, P.; Molina, T.; Dort, J.; Orfi, Z.; Denault, N.; Boutin, J.; et al. Apelin Stimulation of the Vascular Skeletal Muscle Stem Cell Niche Enhances Endogenous Repair in Dystrophic Mice. Sci. Transl. Med. 2024, 16, eabn8529. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Yu, J.; Zhang, Y.; Li, Y.; Fu, R.; Sun, Y.; Zhao, K.; Xiao, Q. Irisin Ameliorates D-Galactose-Induced Skeletal Muscle Fibrosis via the PI3K/Akt Pathway. Eur. J. Pharmacol. 2023, 939, 175476. [Google Scholar] [CrossRef] [PubMed]
- Hoier, B.; Hellsten, Y. Exercise-Induced Capillary Growth in Human Skeletal Muscle and the Dynamics of VEGF. Microcirculation 2014, 21, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, W.; Kassolik, K.; Kobierzycki, C.; Grzegrzolka, J.; Ratajczak-Wielgomas, K.; Jablonska, K.; Halski, T.; Dziegiel, P.; Gworys, B.; Podhorska-Okolow, M. Increased Skeletal Muscle Expression of VEGF Induced by Massage and Exercise. Folia. Histochem. Cytobiol. 2015, 53, 145–151. [Google Scholar] [CrossRef]
- Kivelä, R.; Kyröläinen, H.; Selänne, H.; Komi, P.V.; Kainulainen, H.; Vihko, V. A Single Bout of Exercise with High Mechanical Loading Induces the Expression of Cyr61/CCN1 and CTGF/CCN2 in Human Skeletal Muscle. J. Appl. Physiol. 2007, 103, 1395–1401. [Google Scholar] [CrossRef]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.W.; Wittorf, A.; Richartz, E.; Bartels, M.; Buchkremer, G.; Schott, K. Stage-Dependent BDNF Serum Concentrations in Alzheimer’s Disease. J. Neural. Transm. (Vienna) 2006, 113, 1217–1224. [Google Scholar] [CrossRef]
- Matthews, V.B.; Aström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Akerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Wahren, J. GLUCOSE TURNOVER DURING EXERCISE IN MAN*. Ann. N. Y. Acad. Sci. 1977, 301, 45–55. [Google Scholar] [CrossRef]
- Das, A.M.; Steuerwald, U.; Illsinger, S. Inborn Errors of Energy Metabolism Associated with Myopathies. J. Biomed. Biotechnol. 2010, 2010, 340849. [Google Scholar] [CrossRef]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal Muscle: Energy Metabolism, Fiber Types, Fatigue and Adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M. Family of Glucose-Transporter Genes: Implications for Glucose Homeostasis and Diabetes. Diabetes 1990, 39, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; Volchuk, A.; He, L.; Tsakiridis, T. The Glucose Transporters of Skeletal Muscle. Semin. Cell Dev. Biol. 1996, 7, 229–237. [Google Scholar] [CrossRef]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar] [CrossRef]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.P.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted Disruption of the Glucose Transporter 4 Selectively in Muscle Causes Insulin Resistance and Glucose Intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Ryder, J.W.; Yang, J.; Galuska, D.; Rincón, J.; Björnholm, M.; Krook, A.; Lund, S.; Pedersen, O.; Wallberg-Henriksson, H.; Zierath, J.R.; et al. Use of a Novel Impermeable Biotinylated Photolabeling Reagent to Assess Insulin- and Hypoxia-Stimulated Cell Surface GLUT4 Content in Skeletal Muscle from Type 2 Diabetic Patients. Diabetes 2000, 49, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hp, L. In Vivo Imaging of GLUT4 Translocation. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2009, 34, 420–423. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty Sweet Years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef]
- Jensen, T.E.; Sylow, L.; Rose, A.J.; Madsen, A.B.; Angin, Y.; Maarbjerg, S.J.; Richter, E.A. Contraction-Stimulated Glucose Transport in Muscle Is Controlled by AMPK and Mechanical Stress but Not Sarcoplasmatic Reticulum Ca2+ Release. Mol. Metab. 2014, 3, 742. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of Glycogen Metabolism for Coaches and Athletes. Nutr. Rev. 2018, 76, 243. [Google Scholar] [CrossRef] [PubMed]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Wu, C.-L.; Satomi, Y.; Walsh, K. RNA-Seq and Metabolomic Analyses of Akt1-Mediated Muscle Growth Reveals Regulation of Regenerative Pathways and Changes in the Muscle Secretome. BMC Genom. 2017, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Battistuzzi, G.; D’Urso, M.; Toniolo, D.; Persico, G.M.; Luzzatto, L. Tissue-Specific Levels of Human Glucose-6-Phosphate Dehydrogenase Correlate with Methylation of Specific Sites at the 3’ End of the Gene. Proc. Natl. Acad. Sci. USA 1985, 82, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical Overview of the Interleukin-6 Family Cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef]
