Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Chronic Inflammation and Tumour Specific Immune Response in HCC
3. Neoantigens and Tumor-Associated Antigens in HCC
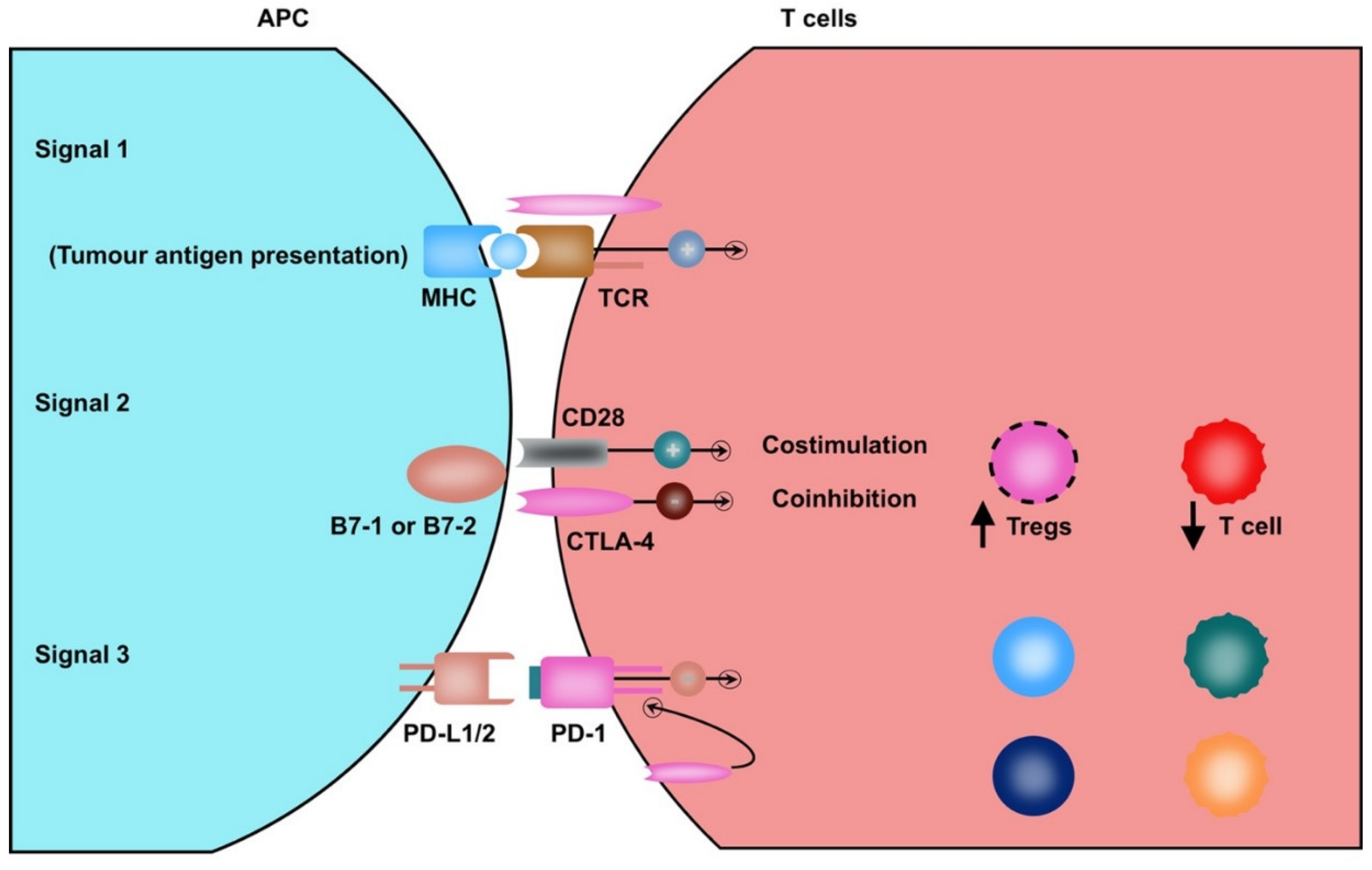
4. Cell- and Cytokine-Mediated Changes in HCC Tumour Microenvironment
5. Macrophages and Associated Cytokines
5.1. Tumour-Associated Macrophages and Associated Cytokines
5.2. Myeloid-Derived Suppressor Cells (MDSCs) and Associated Cytokines
5.3. Dendritic Cells and Associated Cytokines
5.4. T Lymphocytes and Associated Cytokines
5.5. Regulatory T Cells and Associated Cytokines
5.6. NK Cells and Associated Cytokines
5.7. Hepatic Stellate Cells, Endothelial Cells, and Cancer-Associated Fibroblasts
6. Future Implications
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Fuster, J.; Bruix, J. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant. 2004, 10, S115–S120. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrer, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Karaman, B.; Battal, B.; Sari, S.; Verim, S. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 18059–18060. [Google Scholar] [CrossRef]
- Breous, E.; Thimme, R. Potential of immunotherapy for hepatocellular carcinoma. J. Hepatol. 2011, 54, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M. Immuno-Oncology in Hepatocellular Carcinoma: 2017 Update. Oncology 2017, 93, 147–159. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Yoon, S.K.; Lencioni, R. The Etiology of Hepatocellular Carcinoma and Consequences for Treatment. Oncologist 2010, 15, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef]
- Reccia, I.; Kumar, J.; Akladios, C.; Virdis, F.; Pai, M.; Habib, N.; Spalding, D. Non-alcoholic fatty liver disease: A sign of systemic disease. Metab. Clin. Exp. 2017, 72, 94–108. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma review-article. Nat. Immunol. 2018, 19, 222–232. [Google Scholar]
- Hoshida, Y. Molecular signatures and prognosis of hepatocellular carcinoma. Minerva Gastroenterol. Dietol. 2011, 57, 311–322. [Google Scholar]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2011, 56, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef] [Green Version]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The Inflammatory Microenvironment in Hepatocellular Carcinoma: A Pivotal Role for Tumor-Associated Macrophages. BioMed Res. Int. 2012, 2013, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A. Progress in human tumour immunology and immunotherapy. Nature 2001, 411, 380–384. [Google Scholar] [CrossRef]
- Kalialis, L.V.; Drzewiecki, K.T.; Klyver, H. Spontaneous regression of metastases from melanoma: Review of the literature. Melanoma Res. 2009, 19, 275–282. [Google Scholar] [CrossRef]
- Bramhall, R.J.; Mahady, K.; Peach, A.H.S. Spontaneous regression of metastatic melanoma—Clinical evidence of the abscopal effect. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 34–41. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.W.H.; Lo, R.C.L.; Chan, L.K.; Ng, I.O.-L. Molecular Pathogenesis of Hepatocellular Carcinoma. Liver Cancer 2016, 5, 290–302. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Cressman, E.N.K.; Steer, C.J. Cellular and molecular mechanisms of hepatocellular carcinoma: An update. Arch. Toxicol. 