A Comprehensive Approach to Predicting the Outcomes of Transsphenoidal Endoscopic Adenomectomy in Patients with Cushing’s Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Comparison of Preoperative and Postoperative Examination Data in Patients with Remission and Persistence of CD One Year after TSS
3.2.1. Pituitary MRI
3.2.2. HDDST
3.2.3. Morning Serum Cortisol Collected 24 h after TSS
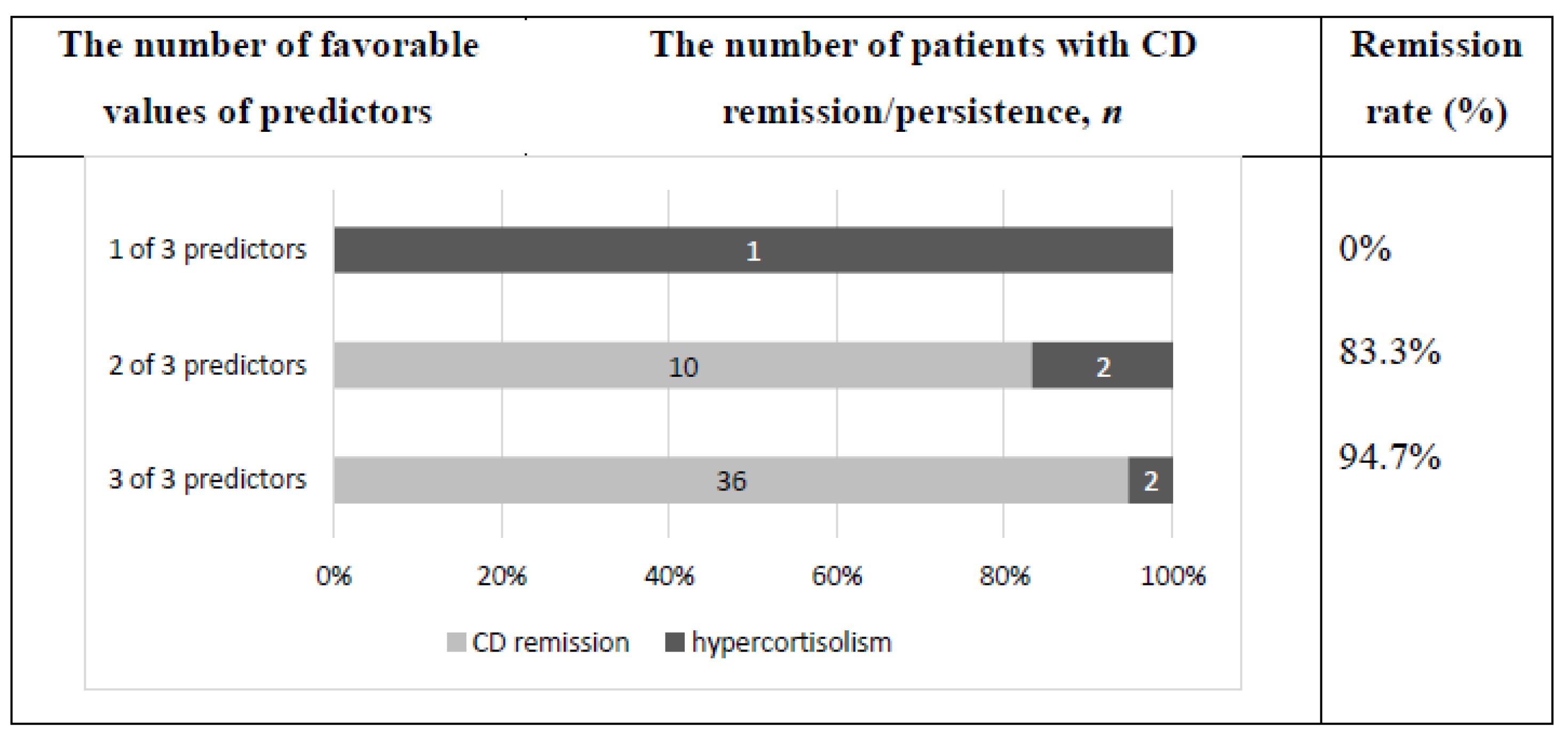
3.3. Combination of Identified Predictors for Forecast of CD Remission One Year after TSS
3.4. Results of the Long-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newell-Price, J.; Trainer, P.J.; Besser, G.M.; Grossman, A.B. The diagnosis and differential diagnosis of Cushing’s and pseudo-Cushing’s states. Endocr. Rev. 1999, 19, 647–672. [Google Scholar] [CrossRef] [Green Version]
- Bertagna, X.; Guignat, L.; Groussin, L.; Bertherat, J. Cushing’s disease. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 607–623. [Google Scholar] [CrossRef]
- Arnaldi, G.; Angeli, A.; Atkinson, A.B.; Bertagna, X.; Cavagnini, F.; Chrousos, G.P.; Fava, G.A.; Findling, J.W.; Gaillard, R.C.; Grossman, A.B.; et al. Diagnosis and complications of Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2003, 88, 5593–5602. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Cushing’s syndrome: Epidemiology and developments in disease management. Clin. Epidemiol. 2015, 7, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Invitti, C.; Giraldi, F.P.; De Martin, M.; Cavagnini, F. The Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypotalamic-Pituitary-Adrenal Axis Diagnosis and Management of Cushing’s syndrome: Results of an Italian Multicentre Study. J. Clin. Endocrinol. Metab. 1999, 84, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Clayton, R.N.; Raskauskiene, D.; Reulen, R.C.; Jones, P.W. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: Audit and meta-analysis of literature. J. Clin. Endocrinol. Metab. 2011, 96, 632–642. [Google Scholar] [CrossRef]
- Abellán, G.P.; Fajardo, M.C.; Suárez, P.A.R.; Gómez, V.J.; Escrivá, C.M.; Lillo, V.R. Predictors of longterm remission after transsphenoidal surgery in Cushing’s disease. Endocrinol. Nutr. 2013, 60, 475–482. [Google Scholar] [CrossRef]
- Biller, B.M.K.; Grossman, A.B.; Stewar, P.M.; Melmed, S.; Bertagna, X.; Bertherat, J.; Buchfelder, M.; Colao, A.; Hermus, A.R.; Hofland, L.J.; et al. Treatment of adrenocorticotropindependent Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2008, 93, 2454–2462. [Google Scholar] [CrossRef] [Green Version]
- Petersenn, S.; Beckers, A.; Ferone, D.; van der Lely, A.; Bollerslev, J.; Boscaro, M.; Brue, T.; Bruzzi, T.P.; Casanueva, F.F.; Chanson, P. Outcomes in patients with Cushing’s disease undergoing transsphenoidal surgery: Systematic review assessing criteria used to defineremission and recurrence. Eur. J. Endocrinol. 2015, 172, R227–R239. [Google Scholar] [CrossRef] [Green Version]
