Soluble High Mobility Group Box 1 (HMGB1) Is a Promising Biomarker for Prediction of Therapy Response and Prognosis in Advanced Lung Cancer Patients
Abstract
:1. Introduction
2. Patients, Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef]
- Štros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Malarkey, C.S.; Churchill, M.E.A. The high mobility group box: The ultimate utility player of a cell. Trends Biochem. Sci. 2012, 37, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Garg, A.D.; Nowis, D.; Golab, J.; Vandenabeele, P.; Krysko, D.V.; Agostinis, P. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim. Biophys. Acta 2010, 1805, 53–71. [Google Scholar] [CrossRef]
- Kepp, O.; Senovilla, L.; Vitale, I.; Vacchelli, E.; Adjemian, S.; Agostinis, P.; Apetoh, L.; Aranda, F.; Barnaba, V.; Bloy, N.; et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014, 3, e955691. [Google Scholar] [CrossRef] [Green Version]
- Antoine, D.J.; Harris, H.E.; Andersson, U.; Tracey, K.J.; Bianchi, M.E. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol. Med. 2014, 20, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Rovere-Querini, P.; Baldini, M.; Baldissera, E.; Sabbadini, M.G.; Bianchi, M.E.; Manfredi, A.A. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: A candidate for microvessel injury in sytemic sclerosis. Antioxid. Redox Signal. 2014, 20, 1060–1074. [Google Scholar] [CrossRef]
- Huang, J.; Ni, J.; Liu, K.; Yu, Y.; Xie, M.; Kang, R.; Vernon, P.; Cao, L.; Tang, D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012, 72, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cheng, Y.; Ren, X.; Zhang, L.; Yap, K.L.; Wu, H.; Patel, R.; Liu, D.; Qin, Z.-H.; Shih, I.-M.; et al. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene 2012, 31, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Huang, J.; Xie, M.; Yu, Y.; Zhu, S.; Kang, R.; Cao, L.; Tang, D.; Duan, X. MIR34A regulates autophagy and apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy 2014, 10, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Hong, L.; Sun, H.; Shi, M.; Song, Y. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol. Rep. 2009, 22, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Holdenrieder, S.; Nagel, D.; Stieber, P. Estimation of prognosis by circulating biomarkers in patients with non-small cell lung cancer. Cancer Biomark. 2010, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-L.; Tsai, J.-R.; Hwang, J.-J.; Chou, S.-H.; Cheng, Y.-J.; Lin, F.-Y.; Chen, Y.-L.; Hung, C.-Y.; Chen, W.-C.; Chen, Y.-H.; et al. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am. J. Respir. Cell Mol. Biol. 2010, 43, 530–538. [Google Scholar] [CrossRef]