- Whitham, M.; Chan, M.H.S.; Pal, M.; Matthews, V.B.; Prelovsek, O.; Lunke, S.; El-Osta, A.; Broenneke, H.; Alber, J.; Brüning, J.C.; et al. Contraction-Induced Interleukin-6 Gene Transcription in Skeletal Muscle Is Regulated by c-Jun Terminal Kinase/Activator Protein-1. J. Biol. Chem. 2012, 287, 10771–10779. [Google Scholar] [CrossRef] [PubMed]
- Welc, S.S.; Clanton, T.L. The Regulation of Interleukin-6 Implicates Skeletal Muscle as an Integrative Stress Sensor and Endocrine Organ. Exp. Physiol. 2013, 98, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Brolin, C.; Nørgaard-Christensen, N.; Dethlefsen, C.; Lauenborg, B.; Olsen, C.K.; Åbom, M.M.; Krag, T.; Gehl, J.; Pedersen, B.K. IL-6 Release from Muscles during Exercise Is Stimulated by Lactate-Dependent Protease Activity. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E940–E947. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ma, Y.; Gao, M.; Liu, D. IL-15/sIL-15Rα Gene Transfer Induces Weight Loss and Improves Glucose Homeostasis in Obese Mice. Gene Ther. 2016, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, G.; Rakotoarivelo, V.; Labbé, S.M.; Vernier, M.; Noll, C.; Mayhue, M.; Stankova, J.; Schwertani, A.; Grenier, G.; Carpentier, A.; et al. Correction: Deficiency of Interleukin-15 Confers Resistance to Obesity by Diminishing Inflammation and Enhancing the Thermogenic Function of Adipose Tissues. PLoS ONE 2016, 11, e0166537. [Google Scholar] [CrossRef]
- Yang, H.; Chang, J.; Chen, W.; Zhao, L.; Qu, B.; Tang, C.; Qi, Y.; Zhang, J. Treadmill Exercise Promotes Interleukin 15 Expression in Skeletal Muscle and Interleukin 15 Receptor Alpha Expression in Adipose Tissue of High-Fat Diet Rats. Endocrine 2013, 43, 579–585. [Google Scholar] [CrossRef]
- Riechman, S.E.; Balasekaran, G.; Roth, S.M.; Ferrell, R.E. Association of Interleukin-15 Protein and Interleukin-15 Receptor Genetic Variation with Resistance Exercise Training Responses. J. Appl. Physiol. 2004, 97, 2214–2219. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Mounier, R.; Plomgaard, P.; Mortensen, O.H.; Penkowa, M.; Speerschneider, T.; Pilegaard, H.; Pedersen, B.K. Expression of Interleukin-15 in Human Skeletal Muscle—Effect of Exercise and Muscle Fibre Type Composition. J. Physiol. 2007, 584, 305–312. [Google Scholar] [CrossRef]
- Hingorjo, M.R.; Zehra, S.; Saleem, S.; Qureshi, M.A. Serum Interleukin-15 and Its Relationship with Adiposity Indices before and after Short-Term Endurance Exercise. Pak. J. Med. Sci. 2018, 34, 1125–1131. [Google Scholar] [CrossRef]
- Khalafi, M.; Maleki, A.H.; Symonds, M.E.; Sakhaei, M.H.; Rosenkranz, S.K.; Ehsanifar, M.; Korivi, M.; Liu, Y. Interleukin-15 Responses to Acute and Chronic Exercise in Adults: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 14, 1288537. [Google Scholar] [CrossRef]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and Regulation of Adipokine and Myokine Production. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 135, pp. 313–336. ISBN 978-0-12-803991-5. [Google Scholar]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and Adipokines: Involvement in the Crosstalk between Skeletal Muscle and Adipose Tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lee, S.-J. Double Muscling in Cattle Due to Mutations in the Myostatin Gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A Mutation in the Myostatin Gene Increases Muscle Mass and Enhances Racing Performance in Heterozygote Dogs. PLoS Genet. 2007, 3, e79. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F. The Correlation of Resistance Exercise-Induced Myostatin with Insulin Resistance and Plasma Cytokines in Healthy Young Men. J. Endocrinol. Investig. 2016, 39, 383–388. [Google Scholar] [CrossRef]
- Saremi, A.; Gharakhanloo, R.; Sharghi, S.; Gharaati, M.R.; Larijani, B.; Omidfar, K. Effects of Oral Creatine and Resistance Training on Serum Myostatin and GASP-1. Mol. Cell. Endocrinol. 2010, 317, 25–30. [Google Scholar] [CrossRef]
