Pancreatic Metastases of Esophageal Squamous Cell Carcinoma: A Case Report and Review of the Literature
Abstract
:1. Introduction
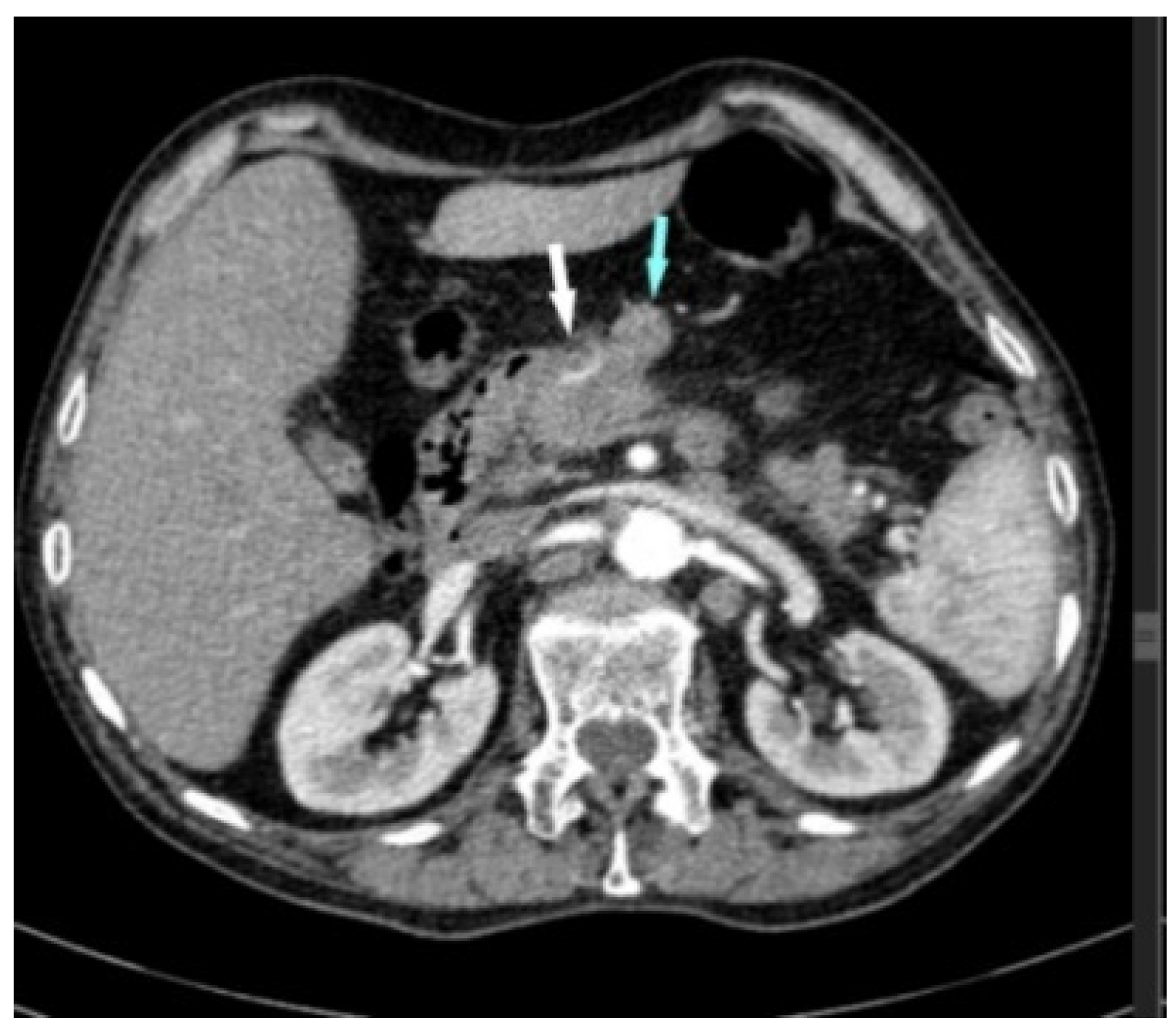
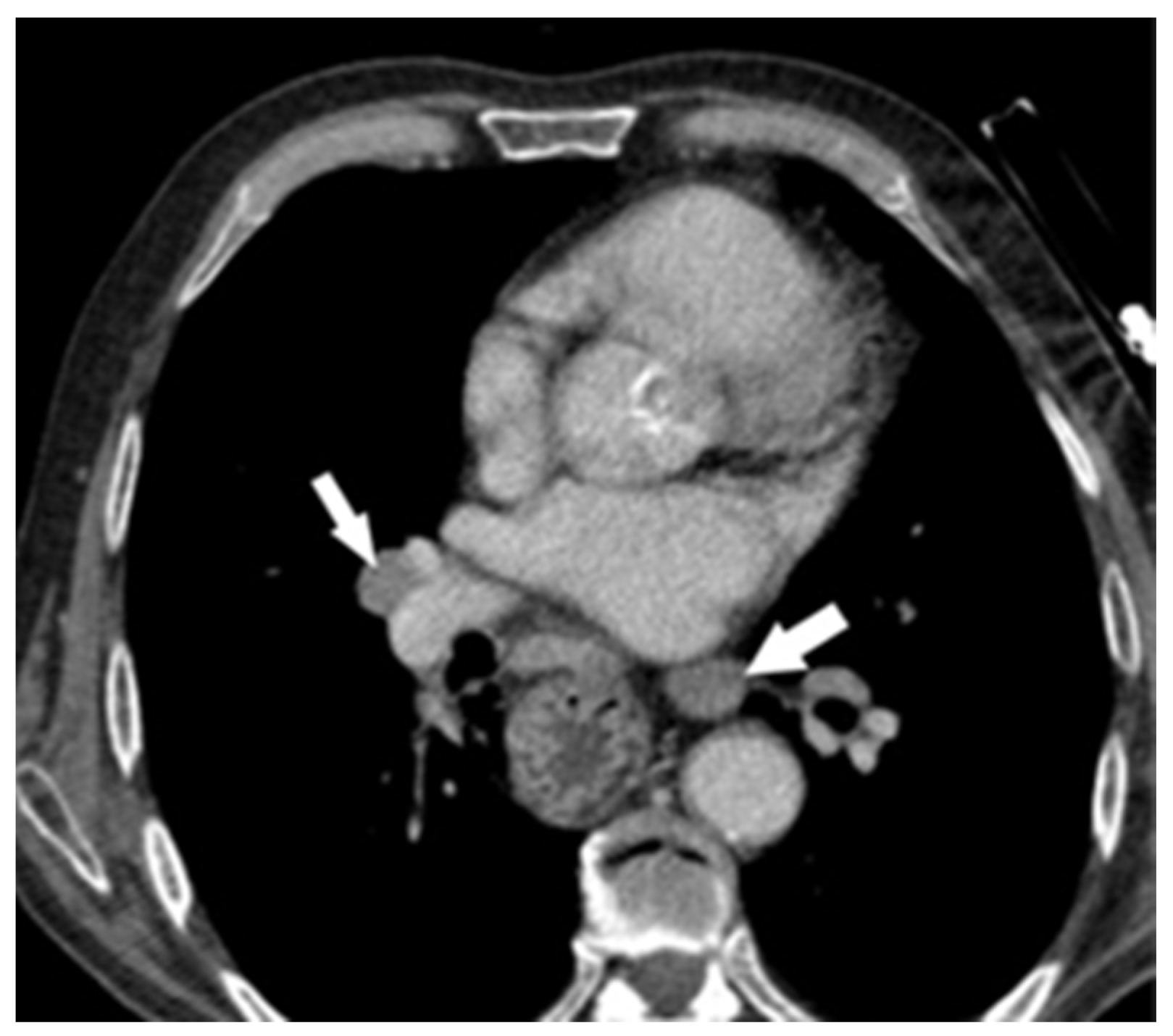
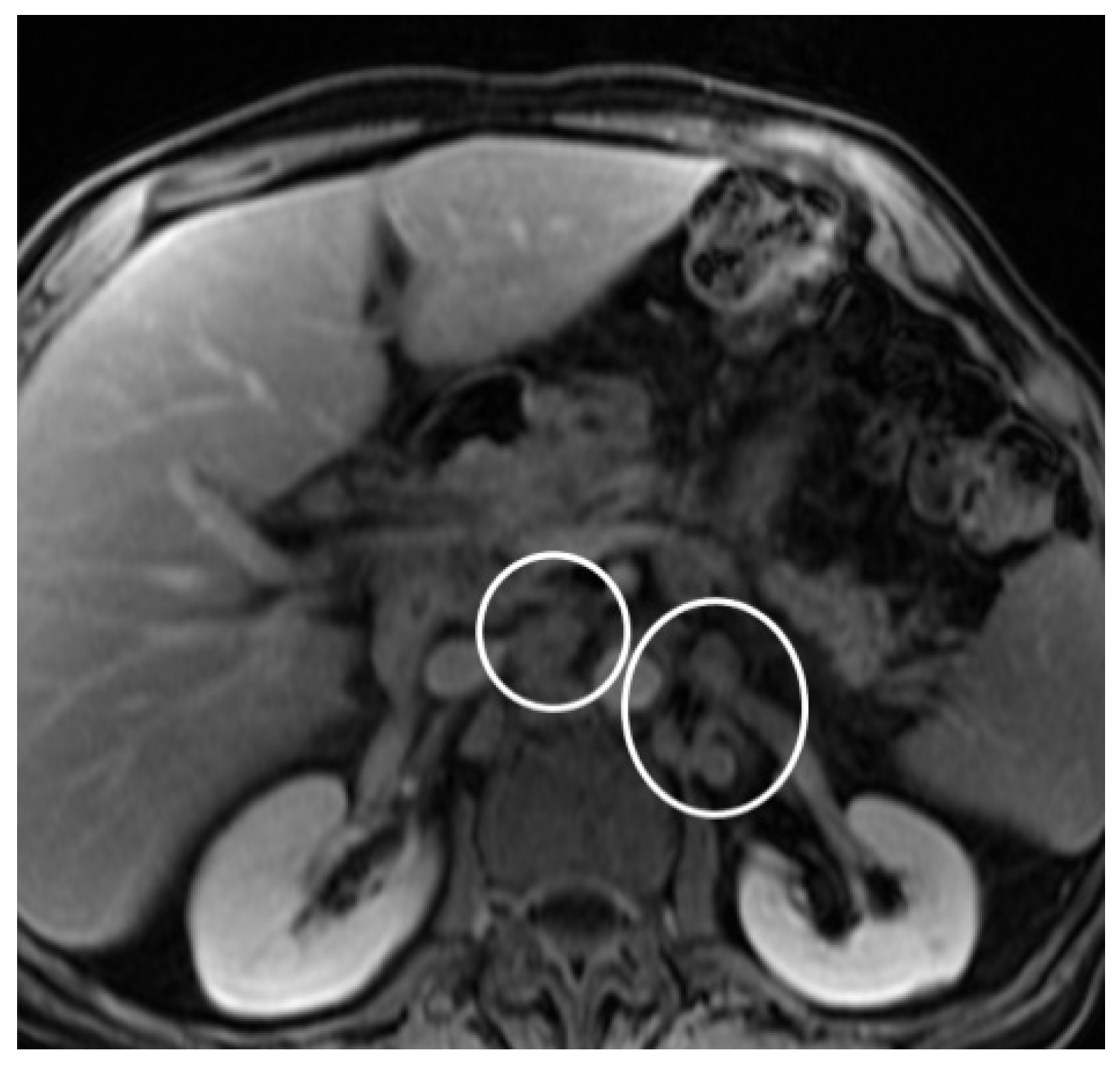
2. Case Report
Search Strategy
3. Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaheen, O.; Ghibour, A.; Alsaid, B. Esophageal Cancer Metastases to Unexpected Sites: A Systematic Review. Gastroenterol. Res. Prac. 2017, 2017, 1657310. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Hara, Y.; Chin, M.; Hagiwara, M.; Onodera, Y.; Horii, S.; Shirahata, Y.; Kamei, T.; Hashizume, E.; Ohuchi, N. An extremely rare case of pancreatic metastasis of esophageal squamous cell carcinoma. World J. Gastroenterol. 2014, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jang, J.Y.; Kim, Y.H.; Hwang, E.J.; Na, K.Y.; Kim, K.Y.; Park, J.H.; Chang, Y.W. A case of esophageal squamous cell carcinoma with pancreatic metastasis. Clin. Endosc. 2013, 46, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Nassar, Y.; Tejada-Almonte, J.; Mansoor, M.S.; Umrau, K.; Hida, S. An Entirely Atypical Presentation of Esophageal Squamous Cell Cancer with Pancreatic and Bone Metastases. Case Rep. Gastroenterol. 2019, 13, 423–429. [Google Scholar] [CrossRef]
- Sawada, T.; Adachi, Y.; Noda, M.; Akino, K.; Kikuchi, T.; Mita, H.; Ishii, Y.; Endo, T. Hepatic portal venous gas in pancreatic solitary metastasis from an esophageal squamous cell carcinoma. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 103–105. [Google Scholar] [CrossRef]
- Ahmadi Somaghian, S.; Mirzaei, S.; Aaliehpour, A.; Baharvand IranNia, Y. Solitary synchronous pancreatic metastasis from esophageal squamous cell carcinoma: A rare case report. Middle East. J. Cancer 2021, 12, 310–314. [Google Scholar] [CrossRef]
- Zhang, L.; Long, X.; Hu, Z.N.; Wu, Y.; Song, J.; Zhang, B.X.; Chen, W.X. An extremely atypical presentation of esophageal squamous cell carcinoma with pancreatic and hepatic metastases: A case report and overview of the literature. Medicine 2021, 100, e25785. [Google Scholar] [CrossRef]
