Assessment of the Trabecular Bone Microstructure Surrounding Impacted Maxillary Canines Using Fractal Analysis on Cone-Beam Computed Tomography Images †
Abstract
:1. Introduction
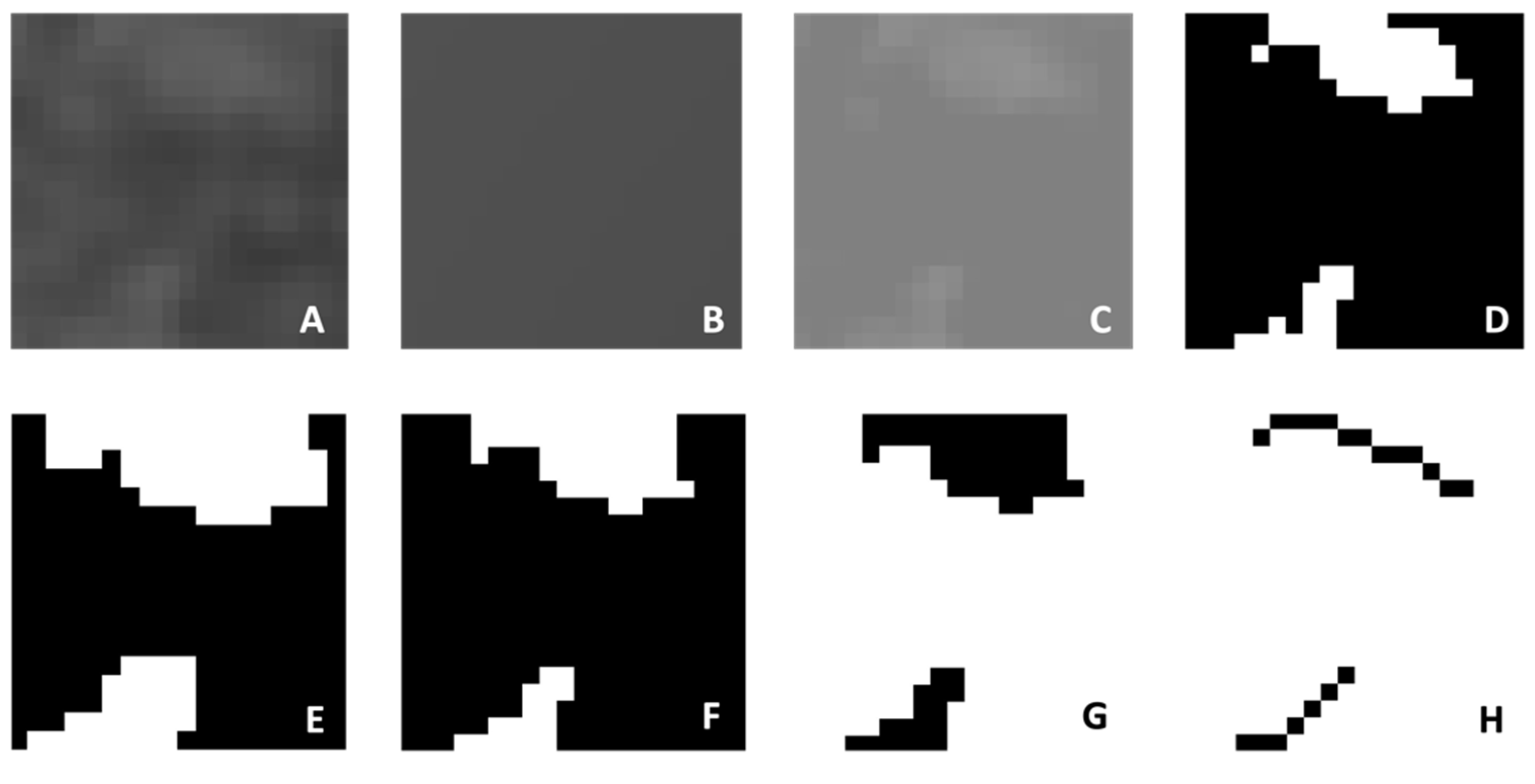
2. Materials and Methods
- Availability of CBCT images of unilateral maxillary impacted canines.
- Complete eruption of the contralateral canine.
- Absence of systemic or genetic diseases affecting bone metabolism.
- No orthodontic treatment history.
- CBCT scans acquired at a different clinic.
- Congenitally missing or extracted teeth other than third molars.
- Presence of supernumerary teeth or any cystic formations in CBCT.
- Presence of artifacts on CBCT images.