2012, 87, 227–247. [Google Scholar] [CrossRef]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Moreno-Otero, R.; Sanz-Cameno, P.; Trapero-Marugán, M.; Chaparro, M.; Jones, E.A. Angiogenesis: From Chronic Liver Inflammation to Hepatocellular Carcinoma. J. Oncology 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro De Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Greten, T.F.; Wang, X.W.; Korangy, F. Current concepts of immune based treatments for patients with HCC: From basic science to novel treatment approaches. Gut 2015, 64, 842–848. [Google Scholar] [CrossRef]
- Thomson, A.W.; O’Connell, P.J.; Steptoe, R.J.; Lu, L. Immunobiology of liver dendritic cells. Immunol. Cell Boil. 2002, 80, 65–73. [Google Scholar] [CrossRef]
- Lau, A.H.; Thomson, A.W. Dendritic cells and immune regulation in the liver. Gut 2003, 52, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Funami, K.; Oshiumi, H.; Seya, T. Toll-IL-1-Receptor-Containing Adaptor Molecule-1: A signaling adaptor linking innate immunity to adaptive immunity. Prog. Mol. Biol. Transl. Sci. 2013, 117, 487–510. [Google Scholar] [CrossRef]
- Nace, G.; Evankovich, J.; Eid, R.; Tsung, A. Dendritic Cells and Damage-Associated Molecular Patterns: Endogenous Danger Signals Linking Innate and Adaptive Immunity. J. Innate Immun. 2012, 4, 6–15. [Google Scholar] [CrossRef]
- Khochenkov, D.A. Biology of dendritic cells. Biochem. Moscow Suppl. Ser. A 2008, 296–311. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Acuto, O.; Michel, F. CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat. Rev. Immunol. 2003, 3, 939–951. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Muraille, E.; Leo, O.; Moser, M. Th1/Th2 paradigm extended: Macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar]
- Chen, M.M.; Xiao, X.; Lao, X.M.; Wei, Y.; Liu, R.X.; Zeng, Q.H.; Wang, J.C.; Ouyang, F.Z.; Chen, D.P.; Chan, K.W.; et al. Polarization of Tissue-Resident TFH-Like Cells in Human Hepatoma Bridges Innate Monocyte Inflammation and M2b Macrophage Polarization. Cancer Discov. 2016, 6, 1182–1195. [Google Scholar] [CrossRef] [Green Version]
- Heath, W.R.; Carbone, F.R. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 2001, 1, 126–134. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Cresswell, P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 2004, 5, 678–684. [Google Scholar] [CrossRef]
- Shresta, S.; Pham, C.T.; Thomas, D.A.; Graubert, T.A.; Ley, T.J. How do cytotoxic lymphocytes kill their targets? Curr. Opin. Immunol. 1998, 10, 581–587. [Google Scholar]
- Groscurth, P.; Filgueira, L. Killing mechanisms of cytotoxic T lymphocytes. News Physiol. Sci. 1998, 13, 17–21. [Google Scholar]
- Shuai, Z.; Leung, M.W.Y.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.C.; Gershwin, M.E. Adaptive immunity in the liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef] [Green Version]
- Ishizawa, T.; Hasegawa, K.; Aoki, T.; Takahashi, M.; Inoue, Y.; Sano, K.; Imamura, H.; Sugawara, Y.; Kokudo, N.; Makuuchi, M. Neither Multiple Tumors Nor Portal Hypertension Are Surgical Contraindications for Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1908–1916. [Google Scholar] [CrossRef]