- Louis, E.; Raue, U.; Yang, Y.; Jemiolo, B.; Trappe, S. Time Course of Proteolytic, Cytokine, and Myostatin Gene Expression after Acute Exercise in Human Skeletal Muscle. J. Appl. Physiol. 2007, 103, 1744–1751. [Google Scholar] [CrossRef]
- Seldin, M.M.; Wong, G.W. Regulation of Tissue Crosstalk by Skeletal Muscle-Derived Myonectin and Other Myokines. Adipocyte 2012, 1, 200–202. [Google Scholar] [CrossRef]
- Seldin, M.M.; Lei, X.; Tan, S.Y.; Stanson, K.P.; Wei, Z.; Wong, G.W. Skeletal Muscle-Derived Myonectin Activates the Mammalian Target of Rapamycin (mTOR) Pathway to Suppress Autophagy in Liver. J. Biol. Chem. 2013, 288, 36073–36082. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Fujie, S.; Horii, N.; Uchida, M.; Kurihara, T.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic Exercise Training-Induced Changes in Serum C1q/TNF-Related Protein Levels Are Associated with Reduced Arterial Stiffness in Middle-Aged and Older Adults. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R94–R101. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, J.H.; Ryu, H.S.; Pak, Y.K.; Park, K.S.; Lee, H.K.; Lee, W. C1q Tumor Necrosis Factor α-Related Protein Isoform 5 Is Increased in Mitochondrial DNA-Depleted Myocytes and Activates AMP-Activated Protein Kinase. J. Biol. Chem. 2009, 284, 27780–27789. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Modan, M.; Halkin, H.; Almog, S.; Lusky, A.; Eshkol, A.; Shefi, M.; Shitrit, A.; Fuchs, Z. Hyperinsulinemia. A Link between Hypertension Obesity and Glucose Intolerance. J. Clin. Investig. 1985, 75, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What Causes the Insulin Resistance Underlying Obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, L.; Hassan, W.; Abdelkader, D.; Shang, J. Adipokines and Hepatic Insulin Resistance. J. Diabetes Res. 2013, 2013, 170532. [Google Scholar] [CrossRef]
- Maury, E.; Ehala-Aleksejev, K.; Guiot, Y.; Detry, R.; Vandenhooft, A.; Brichard, S.M. Adipokines Oversecreted by Omental Adipose Tissue in Human Obesity. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E656–E665. [Google Scholar] [CrossRef]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and Insulin Resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Lui, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.-Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and Perimuscular Fat Expansion in Obesity Correlates with Skeletal Muscle T Cell and Macrophage Infiltration and Insulin Resistance. Int. J. Obes (Lond) 2015, 39, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Fink, L.N.; Costford, S.R.; Lee, Y.S.; Jensen, T.E.; Bilan, P.J.; Oberbach, A.; Blüher, M.; Olefsky, J.M.; Sams, A.; Klip, A. Pro-Inflammatory Macrophages Increase in Skeletal Muscle of High fat-Fed Mice and Correlate with Metabolic Risk Markers in Humans. Obesity 2014, 22, 747–757. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z. Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within. Int. J. Mol. Sci. 2019, 20, 562. [Google Scholar] [CrossRef]
- Vincent, M.A.; Dawson, D.; Clark, A.D.H.; Lindner, J.R.; Rattigan, S.; Clark, M.G.; Barrett, E.J. Skeletal Muscle Microvascular Recruitment by Physiological Hyperinsulinemia Precedes Increases in Total Blood Flow. Diabetes 2002, 51, 42–48. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin Resistance and Its Treatment by Thiazolidinediones. Recent Prog. Horm. Res. 2001, 56, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.K.; Watt, M.J.; Le, J.; Hevener, A.L.; Turcotte, L.P. Thiazolidinediones Enhance Skeletal Muscle Triacylglycerol Synthesis While Protecting against Fatty Acid-Induced Inflammation and Insulin Resistance. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E485–E493. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Dabadghao, P. Polycystic Ovary Syndrome in Adolescents. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101272. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Shah, N.; Deshmukh, H.; Sahebkar, A.; Östlundh, L.; Al-Rifai, R.H.; Atkin, S.L.; Sathyapalan, T. The Effect of Thiazolidinediones in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Adv. Ther. 2024, 41, 2168–2195. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, D.A.; Schneider, D.J.; Sobel, B.E.; Cavaghan, M.K.; Imperial, J.; Rosenfield, R.L.; Polonsky, K.S. Troglitazone Improves Defects in Insulin Action, Insulin Secretion, Ovarian Steroidogenesis, and Fibrinolysis in Women with Polycystic Ovary Syndrome 1. J. Clin. Endocrinol. Metab. 1997, 82, 2108–2116. [Google Scholar] [CrossRef]