- Zhang, C.; Ge, S.; Hu, C.; Yang, N.; Zhang, J. MiRNA-218, a new regulator of HMGB1, suppresses cell migration and invasion in non-small cell lung cancer. Acta Biochim. Biophys. Sin. 2013, 45, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Brezniceanu, M.-L.; Völp, K.; Bösser, S.; Solbach, C.; Lichter, P.; Joos, S.; Zörnig, M. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003, 17, 1295–1297. [Google Scholar] [CrossRef]
- Akaike, H.; Kono, K.; Sugai, H.; Takahashi, A.; Mimura, K.; Kawaguchi, Y.; Fujii, H. Expression of high mobility group box chromosomal protein-1 (HMGB-1) in gastric cancer. Anticancer Res. 2007, 27, 449–457. [Google Scholar]
- Chung, H.W.; Lee, S.-G.; Kim, H.; Hong, D.J.; Chung, J.B.; Stroncek, D.; Lim, J.-B. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J. Transl. Med. 2009, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.-D.; Cui, L.; Peng, C.-H.; Cheng, D.-F.; Han, B.-S.; Huang, F. Expression and clinical significance of HMGB1 in human liver cancer: Knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol. Rep. 2013, 29, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Ding, Y.; Huang, J.; Li, Q.; Liu, Y.; Ni, W.; Zhang, Y.; Zhu, Y.; Chen, L.; Chen, B. The association of HMGB1 gene with the prognosis of HCC. PLoS ONE 2014, 9, e89097. [Google Scholar] [CrossRef]
- Dong, X.D.E.; Ito, N.; Lotze, M.T.; Demarco, R.A.; Popovic, P.; Shand, S.H.; Watkins, S.; Winikoff, S.; Brown, C.K.; Bartlett, D.L.; et al. High mobility group box I (HMGB1) release from tumor cells after treatment: Implications for development of targeted chemoimmunotherapy. J. Immunother. 2007, 30, 596–606. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Zhang, Q.; Ding, Y. Identification of potential genes/proteins regulated by Tiam1 in colorectal cancer by microarray analysis and proteome analysis. Cell Biol. Int. 2008, 32, 1215–1222. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Expression of HMG1 and metastasis of colorectal cancer. Int. J. Colorectal Dis. 2010, 25, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Staratschek-Jox, A.; Springwald, A.; Wenk, H.; Wolf, J.; Wickenhauser, C.; Bullerdiek, J. Non-Hodgkin lymphoma expressing high levels of the danger-signalling protein HMGB1. Leuk. Lymphoma 2008, 49, 1184–1189. [Google Scholar] [CrossRef]