- Fuks, D.; Dokmak, S.; Paradis, V.; Diouf, M.; Durand, F.; Belghiti, J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: An intention-to-treat analysis. Hepatology 2011, 55, 132–140. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Realising the promise: Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar]
- Gubin, M.M.; Artyomov, M.N.; Mardis, E.R.; Schreiber, R.D. Tumor neoantigens: Building a framework for personalized cancer immunotherapy. J. Clin. Investig. 2015, 125, 3413–3421. [Google Scholar] [CrossRef]
- Ward, J.P.; Gubin, M.M.; Schreiber, R.D. The Role of Neoantigens in Naturally Occurring and Therapeutically Induced Immune Responses to Cancer. Adv. Immunol. 2016, 130, 25–74. [Google Scholar]
- Wirth, T.C.; Kühnel, F. Neoantigen targeting—Dawn of a new era in cancer immunotherapy? Front. Immunol. 2017, 8, 1848. [Google Scholar]
- Stenner, F.; Luo, G.; Sahin, U.; Tureci, O.; Koslovski, M.; Kautz, I.; Liewen, H.; Pfreunschuh, M. Definition of tumor-associated antigens in hepatocellular carcinoma. Cancer Epidemiol. Biomark. Prev. 2000, 9, 285–290. [Google Scholar]
- Dai, L.; Lei, N.; Liu, M.; Zhang, J.-Y. Autoantibodies to tumor-associated antigens as biomarkers in human hepatocellular carcinoma (HCC). Exp. Hematol. Oncol. 2013, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, K.J.; Pang, X.-W.; Vaughan, H.A.; Qu, W.; Dong, X.Y.; Peng, J.R.; Zhao, H.T.; Rui, J.A.; Leng, X.S.; et al. Large scale identification of human hepatocellular carcinoma-associated antigens by autoantibodies. J. Immunol. 2002, 169, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Huang, J. Autoantibodies against tumor-associated antigens for detection of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1581–1585. [Google Scholar] [CrossRef]
- Shimizu, Y.; Suzuki, T.; Yoshikawa, T.; Tsuchiya, N.; Sawada, Y.; Endo, I.; Nakatsura, T. Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci. 2018, 109, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef] [Green Version]
- Mizukoshi, E.; Nakamoto, Y.; Arai, K.; Yamashita, T.; Sakai, A.; Sakai, Y.; Kagaya, T.; Honda, M.; Kaneko, S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011, 53, 1206–1216. [Google Scholar] [CrossRef]
- Sideras, K.; Bots, S.J.; Biermann, K.; Sprengers, D.; Polak, W.G.; Ijzermans, J.N.M.; Man, R.A.; Pan, Q.; Sleijfer, S.; Bruno, M.J.; et al. Tumour antigen expression in hepatocellular carcinoma in a low-endemic western area. Br. J. Cancer 2015, 112, 1911–1920. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Ding, T.; Guo, Z.; Yu, X.-J.; Hu, Y.-Z.; Zheng, L.; Xu, J. Expression pattern of tumour-associated antigens in hepatocellular carcinoma: Association with immune infiltration and disease progression. Br. J. Cancer 2013, 109, 1031–1039. [Google Scholar] [CrossRef]
- Kalathil, S.G.; Hutson, A.; Barbi, J.; Iyer, R.V.; Thanavala, Y.M. Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Yang, J.D.; Nakamura, I.; Roberts, L.R. The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. Semin. Cancer Boil. 2010, 21, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Prieto, J.; Melero, I.; Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 681–700. [Google Scholar] [CrossRef]