- Koizumi, W.; Kitago, M.; Shinoda, M.; Yagi, H.; Abe, Y.; Oshima, G.; Hori, S.; Inomata, K.; Kawakubo, H.; Kawaida, M.; et al. Successful resection of pancreatic metastasis from oesophageal squamous cell carcinoma: A case report and review of the literature. BMC Cancer 2019, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Esfehani, M.H.; Mahmoodzadeh, H.; Alibakhshi, A.; Safavi, F. Esophageal squamous cell carcinoma with pancreatic metastasis: A case report. Acta Med. Iran. 2011, 49, 760–762. [Google Scholar]
- Denda, Y.; Matsuo, Y.; Nonoyama, K.; Murase, H.; Kato, T.; Hayashi, Y.; Imafuji, H.; Saito, K.; Morimoto, M.; Kato, H.; et al. Simultaneous presentation and resection of esophageal cancer and metastasis to the pancreas: A case report and literature review. Mol. Clin. Oncol. 2023, 20, 2. [Google Scholar] [CrossRef]
- Akiyama, H.; Miyazono, H.; Tsurumaru, M.; Hashimoto, C.; Kawamura, T. Use of the stomach as an esophageal substitute. Ann. Surg. 1978, 188, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lee, Y.; Rodriguez, T.; Lee, J.; Saif, M.W. Secondary tumors of the pancreas: A case series. Anticancer Res. 2012, 32, 1449–1452. [Google Scholar] [PubMed]
- Yoon, W.J.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B.; Kim, S.W.; Kim, W.H. Clinical Features of Metastatic Tumors of the Pancreas in Korea: A SingleCenter Study. Gut Liver 2011, 5, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, A.A.; Shin, S.H.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Park, K.M.; Lee, Y.J.; Kim, S.C. Pancreatectomy for a secondary metastasis to the pancreas: A single-institution experience. Medicine 2018, 97, e12653. [Google Scholar] [CrossRef] [PubMed]