- Müller, S.; Ronfani, L.; Bianchi, M.E. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J. Intern. Med. 2004, 255, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report; Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Jemal, A.; Thun, M.J.; Ries, L.A.G.; Howe, H.L.; Weir, H.K.; Center, M.M.; Ward, E.; Wu, X.-C.; Eheman, C.; Anderson, R.; et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J. Natl. Cancer Inst. 2008, 100, 1672–1694. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef]
- Roointan, A.; Mir, T.A.; Wani, S.I.; Rehman, M.-U.; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early detection of lung cancer biomarkers through biosensor technology: A review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef]
- Therasse, P.; Eisenhauer, E.A.; Verweij, J. RECIST revisited: A review of validation studies on tumour assessment. Eur. J. Cancer 2006, 42, 1031–1039. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Brambs, H.J.; Claussen, C.D. Pancreatic and ampullary carcinoma. Ultrasound, computed tomography, magnetic resonance imaging and angiography. Endoscopy 1993, 25, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Stieber, P.; Holdenrieder, S. Lung cancer biomarkers—Where we are and what we need. Cancer Biomark. 2010, 6, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdenrieder, S.; von Pawel, J.; Dankelmann, E.; Duell, T.; Faderl, B.; Markus, A.; Siakavara, M.; Wagner, H.; Feldmann, K.; Hoffmann, H.; et al. Nucleosomes and CYFRA 21-1 indicate tumor response after one cycle of chemotherapy in recurrent non-small cell lung cancer. Lung Cancer 2009, 63, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef]
- Naumnik, W.; Nilklińska, W.; Ossolińska, M.; Chyczewska, E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem. Cytobiol. 2009, 47, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Shang, G.-H.; Jia, C.-Q.; Tian, H.; Xiao, W.; Li, Y.; Wang, A.-H.; Dong, L.; Lin, D.-J. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir. Med. 2009, 103, 1949–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Xu, J.; Chen, H.; Gao, Y.; Gong, F.; Hu, L.; Yang, L. Association between an elevated level of HMGB1 and non-small-cell lung cancer: A meta-analysis and literature review. OncoTargets Ther. 2016, 9, 3917–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.