- Buonaguro, L.; Mauriello, A.; Cavalluzzo, B.; Petrizzo, A.; Tagliamonte, M. Immunotherapy in hepatocellular carcinoma. Ann. Hepatol. 2019, 18, 291–297. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Porcheray, F.; Viaud, S.; Rimaniol, A.-C.; Leone, C.; Samah, B.; Dereuddre-Bosquet, N.; Dermont, D.; Gras, G. Macrophage activation switching: An asset for the resolution of inflammation. Clin. Exp. Immunol. 2005, 142, 481–489. [Google Scholar] [CrossRef]
- Goerdt, S.; Politz, O.; Schledzewski, K.; Birk, R.; Gratchev, A.; Guillot, P.; Hakiy, N.; Klemke, C.D.; Dippel, E.; Kodelja, V. Alternative versus classical activation of macrophages. Pathobiology 1999, 67, 222–226. [Google Scholar] [CrossRef]
- Classen, A.; Lloberas, J.; Celada, A. Macrophage Activation: Classical Vs. Alternative. Methods Mol. Biol. 2009, 531, 29–43. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, T.; Zheng, B.; Chen, L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019, 458, 86–91. [Google Scholar] [CrossRef]
- Shirabe, K.; Mano, Y.; Muto, J.; Matono, R.; Motomura, T.; Toshima, T.; Takeishi, K.; Uchiyama, H.; Yoshizumi, T.; Taketomi, A.; et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg. Today 2011, 42, 1–7. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zhou, Z.J.; Hu, Z.Q.; Huang, X.W.; Wang, Z.; Chen, E.B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.-T.; Song, K.; Zhou, J.; Shi, Y.; Liu, W.-R.; Shi, G.; Gao, Q.; Wang, X.-Y.; Ding, Z.; Fan, J. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Ribechini, E.; Greifenberg, V.; Sandwick, S.; Lutz, M.B. Subsets, expansion and activation of myeloid-derived suppressor cells. Med. Microbiol. Immunol. 2010, 199, 273–281. [Google Scholar] [CrossRef]
- Solito, S.; Marigo, I.; Pinton, L.; Damuzzo, V.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann. New York Acad. Sci. 2014, 1319, 47–65. [Google Scholar] [CrossRef]
- Schrader, J. The role of MDSCs in hepatocellular carcinoma--in vivo veritas? J. Hepatol. 2013, 59, 921–923. [Google Scholar] [CrossRef]
- Hammerich, L.; Tacke, F. Emerging roles of myeloid derived suppressor cells in hepatic inflammation and fibrosis. World J. Gastrointest. Pathophysiol. 2015, 6, 43–50. [Google Scholar] [CrossRef]
- Condamine, T.; Ramachandran, I.; Youn, J.-I.; Gabrilovich, D.I. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu. Rev. Med. 2014, 66, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2010, 32, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Kuang, N.-M.; Wu, Y.; Xiao, X.; Li, X.-F.; Li, T.-J.; Zheng, L. Activated CD69+ T Cells Foster Immune Privilege by Regulating IDO Expression in Tumor-Associated Macrophages. J. Immunol. 2011, 188, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Chambers, S.E.; O’Neill, C.L.; O’Doherty, T.M.; Medina, R.J.; Stitt, A.W. The role of immune-related myeloid cells in angiogenesis. Immunobiology 2013, 218, 1370–1375. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Terness, P.; Chuang, J.-J.; Opelz, G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 2006, 27, 68–73. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, Y.; Eynde, B.J.V. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Shibata, Y.; Hara, T.; Nagano, J.; Nakamura, N.; Ohno, T.; Ninomiya, S.; Ito, H.; Tanaka, T.; Saito, K.; Seishima, M.; et al. The Role of Indoleamine 2,3-Dioxygenase in Diethylnitrosamine-Induced Liver Carcinogenesis. PLoS ONE 2016, 11, e0146279. [Google Scholar] [CrossRef]