- Hameed, N.; Yedinak, C.G.; Brzana, J.; Gultekin, S.H.; Coppa, N.D.; Dogan, A.; Delashaw, J.B.; Fleseriu, M. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: A large single center experience. Pituitary 2013, 16, 452–458. [Google Scholar] [CrossRef]
- Hofmann, B.M.; Hlavac, M.; Martinez, R.; Buchfelde, M.; Muller, O.A.; Fahlbusch, R. Long-term results after microsurgery for Cushing disease: Experience with 426 primary operations over 35 years. J. Neurosurg. 2008, 108, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Mathioudakis, N.; Pendleton, C.; Quinones-Hinojosa, A.; Wand, G.S.; Salvatori, R. ACTH-secreting pituitary adenomas: Size does not correlate with hormonal activity. Pituitary 2012, 15, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Fomekong, E.; Maiter, D.; Grandin, C.; Raftopoulos, C. Outcome of transsphenoidal surgery for Cushing’s disease: A high remission rate in ACTH-secreting macroadenomas. Clin. Neurol. Neurosurg. 2009, 111, 442–449. [Google Scholar] [CrossRef]
- Esposito, F.; Dusick, J.R.; Cohan, P.; Moftakhar, P.; McArthur, D.; Wang, C.; Swerdloff, R.S.; Kelly, D.F. Clinical review: Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 2006, 91, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Pendharkar, A.V.; Sussman, E.S.; Ho, A.L.; Gephart, M.G.H.; Katznelson, L. Cushing’s disease: Predicting long-term remission after surgical treatment. Neurosurg. Focus 2015, 38, E13. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Oldfield, E.H.; Stratakis, C.A.; Nieman, L.K. The postoperative basal cortisol and CRH tests for prediction of longterm remission from Cushing’s disease after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2011, 96, 2057–2064. [Google Scholar] [CrossRef]
- Carrasco, C.A.; Coste, J.; Guignat, L.; Groussin, L.; Dugué, M.A.; Gaillard, S. Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J. Clin. Endocrinol. Metab. 2008, 93, 4728–4734. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, L.; Laws, E.R.; Dodd, R.L.; Monita, M.M.; Tannenbaum, C.E.; Kirkeby, K.M.; Chu, O.S.; Harsh, G.R., IV; Katznelson, L. The dynamics of post-operative plasma ACTH values following transsphenoidal surgery for Cushing’s disease. Pituitary 2011, 14, 312–317. [Google Scholar] [CrossRef]
- Alwani, R.A.; de Herder, W.W.; van Aken, M.O.; van den Berge, J.H.; Delwel, E.J.; Dallenga, A.H.G. Biochemical predictors of outcome of pituitary surgery for Cushing’s disease. Neuroendocrinology 2010, 91, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Colombo, P.; Dall’Asta, C.; Barbetta, L.; Re, T.; Passini, E.; Faglia, G.; Ambrosi, B. Usefulness of the desmopressin test in the postoperative evaluation of patients with Cushing’s disease. Eur. J. Endocrinol. 2000, 143, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Romanholi, D.J.P.C.; Machado, M.C.; Pereira, C.C.; Danilovic, D.S.; Pereira, M.A.A.; Cescato, V.A.S.; Neto, M.B.C.C.; Musolino, N.R.C.; de Mendonça, B.B.; Salgado, L.R. Role for postoperative cortisol response to desmopressin in predicting the risk for recurrent Cushing’s disease. Clin. Endocrinol. 2008, 69, 117–122. [Google Scholar] [CrossRef]