-Q.; Jia, C.-Q.; Liu, C.-T.; Lu, X.-F.; Zhong, N.; Zhang, Z.-L.; Fan, W.; Li, Y.-Q. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Dig. Liver Dis. 2008, 40, 446–452. [Google Scholar] [CrossRef]
- Kohles, N.; Nagel, D.; Jüngst, D.; Durner, J.; Stieber, P.; Holdenrieder, S. Relevance of circulating nucleosomes and oncological biomarkers for predicting response to transarterial chemoembolization therapy in liver cancer patients. BMC Cancer 2011, 11, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmueller, Y.N.; Nagel, D.; Hoffmann, R.-T.; Tatsch, K.; Jakobs, T.; Stieber, P.; Holdenrieder, S. Predictive and prognostic value of circulating nucleosomes and serum biomarkers in patients with metastasized colorectal cancer undergoing Selective Internal Radiation Therapy. BMC Cancer 2012, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Sheng, X.; Du, X.; Zhang, X.; Li, D.; Lu, C.; Li, Q.; Ma, Z.; Song, Q.; Wang, C. Clinical value of serum HMGB1 levels in early detection of recurrent squamous cell carcinoma of uterine cervix: Comparison with serum SCCA, CYFRA21-1, and CEA levels. Croat. Med. J. 2009, 50, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Stoetzer, O.J.; Fersching, D.M.I.; Salat, C.; Steinkohl, O.; Gabka, C.J.; Hamann, U.; Braun, M.; Feller, A.-M.; Heinemann, V.; Siegele, B.; et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol. 2013, 34, 81–90. [Google Scholar] [CrossRef]
- Wittwer, C.; Boeck, S.; Heinemann, V.; Haas, M.; Stieber, P.; Nagel, D.; Holdenrieder, S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. Int. J. Cancer 2013, 133, 2619–2630. [Google Scholar] [CrossRef] [Green Version]
- Stieber, P.; Heinemann, V. Sinnvoller Einsatz von Tumormarkern/Sensible use of tumor markers. LaboratoriumsMedizin 2008, 32, 339–360. [Google Scholar] [CrossRef] [Green Version]
- Pilzweger, C.; Holdenrieder, S. Circulating HMGB1 and RAGE as Clinical Biomarkers in Malignant and Autoimmune Diseases. Diagnostics 2015, 5, 219–253. [Google Scholar] [CrossRef] [Green Version]