- Dewanwala, A.; Kotowski, A.; Levea, C.M. Secondary tumors of the pancreas: Case report and a single-center experience. J. Gastrointest. Cancer 2012, 43 (Suppl. S1), 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pozza, G.; Brazzale, A.R.; Buratin, A.; Moletta, L.; Beltrame, V.; Valmasoni, M. Metastatic tumors to the pancreas: A systematic review and meta-analysis. Minerva Chir. 2016, 71, 337–344. [Google Scholar]
- Geramizadeh, B.; Kashkooe, A.; Nikeghbalian, S.; Malek-Hosseini, S.A. Metastatic Tumors to the Pancreas, a Single Center Study. Arch. Iran. Med. 2019, 22, 50–52. [Google Scholar]
- Waters, L.; Si, Q.; Caraway, N.; Mody, D.; Staerkel, G.; Sneige, N. Secondary tumors of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: A 10-year experience. Diagn. Cytopathol. 2014, 42, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Alomari, A.K.; Ustun, B.; Aslanian, H.R.; Ge, X.; Chhieng, D.; Cai, G. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of secondary tumors involving the pancreas: An institution’s experience. Cytojournal 2016, 13, 1. [Google Scholar] [CrossRef]
- Hou, Y.; Shen, R.; Tonkovich, D.; Li, Z. Endoscopic ultrasound-guided fine-needle aspiration diagnosis of secondary tumors involving pancreas: An institution’s experience. J. Am. Soc. Cytopathol. 2018, 7, 261–267. [Google Scholar] [CrossRef]
- Endo, Y.; Noda, H.; Watanabe, F.; Kato, T.; Kakizawa, N.; Ichida, K.; Kasahara, N.; Rikiyama, T. A Retrospective Analysis of Preoperative Evaluation and Surgical Resection for Metastatic Tumors of the Pancreas. Indian. J. Surg. Oncol. 2019, 10, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Moletta, L.; Patanè, G. Metastatic tumors to the pancreas: The role of surgery. World J. Gastrointest. Oncol. 2014, 6, 381–392. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, H.; Liu, C.; Jin, K.; Fan, K.; Cheng, H.; Fan, Z.; Yang, C.; Liu, L.; Long, J.; et al. Surgical Resection for Metastatic Tumors in the Pancreas: A Single-Center Experience and Systematic Review. Ann. Surg. Oncol. 2019, 26, 1649–1656. [Google Scholar] [CrossRef]