- Pan, K.; Wang, H.; Chen, M.-S.; Zhang, H.K.; Weng, D.S.; Zhou, J.; Huang, W.; Li, J.J.; Song, H.F.; Xia, J.C. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J.Cancer Res. Clin. Oncol. 2008, 134, 1247–1253. [Google Scholar] [CrossRef]
- Komiya, T.; Huang, C.H. Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front. Oncol. 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Joffre, O.; Nolte, M.A.; Spörri, R.; Sousa, C.R.E. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009, 227, 234–247. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Prosser, A.C.; Van Bruggen, I.; Robinson, B.W.S.; Currie, A.J. CD8α+ DC are not the sole subset cross-presenting cell-associated tumor antigens from a solid tumor. Eur. J. Immunol. 2010, 40, 1617–1627. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- Villanueva, A.; Chiang, D.Y.; Newell, P.; Peix, J.; Thung, S.; Alsinet, C.; Tovar, V.; Roayaie, S.; Minguez, B.; Sole, M.; et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology 2008, 135, 1972–1983.e11. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X.; Liu, S.; Guo, L.; Zhang, B.; Zhang, J.; Ye, Q. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 2017, 66, 1920–1933. [Google Scholar] [CrossRef] [Green Version]
- Mossanen, J.C.; Tacke, F. Role of lymphocytes in liver cancer. OncoImmunology 2013, 2, e26468. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chong, M.M.W.; Littman, D.R. Plasticity of CD4+ T Cell Lineage Differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.D.; Suresh, R.; Vakil, V.; Gomer, R.H.; Pilling, D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J. Leukoc. Biol. 2008, 83, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- Budhu, A.; Forgues, M.; Ye, Q.H.; Jia, H.L.; He, P.; Zanetti, K.A.; Kammula, U.S.; Qin, L.X.; Tang, Z.Y.; Wang, X.W. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006, 10, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.D.; Zhang, J.-B.; Zhuang, P.Y.; Zhu, H.G.; Zhang, W.; Xiong, Y.Q.; Wu, W.Z.; Tang, Z.Y.; Sun, H.C. High Expression of Macrophage Colony-Stimulating Factor in Peritumoral Liver Tissue Is Associated With Poor Survival After Curative Resection of Hepatocellular Carcinoma. J. Clin. Oncol. 2008, 26, 2707–2716. [Google Scholar] [CrossRef]
- Ji, L.; Gu, J.; Chen, L.; Miao, D. Changes of Th1/Th2 cytokines in patients with primary hepatocellular carcinoma after ultrasound-guided ablation. Int. J. Clin. Exp. Pathol. 2017, 10, 8715–8720. [Google Scholar]
- Lee, H.L.; Jang, J.W.; Lee, S.W.; Yoo, S.H.; Kwon, J.H.; Nam, S.W.; Bae, S.H.; Choi, J.Y.; Han, N.I.; Yoon, S.K. Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci. Rep. 2019, 9, 1–8. [Google Scholar]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Unitt, E.; Rushbrook, S.M.; Marshall, A.; Davies, S.; Gibbs, P.; Morris, L.S.; Coleman, N.; Alexander, G.J.M. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology 2005, 41, 722–730. [Google Scholar] [CrossRef]
- Ormandy, L.A. Increased Populations of Regulatory T Cells in Peripheral Blood of Patients with Hepatocellular Carcinoma. Cancer Res. 2005, 65, 2457–2464. [Google Scholar] [CrossRef] [Green Version]
- Cao, X. Regulatory T cells and immune tolerance to tumors. Immunol. Res. 2009, 46, 79–93. [Google Scholar] [CrossRef]