- Goeckenjan, G.; Sitter, H.; Thomas, M.; Branscheid, D.; Flentje, M.; Griesinger, F.; Niederle, N.; Stuschke, M.; Blum, T.; Deppermann, K.-M.; et al. Prevention, diagnosis, therapy, and follow-up of lung cancer: Interdisciplinary guideline of the German Respiratory Society and the German Cancer Society. Pneumologie 2011, 65, 39–59. [Google Scholar] [CrossRef] [Green Version]
- García-Campelo, R.; Bernabé, R.; Cobo, M.; Corral, J.; Coves, J.; Dómine, M.; Nadal, E.; Rodriguez-Abreu, D.; Viñolas, N.; Massuti, B. SEOM clinical guidelines for the treatment of non-small cell lung cancer (NSCLC) 2015. Clin. Transl. Oncol. 2015, 17, 1020–1029. [Google Scholar] [CrossRef] [Green Version]
- Jakubowska, K.; Naumnik, W.; Niklińska, W.; Chyczewska, E. Clinical Significance of HMGB-1 and TGF-β Level in Serum and BALF of Advanced Non-Small Cell Lung Cancer. Adv. Exp. Med. Biol. 2015, 852, 49–58. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, W.; Yang, G.; Li, H.; Chen, Q.; Song, R.; Zhao, L. HMGB1 overexpression as a prognostic factor for survival in cancer: A meta-analysis and systematic review. Oncotarget 2016, 7, 50417–50427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pujol, J.-L.; Molinier, O.; Ebert, W.; Daurès, J.-P.; Barlesi, F.; Buccheri, G.; Paesmans, M.; Quoix, E.; Moro-Sibilot, D.; Szturmowicz, M.; et al. CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: Results of a meta-analysis in 2063 patients. Br. J. Cancer 2004, 90, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, S.; Wehnl, B.; Hettwer, K.; Simon, K.; Uhlig, S.; Dayyani, F. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: A systematic review and meta-analysis. Br. J. Cancer 2017, 116, 1037–1045. [Google Scholar] [CrossRef]
- Muley, T.; Rolny, V.; He, Y.; Wehnl, B.; Escherich, A.; Warth, A.; Stolp, C.; Schneider, M.A.; Dienemann, H.; Meister, M.; et al. The combination of the blood based tumor biomarkers cytokeratin 19 fragments (CYFRA 21-1) and carcinoembryonic antigen (CEA) as a potential predictor of benefit from adjuvant chemotherapy in early stage squamous cell carcinoma of the lung (SCC). Lung Cancer 2018, 120, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritz, R.; Muller, M.; Korse, C.M.; van den Broek, D.; Baas, P.; van den Noort, V.; ten Hoeve, J.J.; van den Heuvel, M.M.; van Rossum, H.H. Diagnostic validation and interpretation of longitudinal circulating biomarkers using a biomarker response characteristic plot. Clin. Chim. Acta 2018, 487, 6–14. [Google Scholar] [CrossRef]