- Reiser, E.; Lanbach, J.; Böttcher, B.; Toth, B. Non-Hormonal Treatment Options for Regulation of Menstrual Cycle in Adolescents with PCOS. J. Clin. Med. 2022, 12, 67. [Google Scholar] [CrossRef]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic Ovary Syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Liu, D.; Li, N.; Zhou, Y.; Wang, M.; Song, P.; Yuan, C.; Shi, Q.; Chen, H.; Zhou, K.; Wang, H.; et al. Sex-specific Associations between Skeletal Muscle Mass and Incident Diabetes: A Population-based Cohort Study. Diabetes Obes. Metab. 2024, 26, 820–828. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- Lee, J.M. Insulin Resistance in Children and Adolescents. Rev. Endocr. Metab. Disord. 2007, 7, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Way, K.L.; Hackett, D.A.; Baker, M.K.; Johnson, N.A. The Effect of Regular Exercise on Insulin Sensitivity in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. J. 2016, 40, 253. [Google Scholar] [CrossRef]
- Wahren, J.; Felig, P.; Ahlborg, G.; Jorfeldt, L. Glucose Metabolism during Leg Exercise in Man. J. Clin. Investig. 1971, 50, 2715–2725. [Google Scholar] [CrossRef]
- Soo, J.; Raman, A.; Lawler, N.G.; Goods, P.S.R.; Deldicque, L.; Girard, O.; Fairchild, T.J. The Role of Exercise and Hypoxia on Glucose Transport and Regulation. Eur. J. Appl. Physiol. 2023, 123, 1147–1165. [Google Scholar] [CrossRef]
- Wallberg-Henriksson, H. Repeated Exercise Regulates Glucose Transport Capacity in Skeletal Muscle. Acta Physiol. Scand. 1986, 127, 39–44. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular Mechanisms by Which Aerobic Exercise Induces Insulin Sensitivity. J. Cell. Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of Exercise-Induced Mitochondrial Biogenesis in Skeletal Muscle: Implications for Health and Disease. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 1119–1134. ISBN 978-0-470-65071-4. [Google Scholar]
- Masi, L.N.; Serdan, T.D.A.; Levada-Pires, A.C.; Hatanaka, E.; Silveira, L.D.R.; Cury-Boaventura, M.F.; Pithon-Curi, T.C.; Curi, R.; Gorjão, R.; Hirabara, S.M. Regulation of Gene Expression by Exercise-Related Micrornas. Cell. Physiol. Biochem. 2016, 39, 2381–2397. [Google Scholar] [CrossRef]
- Watts, K.; Jones, T.W.; Davis, E.A.; Green, D. Exercise Training in Obese Children and Adolescents: Current Concepts. Sports Med. 2005, 35, 375–392. [Google Scholar] [CrossRef]
- Stabouli, S.; Erdine, S.; Suurorg, L.; Jankauskienė, A.; Lurbe, E. Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link. Nutrients 2021, 13, 4321. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Banks, L.; Schneiderman, J.E.; Caterini, J.E.; Stephens, S.; White, G.; Dogra, S.; Wells, G.D. Physical Activity for Children with Chronic Disease; a Narrative Review and Practical Applications. BMC Pediatr. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Stoner, L.; Rowlands, D.; Morrison, A.; Credeur, D.; Hamlin, M.; Gaffney, K.; Lambrick, D.; Matheson, A. Efficacy of Exercise Intervention for Weight Loss in Overweight and Obese Adolescents: Meta-Analysis and Implications. Sports Med. 2016, 46, 1737–1751. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Son, W.-M.; Headid Iii, R.J.; Pekas, E.J.; Noble, J.M.; Park, S.-Y. The Effects of a 12-Week Jump Rope Exercise Program on Body Composition, Insulin Sensitivity, and Academic Self-Efficacy in Obese Adolescent Girls. J. Pediatr. Endocrinol. Metab. 2020, 33, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.-S.; Carson, L.; Neal, W.; Baylis, C.; Donley, D.; Yeater, R. Effects of an Exercise Intervention Using Dance Dance Revolution on Endothelial Function and Other Risk Factors in Overweight Children. Int. J. Pediatr. Obes. 2009, 4, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, F.; Seabra, A.; Cunha, F.; Montenegro, R.; Penha, J.; Bouskela, E.; Nogueira Neto, J.F.; Collett-Solberg, P.; Farinatti, P. Health Markers in Obese Adolescents Improved by a 12-Week Recreational Soccer Program: A Randomised Controlled Trial. J. Sports Sci. 2016, 34, 564–575. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.; Figueroa, A.; Kim, H.K.; Baek, Y.H.; Kwak, Y.S.; Kim, N.; Choi, T.H.; Rhee, B.D.; Ko, K.S.; et al. Yoga Training Improves Metabolic Parameters in Obese Boys. Korean J. Physiol. Pharmacol. 