- Le Marc’hadour, P.; Muller, M.; Albarel, F.; Coulon, A.L.; Morange, I.; Martinie, M.; Gay, E.; Graillon, T.; Dufour, H.; Conte-Devolx, B.; et al. Postoperative follow-up of Cushing’s disease using cortisol, desmopressin and coupled dexamethasone-desmopressin tests: A head-to-head comparison. Clin. Endocrinol. 2015, 83, 216–222. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, N.; Walia, R.; Bhansali, A.; Dutta, P.; Bhadada, S.K.; Pivonello, R.; Ahuja, C.K.; Dhandapani, S.; Hajela, A.; et al. Remission in Cushing’s disease is predicted by cortisol burden and its withdrawal following pituitary surgery. J. Endocrinol. Investig. 2021, 44, 1869–1878. [Google Scholar] [CrossRef]
- Yap, L.B.; Turner, H.E.; Adams, C.B.; Wass, J.A. Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: A single centre audit. Clin. Endocrinol. 2002, 56, 25–31. [Google Scholar] [CrossRef]
- Patil, C.G.; Prevedello, D.M.; Lad, S.P.; Vance, M.L.; Thorner, M.O.; Katznelson, L.; Laws, E.R., Jr. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2008, 93, 358–362. [Google Scholar] [CrossRef]
- Aranda, G.; Enseñat, J.; Mora, M.; Puig-Domingo, M.; Martínez de Osaba, M.J.; Casals, G.; Verger, E.; Ribalta, M.T.; Hanzu, F.A.; Halperin, I. Long-term remission and recurrence rate in a cohort of Cushing’s disease: The need for long-term follow-up. Pituitary 2015, 18, 142–149. [Google Scholar] [CrossRef]
- Ambrogio, A.G.; Andrioli, M.; De Martin, M.; Cavagnini, F.; Giraldi, F.P. Usefulness of desmopressin testing to predict relapse during long-term follow-up in patients in remission from Cushing’s disease. Endocr. Connect. 2017, 6, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Stroud, A.; Dhaliwal, P.; Alvarado, R.; Winder, M.J.; Jonker, B.P.; Grayson, J.W.; Hamizan, A.; Harvey, R.J.; McCormack, A. Outcomes of pituitary surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary 2020, 23, 595–609. [Google Scholar] [CrossRef]
- Teramoto, A.; Nemoto, S.; Takakura, K.; Sasaki, Y.; Machida, T. Selective venous sampling directly from cavernous sinus in Gushing’s syndrome. J. Clin. Endocrinol. Metab. 1993, 76, 637–641. [Google Scholar] [CrossRef]
- Graham, K.E.; Samuels, M.H.; Nesbit, G.M.; Cook, D.M.; O’Neill, O.R.; Barnwell, S.L.; Loriaux, D.L. Cavernous sinus sampling is highly accurate indistinguishing Cushing’s disease from the ectopic adrenocorticotropin syndrome and in predicting intrapituitary tumor location. J. Clin. Endocrinol. Metab. 1999, 84, 1602–1610. [Google Scholar] [CrossRef] [Green Version]