- Lehner, J.; Wittwer, C.; Fersching, D.; Siegele, B.; Holdenrieder, S.; Stoetzer, O.J. Methodological and preanalytical evaluation of an HMGB1 immunoassay. Anticancer Res. 2012, 32, 2059–2062. [Google Scholar]
- Jube, S.; Rivera, Z.S.; Bianchi, M.E.; Powers, A.; Wang, E.; Pagano, I.; Pass, H.I.; Gaudino, G.; Carbone, M.; Yang, H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012, 72, 3290–3301. [Google Scholar] [CrossRef] [Green Version]
- Schiraldi, M.; Raucci, A.; Muñoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; de Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Pellegrini, L.; Napolitano, A.; Giorgi, C.; Jube, S.; Preti, A.; Jennings, C.J.; de Marchis, F.; Flores, E.G.; Larson, D.; et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis. 2015, 6, e1786. [Google Scholar] [CrossRef]


| Total | NSCLC | SCLC | p-Value | |
|---|---|---|---|---|
| N | 93 | 79 | 14 | |
| Age/gender | ||||
| Age (years) (mean ± SD) | 61.1 ± 10.0 | 61.1 ± 10.6 | 61.0 ± 6.2 | 0.776 |
| Female, N (%) | 32 (34.4) | 26 (32.9) | 6 (42.9) | 0.546 |
| Male, N (%) | 61 (65.6) | 53 (67.1) | 8 (57.1) | |
| Histology, N (%) | ||||
| Large cell cancer | 6 (6.5) | 6 (7.6) | ||
| Adenocarcinoma | 30 (32.3) | 30 (38.0) | ||
| Squamous cell carcinoma | 26 (28.0) | 26 (32.9) | ||
| Other NSCLC | 17 (18.3) | 17 (21.5) | ||
| SCLC | 14 (15.1) | 14 (100) | ||
| Stage, N (%) | ||||
| UICC stage 3 | 27 (29.0) | 21 (26.6) | 6 (42.9) | |
| UICC stage 4 | 64 (68.8) | 56 (70.9) | 8 (57.1) | |
| No staging available | 2 (2.2) | 2 (2.5) | - | |
| Therapy response, N (%) | ||||
| Remission/no change | 51 (54.8) | 41 (51.9) | 10 (71.4) | |
| Progression | 42 (45.2) | 38 (48.1) | 4 (28.6) | |
| Overall survival | ||||
| Mean (days) | 224 | 293 |
| NSCLC | Response | N | Median | IQR | Range | p-Value |
|---|---|---|---|---|---|---|
| Pretherapeutic | ||||||
| HMGB1 | R NR | 41 38 | 2.84 2.84 | 3.9 3.6 | 0.3–13.4 0.3–13.2 | 0.641 |
| CEA | R NR | 35 34 | 5.10 5.85 | 9.6 26.2 | 1.7–2299 1.6–775 | 0.371 |
| CYFRA 21-1 | R NR | 35 34 | 4.40 6.65 | 8.2 10.0 | 0.0–67.0 0.3–72.0 | 0.453 |
| Cycle 2 | ||||||
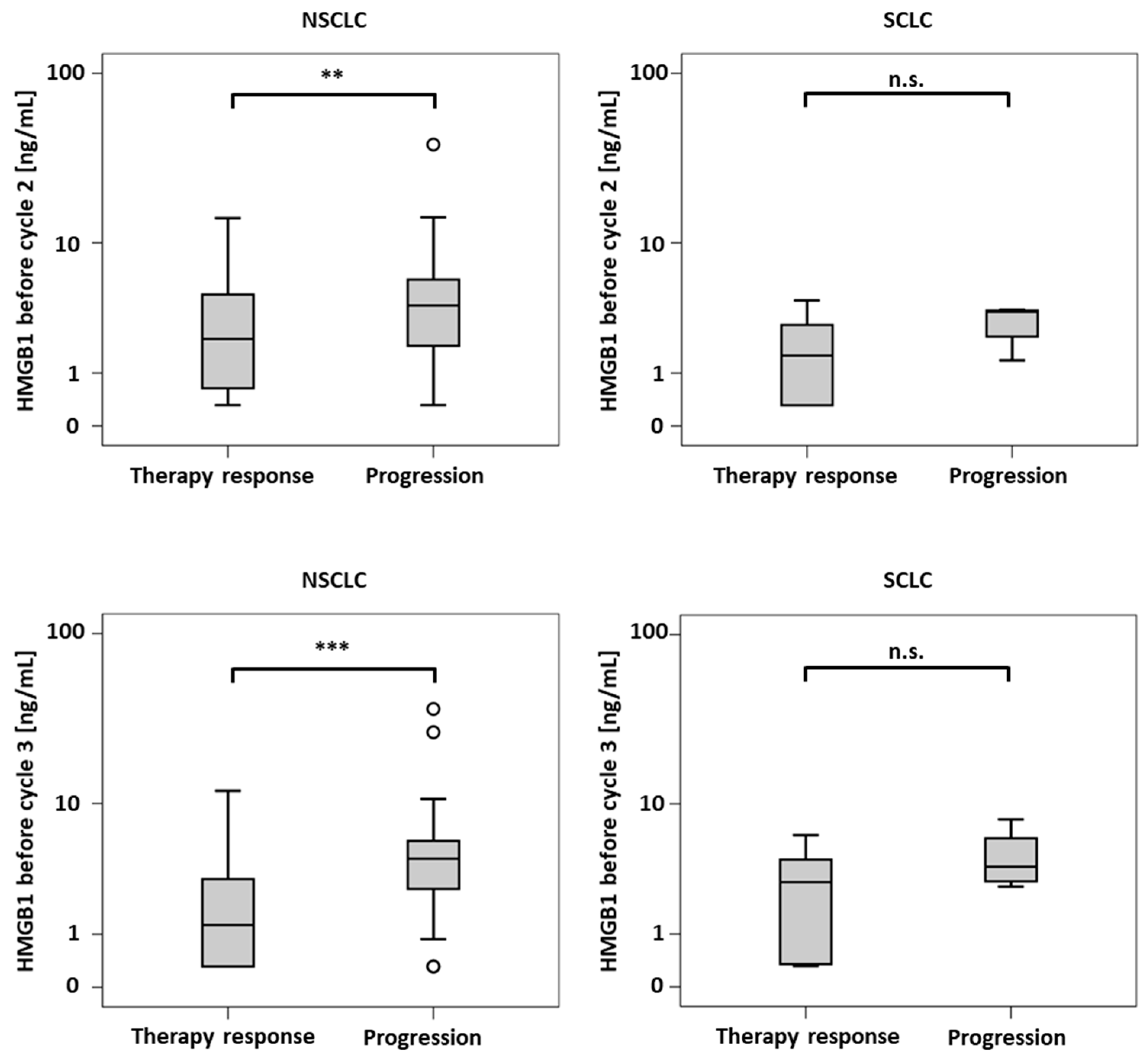
| HMGB1 | R NR | 40 38 | 2.12 3.84 | 4.0 4.1 | 0.3–14.2 0.3–38.8 | 0.009 |
| CEA | R NR | 32 32 | 5.55 5.75 | 10.3 21.7 | 1.6–279 1.6–647 | 0.663 |
| CYFRA 21-1 | R NR | 33 32 | 2.30 6.25 | 4.0 10.2 | 0.3–51.6 1.0–54.0 | 0.006 |
| Cycle 3 | ||||||