- History of dentoalveolar trauma.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thilander, B.; Myrberg, N. The prevalence of malocclusion in Swedish schoolchildren. Eur. J. Oral Sci. 1973, 81, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ericson, S.; Kurol, J. Radiographlc assessment of maxillary canine eruption in children with clinical signs of eruption disturbance. Eur. J. Orthod. 1986, 8, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Celikoglu, M.; Kamak, H.; Oktay, H. Investigation of transmigrated and impacted maxillary and mandibular canine teeth in an orthodontic patient population. J. Oral Maxillofac. Surg. 2010, 68, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Ericson, S.; Kurol, J. Early treatment of palatally erupting maxillary canines by extraction of the primary canines. Eur. J. Orthod. 1988, 10, 283–295. [Google Scholar] [CrossRef]
- Littlewood, S.J.; Mitchell, L. An Introduction to Orthodontics; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Bishara, S.E.; Ortho, D. Impacted maxillary canines: A review. Am. J. Orthod. Dentofac. Orthop. 1992, 101, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, H. The etiology of maxillary canine impactions. Am. J. Orthod. 1983, 84, 125–132. [Google Scholar] [CrossRef]
- Hamada, Y.; Timothius, C.J.C.; Shin, D.; John, V. Canine impaction–A review of the prevalence, etiology, diagnosis and treatment. Semin. Orthod. 2019, 25, 117–123. [Google Scholar] [CrossRef]
- Servais, J.A.; Gaalaas, L.; Lunos, S.; Beiraghi, S.; Larson, B.E.; Leon-Salazar, V. Alternative cone-beam computed tomography method for the analysis of bone density around impacted maxillary canines. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 442–449. [Google Scholar] [CrossRef]
- Arvind, T.P.; Jain, R.K.; Nagi, R.; Tiwari, A. Evaluation of alveolar bone microstructure around impacted maxillary canines using fractal analysis in dravidian population: A retrospective CBCT study. J. Contemp. Dent. Pract. 2022, 23, 593–600. [Google Scholar] [CrossRef]
- Secgin, C.K.; Karslıoglu, H.; Özemre, M.Ö.; Orhan, K. Gray value measurement for the evaluation of local alveolar bone density around impacted maxillary canine teeth using cone beam computed tomography. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e669. [Google Scholar] [CrossRef]
- Richardson, G.; Russell, K.A. A review of impacted permanent maxillary cuspids-diagnosis and prevention. J. Can. Dent. Assoc. 2000, 66, 497–502. [Google Scholar] [PubMed]
- Rossini, G.; Cavallini, C.; Cassetta, M.; Galluccio, G.; Barbato, E. Localization of impacted maxillary canines using cone beam computed tomography. Review of the literature. Ann. Stomatol. 2012, 3, 14–18. [Google Scholar]
- Jung, Y.; Liang, H.; Benson, B.; Flint, D.; Cho, B. The assessment of impacted maxillary canine position with panoramic radiography and cone beam CT. Dentomaxillofac. Radiol. 2012, 41, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Barra, S.G.; Tavares, N.P.; Amaral, T.M.; Brasileiro, C.B.; Mesquita, R.A. Use of fractal analysis in dental images: A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20180457. [Google Scholar] [CrossRef] [PubMed]
- Güngör, E.; Yildirim, D.; Çevik, R. Evaluation of osteoporosis in jaw bones using cone beam CT and dual-energy X-ray absorptiometry. J. Oral Sci. 2016, 58, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Nackaerts, O.; Duyck, J.; Maes, F.; Jacobs, R. Bone quality assessment based on cone beam computed tomography imaging. Clin. Oral Imp. Res. 2009, 20, 767–771. [Google Scholar] [CrossRef]
- Ling, H.; Yang, X.; Li, P.; Megalooikonomou, V.; Xu, Y.; Yang, J. Cross gender–age trabecular texture analysis in cone beam CT. Dentomaxillofac. Radiol. 2014, 43, 20130324. [Google Scholar] [CrossRef]
- Southard, T.E.; Southard, K.A.; Jakobsen, J.R.; Hillis, S.L.; Najim, C.A. Fractal dimension in radiographic analysis of alveolar process bone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 569–576. [Google Scholar] [CrossRef]