2012, 16, 175. [Google Scholar] [CrossRef]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public Health 2021, 18, 7201. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Li, S.; Zou, Y. Effects of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Cardiometabolic Risk Factors in Overweight and Obesity Children and Adolescents: A Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 11905. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Su, Y. Comparative Effectiveness of High-Intensity Interval Training and Moderate-Intensity Continuous Training for Cardiometabolic Risk Factors and Cardiorespiratory Fitness in Childhood Obesity: A Meta-Analysis of Randomized Controlled Trials. Front. Physiol. 2020, 11, 214. [Google Scholar] [CrossRef]
- García-Hermoso, A.; López-Gil, J.F.; Izquierdo, M.; Ramírez-Vélez, R.; Ezzatvar, Y. Exercise and Insulin Resistance Markers in Children and Adolescents With Excess Weight: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1276. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-X.; Huang, X.-Q.; Yan, Y.; Li, B.-W.; Zhong, W.-J.; Chen, J.-F.; Zhang, Y.-M.; Wang, Z.-Z.; Wang, L.; Shi, X.-C.; et al. One-Hour after-School Exercise Ameliorates Central Adiposity and Lipids in Overweight Chinese Adolescents: A Randomized Controlled Trial. Chin. Med. J. (Engl.) 2011, 124, 323–329. [Google Scholar] [PubMed]
- Kelly, A.S.; Wetzsteon, R.J.; Kaiser, D.R.; Steinberger, J.; Bank, A.J.; Dengel, D.R. Inflammation, Insulin, and Endothelial Function in Overweight Children and Adolescents: The Role of Exercise. J. Pediatr. 2004, 145, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, X.; Wang, X. Decrease in Serum Chemerin through Aerobic Exercise plus Dieting and Its Association with Mitigation of Cardio-Metabolic Risk in Obese Female Adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Karacabey, K. The Effect of Exercise on Leptin, Insulin, Cortisol and Lipid Profiles in Obese Children. J. Int. Med. Res. 2009, 37, 1472–1478. [Google Scholar] [CrossRef]
- McCormack, S.E.; McCarthy, M.A.; Harrington, S.G.; Farilla, L.; Hrovat, M.I.; Systrom, D.M.; Thomas, B.J.; Torriani, M.; McInnis, K.; Grinspoon, S.K.; et al. Effects of Exercise and Lifestyle Modification on Fitness, Insulin Resistance, Skeletal Muscle Oxidative Phosphorylation and Intramyocellular Lipid Content in Obese Children and Adolescents. Pediatr. Obes. 2014, 9, 281–291. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, E.S.; Jeon, J.Y.; Jekal, Y. Improved Insulin Resistance, Adiponectin and Liver Enzymes without Change in Plasma Vaspin Level after 12 Weeks of Exercise Training among Obese Male Adolescents. Korean J. Obes. 2011, 20, 138. [Google Scholar] [CrossRef]
- Leite, N.; Tadiotto, M.C.; Corazza, P.R.P.; De Menezes Junior, F.J.; Carli, M.E.C.; Milano-Gai, G.E.; Lopes, W.A.; Gaya, A.R.; Brand, C.; Mota, J.; et al. Responsiveness on Metabolic Syndrome Criteria and Hepatic Parameters after 12 Weeks and 24 Weeks of Multidisciplinary Intervention in Overweight Adolescents. J. Endocrinol. Investig. 2021, 45, 741–752. [Google Scholar] [CrossRef]
- Salahshoornezhad, S.; Sohrabi, Z.; Mani, A.; Abdelbasset, W.K.; Mehrabi, M.; Zare, M.; Mehrabani, S.; Gerami, S.; Haghighat, N.; Akbarzadeh, M.; et al. Effect of a Multi-Disciplinary Program on Anthropometric and Biochemical Parameters in Obese and Overweight Elementary School Girls: A Randomized Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1982–1989. [Google Scholar] [CrossRef]
- Alizadeh, H.; Safarzade, A. Effect of a 6-Week Running Sprint Interval Training Protocol on Serum Meteorin-like Hormone, Insulin Resistance, and Body Composition in Overweight Adolescents. Med. Sport 2019, 72, 79–88. [Google Scholar] [CrossRef]
- Boer, P.-H.; Meeus, M.; Terblanche, E.; Rombaut, L.; Wandele, I.D.; Hermans, L.; Gysel, T.; Ruige, J.; Calders, P. The Influence of Sprint Interval Training on Body Composition, Physical and Metabolic Fitness in Adolescents and Young Adults with Intellectual Disability: A Randomized Controlled Trial. Clin. Rehabil. 2014, 28, 221–231. [Google Scholar] [CrossRef]