| HMGB1 | R NR | 40 34 | 1.25 4.35 | 2.9 3.4 | 0.3–12.0 0.3–36.7 | <0.001 |
| CEA | R NR | 39 24 | 5.30 6.90 | 9.9 15.7 | 1.4–2400 2.7–393 | 0.188 |
| CYFRA 21-1 | R NR | 38 26 | 2.30 8.60 | 3.5 13.3 | 0.5–92.7 1.1–68.1 | <0.001 |
| Changes Cycles 1–2 (%) | ||||||
| HMGB1 | R NR | 41 38 | 62.8 106 | 87.8 119 | 0.0–1764 9.4–2446 | 0.008 |
| CEA | R NR | 35 34 | 85.7 97.4 | 57.4 44.6 | 0.0–290 0.0–437 | 0.086 |
| CYFRA 21-1 | R NR | 34 34 | 61.2 97.9 | 59.0 120 | 0.0–305 0.0–500 | 0.001 |
| Changes Cycles 1–3 (%) | ||||||
| HMGB1 | R NR | 41 38 | 50.2 84.3 | 103 151 | 0.0–1096 0.0–2317 | 0.058 |
| CEA | R NR | 35 34 | 92.3 89.2 | 71.1 138 | 0.0–340 0.0–702 | 0.621 |
| CYFRA 21-1 | R NR | 34 34 | 53.8 79.0 | 63.1 178 | 0.0–461 0.0–1833 | 0.213 |
| SCLC | Response | N | Median | IQR | Range | p-Value |
|---|---|---|---|---|---|---|
| Pretherapeutic | ||||||
| HMGB1 | R NR | 10 4 | 2.84 3.29 | 4.6 3.4 | 1.2–8.9 0.3–4.5 | 0.839 |
| CEA | R NR | 9 3 | 3.90 12.6 | 6.7 - | 1.9–10.9 4.6–93.4 | 0.064 |
| NSE | R NR | 8 4 | 18.7 33.6 | 64.7 91.4 | 8.7–591 18.3–133 | 0.283 |
| Cycle 2 | ||||||
| HMGB1 | R NR | 10 4 | 1.54 3.44 | 2.5 1.7 | 0.3–4.2 1.4–3.6 | 0.106 |
| CEA | R NR | 8 3 | 5.65 47.7 | 6.1 - | 1.8–12.4 7.3–94.1 | 0.048 |
| NSE | R NR | 9 3 | 11.9 35.5 | 4.4 - | 8.9–24.2 34.2–39.6 | 0.009 |
| Cycle 3 | ||||||
| HMGB1 | R NR | 10 4 | 2.95 3.87 | 4.2 4.2 | 0.3–6.3 2.7–8.0 | 0.304 |
| CEA | R NR | 9 4 | 4.70 28.5 | 5.2 111 | 1.5–23.5 6.7–141 | 0.020 |
| NSE | R NR | 8 4 | 12.6 60.7 | 4.4 55.0 | 8.0–15.9 38.4–97.8 | 0.004 |
| Changes Cycles 1–2 (%) | ||||||
| HMGB1 | R NR | 10 4 | 38.0 108 | 74.6 277 | 8.6–218 79.8–437 | 0.054 |
| CEA | R NR | 9 3 | 94.7 101 | 88.0 - | 0.0–154 0.0–379 | 0.727 |
| NSE | R NR | 8 4 | 63.9 127 | 92.7 163 | 0.0–122 0.0–187 | 0.368 |
| Changes Cycles 1–3 (%) | ||||||
| HMGB1 | R NR | 10 4 | 72.1 206 | 111 643 | 19.2–254 72.9–870 | 0.106 |
| CEA | R NR | 9 3 | 94.7 151 | 66.7 - | 0.0–230 146–387 | 0.036 |
| NSE | R NR | 8 4 | 54.2 152 | 71.5 245 | 0.0–120 73.5–356 | 0.028 |
| AUC | 95% CI | Sensitivity at 90% Specificity | Sensitivity at 95% Specificity | p-Value | |
|---|---|---|---|---|---|
| Pretherapeutic | |||||
| HMGB1 | 0.549 | 0.410–0.687 | 3.4% | 2.0% | 0.486 |
| CEA | 0.563 | 0.426–0.699 | 11.4% | 2.9% | 0.371 |
| CYFRA 21-1 | 0.553 | 0.416–0.689 | 14.3% | 2.9% | 0.453 |
| Cycle 2 | |||||
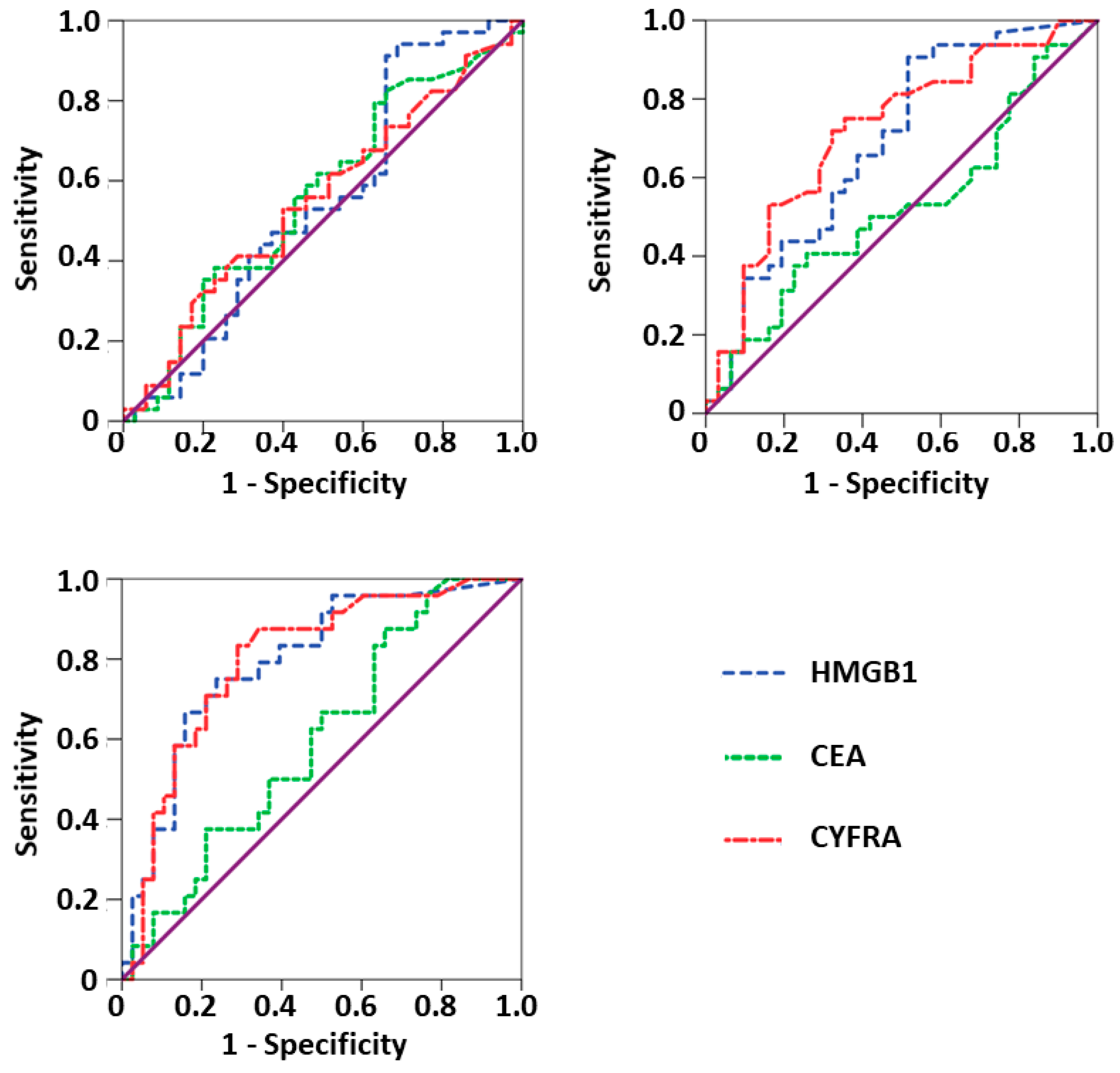