- Chugh, T.; Jain, A.K.; Jaiswal, R.K.; Mehrotra, P.; Mehrotra, R. Bone density and its importance in orthodontics. J. Oral Biol. Craniofac. Res. 2013, 3, 92–97. [Google Scholar] [CrossRef]
- Roberts, W.E. Bone physiology, metabolism, and biomechanics in orthodontic practice. In Orthodontics: Current Principles and Techniques, 4th ed.; Graber, T.M., Vanarsdall, R.L., Vig, K.W.L., Eds.; Mosby: St Louis, MO, USA, 2005; pp. 221–292. [Google Scholar]
- White, S.C.; Rudolph, D.J. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 628–635. [Google Scholar] [CrossRef]
- Ibrahim, N.; Parsa, A.; Hassan, B.; Van der Stelt, P.; Wismeijer, D. Diagnostic imaging of trabecular bone microstructure for oral implants: A literature review. Dentomaxillofac. Radiol. 2013, 42, 20120075. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, W.C.; Farman, A.G.; Sukovic, P. Clinical applications of cone-beam computed tomography in dental practice. J. Can. Dent. Assoc. 2006, 72, 75–80. [Google Scholar] [PubMed]
- Aranyarachkul, P.; Caruso, J.; Gantes, B.; Schulz, E.; Riggs, M.; Dus, I. Bone density assessments of dental implant sites: 2. Quantitative cone-beam computerized tomography. Int. J. Oral Maxillofac. Imp. 2005, 20, 416–424. [Google Scholar]
- Huh, K.-H.; Baik, J.-S.; Yi, W.-J.; Heo, M.-S.; Lee, S.-S.; Choi, S.-C. Fractal analysis of mandibular trabecular bone: Optimal tile sizes for the tile counting method. Imaging Sci. Dent. 2011, 41, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A.K.; Ergün, S.; Güneri, P.; Aktener, B.O.; Boyacıoğlu, H. Mandibular bone changes in sickle cell anemia: Fractal analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, e41–e48. [Google Scholar] [CrossRef]
- Kocak, A.T.Ö.; Bulut, D.G. Measurement of the trabecular bone structure of the TMJ region in patients with transverse maxillary deficiency: A CBCT fractal analysis study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 352–360. [Google Scholar] [CrossRef]

| Impacted Canine Group (n:41) | Control Group (n:39) | ||||
|---|---|---|---|---|---|
| Sex | n | % | n | % | p value |
| Female | 30 | 73.2 | 22 | 56.4 | 0.12 |
| Male | 11 | 26.8 | 17 | 43.6 | |
| Age (year) | Mean | SD | Mean | SD | p value |
| 15.79 | 3.29 | 19.61 | 2.38 | <0.001*** | |
| Mean | SD | p Value | ||
|---|---|---|---|---|
| Impacted Canine Group (n:41) | Impacted Side | 1.02 | 0.12 | 0.02* |
| Nonimpacted Side | 0.96 | 0.12 | ||
| Buccal Impacted Canine Group (n:11) | Impacted Side | 1.07 | 0.08 | 0.02 * |
| Nonimpacted Side | 0.95 | 0.13 | ||
| Palatal Impacted Canine Group (n:30) | Impacted Side | 1.01 | 0.12 | 0.17 |
| Nonimpacted Side | 0.96 | 0.11 | ||
| Control Group (n:39) | Left Side | 0.97 | 0.11 | 0.22 |
| Right Side | 0.94 | 0.11 | ||
| Mean Difference | SD | p Value | |
|---|---|---|---|
| Palatal Impacted Canine Group (n:30) | 0.13 | 0.11 | 0.78 |
| Buccal Impacted Canine Group (n:11) | 0.14 | 0.11 | |
| Control Group (n:20) | 0.10 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunal Akturk, E.; Toktas, A.I.; Can, E.; Kosen, E.; Sarica, I. Assessment of the Trabecular Bone Microstructure Surrounding Impacted Maxillary Canines Using Fractal Analysis on Cone-Beam Computed Tomography Images. Diagnostics 2024, 14, 2143. https://doi.org/10.3390/diagnostics14192143
Sunal Akturk E, Toktas AI, Can E, Kosen E, Sarica I. Assessment of the Trabecular Bone Microstructure Surrounding Impacted Maxillary Canines Using Fractal Analysis on Cone-Beam Computed Tomography Images. Diagnostics. 2024; 14(19):2143. https://doi.org/10.3390/diagnostics14192143
Chicago/Turabian StyleSunal Akturk, Ezgi, Ahsen Irem Toktas, Erkay Can, Ezgi Kosen, and Irfan Sarica. 2024. "Assessment of the Trabecular Bone Microstructure Surrounding Impacted Maxillary Canines Using Fractal Analysis on Cone-Beam Computed Tomography Images" Diagnostics 14, no. 19: 2143. https://doi.org/10.3390/diagnostics14192143