- Yagi, T.; Hashimoto, D.; Taki, K.; Yamamura, K.; Chikamoto, A.; Ohmuraya, M.; Beppu, T.; Baba, H. Surgery for metastatic tumors of the pancreas. Surg. Case Rep. 2017, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Merigliano, S.; Moletta, L. Metastatic pancreatic tumors: What is the optimal treatment? Minerva Chir. 2015, 70, 131–139. [Google Scholar] [PubMed]
- Adler, H.; Redmond, C.E.; Heneghan, H.M.; Swan, N.; Maguire, D.; Traynor, O.; Hoti, E.; Geoghegan, J.G.; Conlon, K.C. Pancreatectomy for metastatic disease: A systematic review. Eur. J. Surg. Oncol. 2014, 40, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Redmond, C.E.; Adler, H.; Heneghan, H.M.; Kelly, R.; Swan, N.; Cantwell, C.P.; Maguire, D.; Traynor, O.; Hoti, E.; Geoghegan, J.G.; et al. Pancreatic metastasectomy: Experience of the Irish National Surgical Centre for Pancreatic Cancer. Ir. J. Med. Sci. 2014, 183, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Gemenetzis, G.; Cooper, M.; Javed, A.A.; Cameron, J.L.; Wolfgang, C.L.; Eckhauser, F.E.; He, J.; Weiss, M.J. Long-Term Outcomes of 98 Surgically Resected Metastatic Tumors in the Pancreas. Ann. Surg. Oncol. 2017, 24, 801–807. [Google Scholar] [CrossRef]
- Chikhladze, S.; Lederer, A.K.; Kühlbrey, C.M.; Hipp, J.; Sick, O.; Fichtner-Feigl, S.; Wittel, U.A. Curative-intent pancreas resection for pancreatic metastases: Surgical and oncological results. Clin. Exp. Metastasis 2020, 37, 313–324. [Google Scholar] [CrossRef]
- Pierzankowski, I.; Bednarczyk, M.; Dmitruk, A.; Hevelke, P.; Maj, T.; Saramak, P.; Surowski, P.; Szpakowski, M.; Zając, L.; Zyskowski, Ł.; et al. Metastatic tumors of pancreas—Whether and when surgical intervention is gainful for diseased people. Retrospective analysis of data from three surgery centers. Nowotw. J. Oncol. 2018, 68, 240–244. [Google Scholar] [CrossRef]
- Cheng, S.K.; Chuah, K.L. Metastatic Renal Cell Carcinoma to the Pancreas: A Review. Arch. Pathol. Lab. Med. 2016, 140, 598–602. [Google Scholar] [CrossRef]