- Benson, A.C.; Torode, M.E.; Fiatarone Singh, M.A. The Effect of High-Intensity Progressive Resistance Training on Adiposity in Children: A Randomized Controlled Trial. Int. J. Obes 2008, 32, 1016–1027. [Google Scholar] [CrossRef]
- Kelly, L.; Holmberg, P.M.; Schroeder, E.T.; Loza, A.; Lin, X.; Moody, A.; Hughes, A.; Gibson, A.-M.; Kirk, A. Effect of Home-Based Strength Training Program on IGF-I, IGFBP-1 and IGFBP-3 in Obese Latino Boys Participating in a 16-Week Randomized Controlled Trial. J. Pediatr. Endocrinol. Metab. 2019, 32, 1121–1129. [Google Scholar] [CrossRef]
- Lee, S.; Deldin, A.R.; White, D.; Kim, Y.; Libman, I.; Rivera-Vega, M.; Kuk, J.L.; Sandoval, S.; Boesch, C.; Arslanian, S. Aerobic Exercise but Not Resistance Exercise Reduces Intrahepatic Lipid Content and Visceral Fat and Improves Insulin Sensitivity in Obese Adolescent Girls: A Randomized Controlled Trial. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E1222–E1229. [Google Scholar] [CrossRef]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of Aerobic Versus Resistance Exercise Without Caloric Restriction on Abdominal Fat, Intrahepatic Lipid, and Insulin Sensitivity in Obese Adolescent Boys. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef]
- Rasooli, S.A.; Fathi, R.; Golzar, F.A.-K.; Baghersalimi, M. The Effect of Circuit Resistance Training on Plasma Levels of Amino Acids, Alpha-Hydroxybutyrate, Mannose, and Urinary Levels of Glycine Conjugated Adducts in Obese Adolescent Boys. Appl. Physiol. Nutr. Metab. 2021, 46, 561–570. [Google Scholar] [CrossRef]
- Chae, H.-W.; Kwon, Y.-N.; Rhie, Y.-J.; Kim, H.-S.; Kim, Y.-S.; Paik, I.-Y.; Suh, S.-H.; Kim, D.-H. Effects of a Structured Exercise Program on Insulin Resistance, Inflammatory Markers and Physical Fitness in Obese Korean Children. J. Pediatr. Endocrinol. Metab. 2010, 23, 1065–1072. [Google Scholar] [CrossRef]
- Vissers, D.; De Meulenaere, A.; Vanroy, C.; Vanherle, K.; Van De Sompel, A.; Truijen, S.; Van Gaal, L. Effect of a Multidisciplinary School-Based Lifestyle Intervention on Body Weight and Metabolic Variables in Overweight and Obese Youth. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2008, 3, e196–e202. [Google Scholar] [CrossRef]
- Wong, A.; Sanchez-Gonzalez, M.A.; Son, W.-M.; Kwak, Y.-S.; Park, S.-Y. The Effects of a 12-Week Combined Exercise Training Program on Arterial Stiffness, Vasoactive Substances, Inflammatory Markers, Metabolic Profile, and Body Composition in Obese Adolescent Girls. Pediatr. Exerc. Sci. 2018, 30, 480–486. [Google Scholar] [CrossRef]
- Mohammadi Sefat, S.; Shabani, R.; Nazari, M. The Effect of Concurrent Aerobic-Resistance Training on Thyroid Hormones, Blood Glucose Hemostasis, and Blood Lipid Indices in Overweight Girls with Hypothyroidism. Horm. Mol. Biol. Clin. Investig. 2019, 40, 20190031. [Google Scholar] [CrossRef]
- Son, W.-M.; Sung, K.-D.; Bharath, L.P.; Choi, K.-J.; Park, S.-Y. Combined Exercise Training Reduces Blood Pressure, Arterial Stiffness, and Insulin Resistance in Obese Prehypertensive Adolescent Girls. Clin. Exp. Hypertens. 2017, 39, 546–552. [Google Scholar] [CrossRef]
- Zehsaz, F.; Farhangi, N.; Ghahramani, M. The Response of Circulating Omentin-1 Concentration to 16-Week Exercise Training in Male Children with Obesity. Physician Sportsmed. 2016, 44, 355–361. [Google Scholar] [CrossRef]
- Davis, J.N.; Gyllenhammer, L.E.; Vanni, A.A.; Meija, M.; Tung, A.; Schroeder, E.T.; Spruijt-Metz, D.; Goran, M.I. Startup Circuit Training Program Reduces Metabolic Risk in Latino Adolescents. Med. Sci. Sports Exerc. 2011, 43, 2195–2203. [Google Scholar] [CrossRef]
- Farpour-Lambert, N.J.; Aggoun, Y.; Marchand, L.M.; Martin, X.E.; Herrmann, F.R.; Beghetti, M. Physical Activity Reduces Systemic Blood Pressure and Improves Early Markers of Atherosclerosis in Pre-Pubertal Obese Children. J. Am. Coll. Cardiol. 2009, 54, 2396–2406. [Google Scholar] [CrossRef]
- Lopes, W.A.; Leite, N.; Da Silva, L.R.; Brunelli, D.T.; Gáspari, A.F.; Radominski, R.B.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R. Effects of 12 Weeks of Combined Training without Caloric Restriction on Inflammatory Markers in Overweight Girls. J. Sports Sci. 2016, 34, 1902–1912. [Google Scholar] [CrossRef]
- Davis, J.N.; Tung, A.; Chak, S.S.; Ventura, E.E.; Byrd-Williams, C.E.; Alexander, K.E.; Lane, C.J.; Weigensberg, M.J.; Spruijt-Metz, D.; Goran, M.I. Aerobic and Strength Training Reduces Adiposity in Overweight Latina Adolescents. Med. Sci. Sports Exerc. 2009, 41, 1494–1503. [Google Scholar] [CrossRef]