| HMGB1 | 0.690 | 0.558–0.821 | 34.4% | 6.3% | 0.010 |
| CEA | 0.523 | 0.378–0.667 | 18.8% | 6.3% | 0.757 |
| CYFRA 21-1 | 0.719 | 0.592–0.847 | 37.5% | 15.6% | 0.003 |
| Cycle 3 | |||||
| HMGB1 | 0.794 | 0.680–0.909 | 37.5% | 20.8% | <0.001 |
| CEA | 0.598 | 0.456–0.739 | 16.7% | 8.3% | 0.198 |
| CYFRA 21-1 | 0.799 | 0.686–0.913 | 41.7% | 4.2% | <0.001 |
| Below Cutoff | Above Cutoff | |||||
|---|---|---|---|---|---|---|
| Cutoff | Median OS (d) | 95% CI | Median OS (d) | 95% CI | p-Value | |
| Pretherapeutic | ||||||
| HMGB1 | 2.84 | 195 | 116–274 | 239 | 165–313 | 0.742 |
| CEA | 5.50 | 180 | 140–220 | 211 | 140–282 | 0.896 |
| CYFRA 21-1 | 4.90 | 291 | 10–572 | 139 | 73–205 | 0.001 |
| Prior to cycle 2 | ||||||
| HMGB1 | 2.52 | 239 | 89–389 | 184 | 51–317 | 0.038 |
| CEA | 5.60 | 195 | 147–233 | 211 | 12–410 | 0.888 |
| CYFRA 21-1 | 4.40 | 643 | 438–848 | 134 | 86–182 | <0.001 |
| Prior to cycle 3 | ||||||
| HMGB1 | 2.69 | 490 | 296–684 | 134 | 98–170 | <0.001 |
| CEA | 6.00 | 279 | 30 -528 | 242 | 130–354 | 0.721 |
| CYFRA 21-1 | 3.85 | 624 | 399–849 | 167 | 103–231 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handke, N.A.; Rupp, A.B.A.; Trimpop, N.; von Pawel, J.; Holdenrieder, S. Soluble High Mobility Group Box 1 (HMGB1) Is a Promising Biomarker for Prediction of Therapy Response and Prognosis in Advanced Lung Cancer Patients. Diagnostics 2021, 11, 356. https://doi.org/10.3390/diagnostics11020356
Handke NA, Rupp ABA, Trimpop N, von Pawel J, Holdenrieder S. Soluble High Mobility Group Box 1 (HMGB1) Is a Promising Biomarker for Prediction of Therapy Response and Prognosis in Advanced Lung Cancer Patients. Diagnostics. 2021; 11(2):356. https://doi.org/10.3390/diagnostics11020356
Chicago/Turabian StyleHandke, Nikolaus A., Alexander B. A. Rupp, Nicolai Trimpop, Joachim von Pawel, and Stefan Holdenrieder. 2021. "Soluble High Mobility Group Box 1 (HMGB1) Is a Promising Biomarker for Prediction of Therapy Response and Prognosis in Advanced Lung Cancer Patients" Diagnostics 11, no. 2: 356. https://doi.org/10.3390/diagnostics11020356
APA StyleHandke, N. A., Rupp, A. B. A., Trimpop, N., von Pawel, J., & Holdenrieder, S. (2021). Soluble High Mobility Group Box 1 (HMGB1) Is a Promising Biomarker for Prediction of Therapy Response and Prognosis in Advanced Lung Cancer Patients. Diagnostics, 11(2), 356. https://doi.org/10.3390/diagnostics11020356