- Machairas, N.; Paspala, A.; Schizas, D.; Ntomi, V.; Moris, D.; Tsilimigras, D.I.; Misiakos, E.P.; Machairas, A. Metastatic squamous cell carcinoma to the pancreas: Report of an extremely rare case. Mol. Clin. Oncol. 2019, 10, 144–146. [Google Scholar] [CrossRef]
- Abdul-Ghafar, J.; Ud Din, N.; Saadaat, R.; Ahmad, Z. Metastatic renal cell carcinoma to pancreas and gastrointestinal tract: A clinicopathological study of 3 cases and review of literature. BMC Urol. 2021, 21, 84. [Google Scholar] [CrossRef]
- Liang, X.K.; Li, L.J.; He, Y.M.; Xu, Z.F. Misdiagnosis of pancreatic metastasis from renal cell carcinoma: A case report. World J. Clin. Cases 2022, 10, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, M.; Myoteri, D.; Kotsis, T.; Polymeneas, G.; Bournakis, E.; Dellaportas, D. Solitary Colorectal Cancer Metastasis to the Pancreas. Case Rep. Surg. 2019, 2019, 4891512. [Google Scholar] [CrossRef]
- Tani, R.; Hori, T.; Yamada, M.; Yamamoto, H.; Harada, H.; Yamamoto, M.; Yazawa, T.; Tani, M.; Kamada, Y.; Aoyama, R.; et al. Metachronous Pancreatic Metastasis from Rectal Cancer that Masqueraded as a Primary Pancreatic Cancer: A Rare and Difficult-to-Diagnose Metastatic Tumor in the Pancreas. Am. J. Case Rep. 2019, 20, 1781–1787. [Google Scholar] [CrossRef]
- Yoon, J.; Petrosyan, A.; Wang, T.; Ameer, A. Colonic adenocarcinoma with synchronous metastasis to the pancreas: A case report and literature review. JGH Open 2022, 6, 274–276. [Google Scholar] [CrossRef]
- Bartosch, C.; Afonso, M.; Pires-Luís, A.S.; Galaghar, A.; Guimarães, M.; Antunes, L.; Lopes, J.M. Distant Metastases in Uterine Leiomyosarcomas: The Wide Variety of Body Sites and Time Intervals to Metastatic Relapse. Int. J. Gynecol. Pathol. 2017, 36, 31–41. [Google Scholar] [CrossRef]
- Polimera, H.; Moku, P.; Abusharar, S.P.; Vasekar, M.; Chintanaboina, J. Metastasis of Ewing Sarcoma to the Pancreas: Case Report and Literature Review. Case Rep. Oncol. Med. 2020, 2020, 7075048. [Google Scholar] [CrossRef]