- De Lira, C.T.; Dos Santos, M.A.; Gomes, P.P.; Fidelix, Y.L.; Dos Santos, A.C.; Tenório, T.R.; Lofrano-Prado, M.C.; Do Prado, W.L. Aerobic Training Performed at Ventilatory Threshold Improves Liver Enzymes and Lipid Profile Related to Non-Alcoholic Fatty Liver Disease in Adolescents with Obesity. Nutr. Health 2017, 23, 281–288. [Google Scholar] [CrossRef]
- Meng, C.; Yucheng, T.; Shu, L.; Yu, Z. Effects of School-Based High-Intensity Interval Training on Body Composition, Cardiorespiratory Fitness and Cardiometabolic Markers in Adolescent Boys with Obesity: A Randomized Controlled Trial. BMC Pediatr. 2022, 22, 112. [Google Scholar] [CrossRef]
- Plavsic, L.; Knezevic, O.M.; Sovtic, A.; Minic, P.; Vukovic, R.; Mazibrada, I.; Stanojlovic, O.; Hrncic, D.; Rasic-Markovic, A.; Macut, D. Effects of High-Intensity Interval Training and Nutrition Advice on Cardiometabolic Markers and Aerobic Fitness in Adolescent Girls with Obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 294–300. [Google Scholar] [CrossRef]
- Abassi, W.; Ouerghi, N.; Ghouili, H.; Haouami, S.; Bouassida, A. Greater Effects of High-Compared with Moderate-Intensity Interval Training on Thyroid Hormones in Overweight/Obese Adolescent Girls. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200031. [Google Scholar] [CrossRef]
- Racil, G.; Zouhal, H.; Elmontassar, W.; Abderrahmane, A.B.; De Sousa, M.V.; Chamari, K.; Amri, M.; Coquart, J.B. Plyometric Exercise Combined with High-Intensity Interval Training Improves Metabolic Abnormalities in Young Obese Females More so than Interval Training Alone. Appl. Physiol. Nutr. Metab. 2016, 41, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.A.; Ingul, C.B.; Tjønna, A.E.; Keating, S.E.; Gomersall, S.R.; Follestad, T.; Hosseini, M.S.; Hollekim-Strand, S.M.; Ro, T.B.; Haram, M.; et al. Effect of High-Intensity Interval Training on Fitness, Fat Mass and Cardiometabolic Biomarkers in Children with Obesity: A Randomised Controlled Trial. Sports Med. 2018, 48, 733–746. [Google Scholar] [CrossRef]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of High vs. Moderate Exercise Intensity during Interval Training on Lipids and Adiponectin Levels in Obese Young Females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.A.; Kundt, G.; Lenschow, U.; Schuff-Werner, P.; Kienast, W. Improvement of Early Vascular Changes and Cardiovascular Risk Factors in Obese Children After a Six-Month Exercise Program. J. Am. Coll. Cardiol. 2006, 48, 1865–1870. [Google Scholar] [CrossRef]
- De Souza, F.; Da Silva, L.A.; Ferreira, G.S.; De Souza, M.M.M.; Bobinski, F.; Palandi, J.; Marcon, C.E.M.; Martins, D.F.; Schuelter-Trevisol, F.; Trevisol, D.J. Karate Training Improves Metabolic Health in Overweight and Obese Adolescents: A Randomized Clinical Trial. Pediatr. Exerc. Sci. 2022, 34, 108–118. [Google Scholar] [CrossRef]

| Myokines | Function |
|---|---|
| Myostatin [22,27,29,30,31,32,33] | It increases with physical inactivity. It is an inhibitor of muscle mass gain and bone healing. It is involved in metabolic equilibrium and the control of adipose tissue activity and mass. It is regulated by decorin and follistatin. |
| IL-6 [4,7,23,28,36,37,39,40,41] | It is involved in autocrine and paracrine signaling in skeletal muscle, particularly activating the STAT3 pathway, which is important for hypertrophic muscle growth and myogenesis. It enhances insulin-stimulated glucose uptake and oxidation, stimulates lipolysis and fat oxidation, promotes pancreatic β-cell expansion, and improves insulin secretion and glycemic control. IL-6 produced by skeletal muscles during exercise exerts anti-inflammatory effects, suppressing the synthesis of IL-1 and TNF-α and stimulating the production of anti-inflammatory cytokines like IL-1ra and IL-10. |
| IL-15 [4,28] | It is an anabolic factor that stimulates muscle growth: IL-15 signaling is involved in the regulation of muscle fiber composition and contractility. It is involved in lipid metabolism regulation, reducing lipid accumulation in preadipocytes and decreasing the mass of white adipose tissue. A negative relationship has been observed between plasma IL-15 levels and total body fat mass, especially trunk fat mass. |