- Potts, M.B.; Shah, J.K.; Molinaro, A.M.; Blevins, L.S.; Tyrrell, J.B.; Kunwar, S.; Dowd, C.D.; Hetts, S.W.; Aghi, M.K. Cavernous and inferior petrosal sinus sampling and dynamic magnetic resonance imaging in the preoperative evaluation of Cushing’s disease. J. Neurooncol. 2014, 116, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Kurimoto, M.; Kubo, M.; Kuwayama, N.; Kurosaki, K.; Nagai, S. The impact of cavernous sinus drainage pattern on the results of venous sampling in patients with suspected Cushing syndrome. Am. J. Neuroradiol. 2008, 29, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin. Neurosurg. 1969, 16, 185–217. [Google Scholar] [CrossRef] [PubMed]
- Knosp, E.; Steiner, E.; Kitz, K.; Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993, 33, 610–617. [Google Scholar] [CrossRef]
- Machado, M.C.; Alcantara, A.E.; Pereira, A.C.; Cescato, V.A.; Castro Musolino, N.R.; de Mendonça, B.B.; Bronstein, M.D.; Fragoso, M.C. Negative correlation between tumour size and cortisol/ACTH ratios in patients with Cushing’s disease harbouring microadenomas or macroadenomas. J. Endocrinol. Investig. 2016, 39, 1401–1409. [Google Scholar] [CrossRef]
- Budan, R.M.; Georgescu, C.E. Multiple Pituitary Adenomas: A Systematic Review. Front. Endocrinol. 2016, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Ratliff, J.K.; Oldfield, E.H. Multiple pituitary adenomas in Cushing’s disease. J. Neurosurg. 2000, 93, 753–761. [Google Scholar] [CrossRef]
- Sud, T.; Kageyama, K.; Nigawara, T.; Sakihara, S. Evaluation of diagnostic tests for ACTH-dependent Cushing’s syndrome. Endocr. J. 2009, 56, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Aron, D.C.; Raff, H.; Findling, J.W. Effectiveness versus efficacy: The limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1997, 82, 1780–1785. [Google Scholar] [CrossRef]
- Mu, Y.M.; Takayanagi, R.; Imasaki, K.; Ohe, K.; Ikuyama, S.; Yanase, T.; Nawata, H. Low level of glucocorticoid receptor messenger ribonucleic acid in pituitary adenomas manifesting Cushing’s disease with resistance to a high dose-dexamethasone suppression test. Clin. Endocrinol. 1998, 49, 301–306. [Google Scholar] [CrossRef]
- Fukuoka, H.; Shichi, H.; Yamamoto, M.; Takahashi, Y. The Mechanisms Underlying Autonomous Adrenocorticotropic Hormone Secretion in Cushing’s Disease. Int. J. Mol. Sci. 2020, 21, 9132. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Cannarile, L.; Migliorati, G.; Nocentini, G.; Delfino, D.V.; Riccardi, C. Mechanisms of the anti-inflammatory effects of glucocorticoids: Genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 2012, 26, 4805–4820. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nakao, T.; Ogawa, W.; Fukuoka, H. Aggressive Cushing’s Disease: Molecular Pathology and Its Therapeutic Approach. Front. Endocrinol. 2021, 12, 650791. [Google Scholar] [CrossRef] [PubMed]




| Remission (N = 63) | Persistence (N = 38) | p | |
|---|---|---|---|
| Male/female, n | 5/58 | 7/31 | 0.208 (χ2 = 1.588) |
| Age, years Me (25%; 75%) (min–max) | 43 (33; 53) (21–68) | 38 (28.75; 47.5) (15–72) | 0.336 |