- Apodaca-Rueda, M.; Chaim, F.H.M.; Garcia, M.D.S.; Saito, H.P.A.; Gestic, M.A.; Utrini, M.P.; Callejas-Neto, F.; Chaim, E.A.; Cazzo, E. Solitary pancreatic metastasis from breast cancer: Case report and review of literature. Sao Paulo Med. J. 2019, 137, 201–205. [Google Scholar] [CrossRef]
- Jaén-Torrejimeno, I.; López-Guerra, D.; Rojas-Holguín, A.; De-Armas-Conde, N.; Blanco-Fernández, G. Resection of isolated pancreatic metastases from pulmonary neoplasia: A systematic review. Updates Surg. 2022, 74, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Tumour Evolution and Seed and Soil Mechanism in Pancreatic Metastases of Renal Cell Carcinoma. Cancers 2021, 13, 1342. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Isolated Pancreatic Metastases of Renal Cell Carcinoma—Clinical Particularities and Seed and Soil Hypothesis. Cancers 2023, 15, 339. [Google Scholar] [CrossRef]
- Hedenström, P.; Demir, A.; Khodakaram, K.; Nilsson, O.; Sadik, R. EUS-guided reverse bevel fine-needle biopsy sampling and open tip fine-needle aspiration in solid pancreatic lesions—A prospective, comparative study. Scand. J. Gastroenterol. 2018, 53, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Meng, Y.; Lu, M.; Tian, C.; Wang, M.; Zhang, J. Primary squamous cell carcinoma of the pancreas with a large pseudocyst of the pancreas as the first manifestation: A rare case report and literature review. BMC Gastroenterol. 2021, 21, 208. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Z.Z.; Xu, G.H.; Jiang, X.; Wang, X.X.; Wang, Q.F. Primary squamous cell carcinoma of the pancreas with effective comprehensive treatment: A case report and literature review. Medicine 2018, 97, e12253. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Exocrine Pancreas, Ampulla of Vater and Distal Common Bile Duct Structured Reporting Protocol, 2nd ed.; Incorporating the: International Collaboration on Cancer Reporting (ICCR) Carcinoma of the Exocrine Pancreas Dataset; RCPA: Surry Hills, NSW, Australia, 2020; ISBN 978-1-76081-426-7. Available online: www.ICCR-Cancer.org (accessed on 18 April 2022).