- Johnston, M.P.; Khakoo, S.I. Immunotherapy for hepatocellular carcinoma: Current and future. World J. Gastroenterol. 2019, 25, 2977–2989. [Google Scholar] [CrossRef]
- Subleski, J.J.; Wiltrout, R.H.; Weiss, J.M. Application of tissue-specific NK and NKT cell activity for tumor immunotherapy. J. Autoimmun. 2009, 33, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.-D.; Ljunggren, H.-G.; La Cava, A.; Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011, 11, 658–671. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, X.; Chen, Y.; Sojka, D.R.K.; Wei, H.; Gao, X.; Sun, R.; Yokoyama, W.M.; Tian, Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Investig. 2013, 123, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Peng, H.; Zhou, J.; Chen, Y.; Wei, H.; Sun, R.; Yokoyama, W.M.; Tian, Z. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J. Autoimmun. 2016, 67, 29–35. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Tian, Z.; Xiao, W. NK cells in immunotolerant organs. Cell. Mol. Immunol. 2013, 10, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Fu, B.; Tian, Z.; Wei, H. Subsets of human natural killer cells and their regulatory effects. Immunology 2014, 141, 483–489. [Google Scholar] [CrossRef]
- Hudspeth, K.; Donadon, M.; Cimino, M.; Pontarini, E.; Tentorio, P.; Preti, M.; Hong, M.; Bertoletti, A.; Bicciato, S.; Invernizzi, P.; et al. Human liver-resident CD56bright/CD16neg NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016, 66, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Long, E.O.; Sik Kim, H.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef] [Green Version]
- Vermijlen, D.; Luo, D.; Froelich, C.J.; Medema, J.P.; Kummer, J.A.; Willems, E.; Braet, F.; Wisse, E. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J. Leukoc. Biol. 2002, 72. [Google Scholar]
- Cai, L.; Zhang, Z.; Zhou, L.; Wang, H.; Fu, J.; Zhang, S.; Shi, M.; Zhang, H.; Yang, Y.; Wu, H.; et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin. Immunol. 2008, 129, 428–437. [Google Scholar] [CrossRef]
- Dessouki, O.; Kamiya, Y.; Nagahama, H.; Tanaka, M.; Suzu, S.; Sasaki, Y.; Okada, S. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: Reversion by anti-viral treatment. Biochem. Biophys. Res. Commun. 2010, 393, 331–337. [Google Scholar] [CrossRef]
- Morishima, C.; Paschal, D.M.; Wang, C.C.; Yoshihara, C.S.; Wood, B.L.; Yeo, A.E.; Emerson, S.S.; Shuhart, M.C.; Gretch, D.R. Decreased NK cell frequency in chronic hepatitis C does not affectex vivo cytolytic killing. Hepatology 2006, 43, 573–580. [Google Scholar] [CrossRef]
- Gao, B.; Radaeva, S.; Park, O. Liver natural killer and natural killer T cells: Immunobiology and emerging roles in liver diseases. J. Leukoc. Biol. 2009, 86, 513–528. [Google Scholar] [CrossRef]
- Baglieri, J.; Brenner, D.A.; Kisseleva, T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1723. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosisin Liver Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 153–186. [Google Scholar]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef]
- Kubo, N.; Araki, K.; Kuwano, H.; Shirabe, K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 6841–6850. [Google Scholar] [CrossRef]