| Decorin [33,43,46] | It plays a role in the regulation of muscle hypertrophy, particularly in response to exercise. Together with follistatin, it is a myostatin inhibitor. |
| Leukemia inhibitory factor (LIF) [22,44,47,48] | It is a contraction-induced myokine. It acts in an autocrine and/or paracrine fashion. It promotes platelet production, the proliferation of hematopoietic cells, bone formation, neural survival and development, and the acute-phase response in liver cells. It supports satellite cell proliferation. |
| Chitinase-3-like protein 1 (CHI3L1) [45,49,50] | It is produced and secreted by skeletal muscle in response to acute physical exercise. Through the CHI3L1/PAR-2 signaling pathway, it stimulates myocyte proliferation, which is important for skeletal muscle remodeling in response to training. It is linked to increased glucose uptake in skeletal muscles both via an AMP-activated protein kinase (AMPK)-dependent mechanism and via the PI3K/AKT pathway. |
| Angiopoietin-like 4 (ANGPTL4) [51,52,53,54,55,56] | It inhibits lipoprotein lipase activity, which reduces lipid accumulation and promotes lipolysis in white adipose tissue. In non-exercising muscles, ANGPTL4 levels increase in response to elevated plasma FFA through PPAR activation, helping to prevent fat overload and ensuring the supply of fatty acids to active skeletal muscles. By regulating its expression through PPARs, ANGPTL4 may contribute to the development of insulin resistance. ANGPTL4 levels can be modified by exercise, such as acute resistance and strength training, as well as by dietary factors, particularly the intake of SFA. It has been implicated in mice as a mediator of pancreatic β-cell hyperplasia. |
| Apelin [57,58,59,60,61] | It enhances glucose uptake in skeletal muscle. It increases mitochondrial oxidative capacity in skeletal muscle. It improves muscle function by enhancing energy production. It reduces inflammation in skeletal muscle. It supports muscle stem cell (MuSC) regeneration, especially stimulating skeletal muscle endothelial cells (ECs). |
| Irisin [22,62,63,64] | It is a polypeptide hormone produced by muscles in response to physical exercise. It promotes the conversion of white adipose tissue into a brown fat-like phenotype. It exerts antifibrotic effects on the heart, liver, pancreas, and muscle. |
| VEGF [65,66] | It is a primary angiogenic factor that promotes the growth of new blood vessels, including the capillaries within skeletal muscle. During physical exercise, VEGF levels rise in the muscle interstitium, enhancing angiogenic activity. It facilitates the development and growth of blood vessels within skeletal muscle tissue, ensuring adequate blood supply to the muscles. It contributes to the regeneration of tissues, supporting repair and recovery processes in skeletal muscle. |
| IL-8 [22] | It induces angiogenesis. |
| CYR61 (CCN1) and CTGF (CCN2) [67] | They increase after physical activity, especially following mechanical loading. They regulate the expression of genes involved in angiogenesis and ECM remodeling. |
| Brain-derived neurotrophic factor (BDNF) [28,68,69] | Its levels increase after physical activity. It plays a critical role in the growth, survival, and maintenance of neurons. It impacts information processing, learning, and memory. It influences energy metabolism by enhancing fat oxidation in skeletal muscles through an AMPK-dependent pathway. It contributes to a decrease in adipose tissue mass. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Magenes, V.C.; Bianchi, A.; Rossi, V.; Gatti, A.; Marin, L.; Vandoni, M.; Zuccotti, G. How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes? Life 2024, 14, 1198. https://doi.org/10.3390/life14091198
Calcaterra V, Magenes VC, Bianchi A, Rossi V, Gatti A, Marin L, Vandoni M, Zuccotti G. How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes? Life. 2024; 14(9):1198. https://doi.org/10.3390/life14091198
Chicago/Turabian StyleCalcaterra, Valeria, Vittoria Carlotta Magenes, Alice Bianchi, Virginia Rossi, Alessandro Gatti, Luca Marin, Matteo Vandoni, and Gianvincenzo Zuccotti. 2024. "How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes?" Life 14, no. 9: 1198. https://doi.org/10.3390/life14091198