- Singla, N.; Xie, Z.; Zhang, Z.; Gao, M.; Yousuf, Q.; Onabolu, O.; McKenzie, T.; Tcheuyap, V.T.; Ma, Y.; Choi, J.; et al. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight 2020, 5, e134564. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.; Li, J.; Qiu, S.; Hao, S.; Yue, Z.Y.; Zhai, S.; Li, M.Y.; Liu, Y. Pancreatic cancer environment: From patient-derived models to single-cell omics. Mol. Omics 2024, 20, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, J.; Qin, K.; Zhou, Y.; Ying, X.; Yuan, F.; Shi, M.; Jin, J.; Wang, D.; Gu, J.; et al. Resection of pancreatic metastatic renal cell carcinoma: Experience and long-term survival outcome from a large center in China. Int. J. Clin. Oncol. 2019, 24, 686–693. [Google Scholar] [CrossRef]
- Letaief Ksontini, F.; Khrouf, S.; Kacem, S.; Haddad, A.; Magherbi, H.; Chaker, Y.; Ayadi, M.; Ben Safta, Z. Pancreatic Metastasis of Renal Cell Carcinoma. A Surgical Indication for a Disseminated Disease. Case Rep. Med. 2021, 2021, 5579385. [Google Scholar] [CrossRef]
- Boussios, S.; Zerdes, I.; Batsi, O.; Papakostas, V.P.; Seraj, E.; Pentheroudakis, G.; Glantzounis, G.K. Pancreatic resection for renal cell carcinoma metastasis: An exceptionally rare coexistence. Int. J. Surg. Case Rep. 2016, 27, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Jaen-Torrejimeno, I.; Rojas-Holguín, A.; López-Guerra, D.; Ramia, J.M.; Blanco-Fernández, G. Pancreatic resection for metastatic renal cell carcinoma. A systematic review. HPB 2020, 22, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, M.; Chua, K.J.; Khizir, L.; Tabakin, A.; Singer, E.A. Role of metastasectomy in the management of renal cell carcinoma. Front. Surg. 2022, 9, 943604. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Williams, G.A.; Sanford, D.E.; Liu, J.; Dageforde, L.A.; Hammill, C.W.; Fields, R.C.; Hawkins, W.G.; Strasberg, S.M.; Doyle, M.B.; et al. A 22-year Experience with Pancreatic Resection for Metastatic Renal Cell Carcinoma. HPB 2020, 22, 312–317. [Google Scholar] [CrossRef]