- Giannelli, G.; Villa, E.; Lahn, M. Transforming Growth factor-β as a Therapeutic Target in Hepatocellular Carcinoma. Cancer Res. 2014, 74, 1890–1894. [Google Scholar] [CrossRef] [Green Version]
- Lau, E.Y.T.; Lo, J.; Cheng, B.Y.L.; Ma, M.K.F.; Lee, J.M.F.; Ng, J.K.Y.; Chai, S.; Lin, C.H.; Tsang, S.Y.; Ma, S.; et al. Cancer-Associated Fibroblasts Regulate Tumor-Initiating Cell Plasticity in Hepatocellular Carcinoma through c-Met/FRA1/HEY1 Signaling. Cell. Rep. 2016, 15, 1175–1189. [Google Scholar]
- Ng, J.; Dai, T. Radiation therapy and the abscopal effect: A concept comes of age. Ann. Transl. Med. 2016, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.W.; Jayant, K.; Lee, P.-H.; Yang, P.-C.; Hsiao, C.-Y.; Habib, N.; Sodergren, M. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Jayant, K.; Sodergren, M.H.; Reccia, I.; Kusano, T.; Zacharoulis, D.; Spalding, D.; Pai, M.; Zhou, L.; Huang, K.W. A systematic review and meta-analysis comparing liver resection with the Rf-based device habibTM-4X with the clamp-crush technique. Cancers 2018, 10, 428. [Google Scholar]
- Huang, K.; Lee, P.H.; Kusano, T.; Reccia, I.; Jayant, K.; Habib, N. Impact of cavitron ultrasonic surgical aspirator (CUSA) and bipolar radiofrequency device (Habib-4X) based hepatectomy for hepatocellular carcinoma on tumour recurrence and disease-free survival. Oncotarget 2017, 8, 93644–93654. [Google Scholar] [CrossRef]
- Mazmishvili, K.; Jayant, K.; Janikashvili, N.; Kikodze, N.; Mizandari, M.; Pantsulaia, I.; Sodergren, M.; Reccia, I.; Pai, M.; Habib, N.; et al. Study to evaluate the immunomodulatory effects of radiofrequency ablation compared to surgical resection for liver cancer. J. Cancer 2018, 9, 3187–3195. [Google Scholar] [CrossRef] [Green Version]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-Q.; Li, W.-M.; Lu, Z.-Q.; Yao, Y.-M. Roles of Tregs in development of hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2014, 20, 7971–7978. [Google Scholar] [CrossRef]
- Harding, J.J.; El Dika, I.; Abou-Alfa, G.K. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer 2015, 122, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Sprengers, D.; Boor, P.P.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; De Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenteroloy 2017, 153, 1107–1119.e10. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; Elgindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2016, 66, 545–551. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayant, K.; Habib, N.; Huang, K.W.; Warwick, J.; Arasaradnam, R. Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma. Diagnostics 2020, 10, 338. https://doi.org/10.3390/diagnostics10050338
Jayant K, Habib N, Huang KW, Warwick J, Arasaradnam R. Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma. Diagnostics. 2020; 10(5):338. https://doi.org/10.3390/diagnostics10050338
Chicago/Turabian StyleJayant, Kumar, Nagy Habib, Kai W. Huang, Jane Warwick, and Ramesh Arasaradnam. 2020. "Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma" Diagnostics 10, no. 5: 338. https://doi.org/10.3390/diagnostics10050338
APA StyleJayant, K., Habib, N., Huang, K. W., Warwick, J., & Arasaradnam, R. (2020). Recent Advances: The Imbalance of Immune Cells and Cytokines in the Pathogenesis of Hepatocellular Carcinoma. Diagnostics, 10(5), 338. https://doi.org/10.3390/diagnostics10050338