| Midnight serum cortisol, nmol/L Me (25%; 75%) (min−max) | 496.6 (381.7; 750.2) (274.5−1453) | 605 (436; 754) (316−1006) | 0.156 |
| 24-h UFC, nmol/24-h Me (25%; 75%) (min–max) | 588.96 (419.1; 921.1) (66–6406) | 762.4 (432; 2096.5) (156.6–8740) | 0.325 |
| Morning plasma ACTH, pg/mL Me (25%; 75%) (min–max) | 75.7 (46.2; 91.4) (12.5–241.9) | 56.99 (47.1; 78) (19.64–213) | 0.201 |
| Midnight salivary cortisol, nmol/L Me (25%; 75%) (min–max) | 14.5 (8.6; 22.3) (5.42–42.11) | 11.76 (8.75; 16.5) (3.8–44.3) | 0.443 |
| Serum cortisol (LDDST), nmol/L Me (25%; 75%) (min–max) | 306.9 (163.5; 537.2) (13.24–883) | 477 (368.8; 584.2) (63.97–770.3) | 0.085 |
| Remission (N = 63) | Persistence (N = 38) | p | |
|---|---|---|---|
| Pituitary adenoma size, mm Me (25%; 75%) (min–max) | 6 (4;8) (2.2–22) | 5.5 (3; 8.5) (2–32) | 0.31 |
| Age, years Me (25%; 75%) (min–max) | 43 (33; 53) (21–68) | 38 (28.75; 47.5) (15–72) | 0.336 |
| Microadenoma/macroadenoma, n | 52/11 | 30/8 | 0.655 (χ2 = 0.2) |
| MRI-invisible adenoma, N | 2 | 3 | 0.584 (χ2 = 0.3) |
| Invasive growth, n | 3 | 21 | <0.001 (χ2 = 33.4) |
| 24 h MSeC | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 50 nmol/L | 53.9% | 100% |
| 140 nmol/L | 76.2% | 89.5% |
| 388 nmol/L | 97.4% | 79.3% |
| Predictor | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Preoperative MRI data (none-invasive adenoma size ≥ 3 mm) | 82.8% | 82.4% |
| Preoperative HDDST (serum cortisol suppression ≥ 74%) | 86.3% | 81.5% |
| 24 h MSeC ≤ 388 nmol/L | 97.4% | 79.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuritsyna, N.V.; Tsoy, U.A.; Cherebillo, V.Y.; Paltsev, A.A.; Ryzhkov, A.V.; Ryazanov, P.A.; Ryzhkov, V.K.; Grineva, E.N. A Comprehensive Approach to Predicting the Outcomes of Transsphenoidal Endoscopic Adenomectomy in Patients with Cushing’s Disease. J. Pers. Med. 2022, 12, 798. https://doi.org/10.3390/jpm12050798
Kuritsyna NV, Tsoy UA, Cherebillo VY, Paltsev AA, Ryzhkov AV, Ryazanov PA, Ryzhkov VK, Grineva EN. A Comprehensive Approach to Predicting the Outcomes of Transsphenoidal Endoscopic Adenomectomy in Patients with Cushing’s Disease. Journal of Personalized Medicine. 2022; 12(5):798. https://doi.org/10.3390/jpm12050798
Chicago/Turabian StyleKuritsyna, Natalia V., Uliana A. Tsoy, Vladislav Y. Cherebillo, Artem A. Paltsev, Anton V. Ryzhkov, Pavel A. Ryazanov, Vladimir K. Ryzhkov, and Elena N. Grineva. 2022. "A Comprehensive Approach to Predicting the Outcomes of Transsphenoidal Endoscopic Adenomectomy in Patients with Cushing’s Disease" Journal of Personalized Medicine 12, no. 5: 798. https://doi.org/10.3390/jpm12050798
APA StyleKuritsyna, N. V., Tsoy, U. A., Cherebillo, V. Y., Paltsev, A. A., Ryzhkov, A. V., Ryazanov, P. A., Ryzhkov, V. K., & Grineva, E. N. (2022). A Comprehensive Approach to Predicting the Outcomes of Transsphenoidal Endoscopic Adenomectomy in Patients with Cushing’s Disease. Journal of Personalized Medicine, 12(5), 798. https://doi.org/10.3390/jpm12050798