| Nr | Authors (Year of Publication) | Age (Years) | Sex | Therapy of ESCC | Interval between ESCC and Pancreatic Metastasis (Months) | Surgery of Pancreatic Metastasis | Follow-Up (Months) | Localization of Pancreatic Metastasis |
|---|---|---|---|---|---|---|---|---|
| 1 | Esfehani et al. (2011) [9] | 59 | F | S + AT | 48 | DP | 4 | body-tail |
| 2 | Park et al. (2013) [3] | 58 | M | S + AT | Synchronous | DP | 6 | tail |
| 3 | Sawada et al. (2013) [5] | 73 | M | No | Synchronous | No | 3 | body |
| 4 | Okamoto et al. (2014) [2] | 68 | M | NAT + S | 24 | DP | 9 | body |
| 5 | Koizumi et al. (2019) [8] | 81 | F | S | 132 | DP | 24 | tail |
| 6 | Yoon et al. (2019) [4] | 67 | M | AT | Synchronous | No | head | |
| 7 | Ahmadi Somaghian et al. (2021) [6] | 78 | F | AT | Synchronous | No | 9 | body-head |
| 8 | Zang et al. (2021) [7] | 67 | M | AT | Synchronous | No | 1 | body-tail |
| 9 | Denda et al. (2023) [10] | 54 | M | S + AT | Synchronous | DP | 9 | tail |
| 10 | Present case (2024) | 75 | M | S | 67 | No | 7 | body-head |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bara, T., Jr.; Scurtu, A.G.; Bara, T.; Fulop, Z.Z.; Moriczi, R.; Simu, P.; Borz, P.; Gurzu, S. Pancreatic Metastases of Esophageal Squamous Cell Carcinoma: A Case Report and Review of the Literature. Diagnostics 2024, 14, 2164. https://doi.org/10.3390/diagnostics14192164
Bara T Jr., Scurtu AG, Bara T, Fulop ZZ, Moriczi R, Simu P, Borz P, Gurzu S. Pancreatic Metastases of Esophageal Squamous Cell Carcinoma: A Case Report and Review of the Literature. Diagnostics. 2024; 14(19):2164. https://doi.org/10.3390/diagnostics14192164
Chicago/Turabian StyleBara, Tivadar, Jr., Alexandra Georgiana Scurtu, Tivadar Bara, Zsolt Zoltan Fulop, Renata Moriczi, Patricia Simu, Paul Borz, and Simona Gurzu. 2024. "Pancreatic Metastases of Esophageal Squamous Cell Carcinoma: A Case Report and Review of the Literature" Diagnostics 14, no. 19: 2164. https://doi.org/10.3390/diagnostics14192164






